Abstract
SUMMARY: The purpose of this case report is to show the diagnostic potential of diffusion-weighted MR imaging in establishing the presence of ischemic optic neuropathy (ION). We report the MR imaging findings in a patient presenting with acute ION in whom diffusion imaging showed decreased water mobility as seen in patients with acute brain ischemia.
Case Report
A 56-year-old, right-handed woman presented with a history of headache for 4 days with subsequent left-sided visual loss. Her headache gradually progressed in severity. It involved the left frontal region and eventually localized to the left retro-orbital region. She described increasingly blurred vision and presented to the emergency department on her fourth day of symptoms primarily because of sudden worsening of headache. On examination, she was found to have decreased visual acuity on the left (20/40 OS) with normal vision on the right (20/20 OD). The left optic disk was swollen. She was referred to the eye clinic for follow-up in 1 week. Her vision deteriorated over that time and, on examination, she was not able to count fingers with the left eye. There was a swelling of the left optic disk, and a left relative afferent papillary defect (RAPD) was present. The right eye remained normal. The rest of the cranial nerves were normal, and no other neurologic deficits were present. She had mild tenderness over the left temporal area in the region of the superficial temporal artery. Intraocular pressure (IOP) was 20 mm Hg bilaterally. Complete blood count, erythrocyte sedimentation rate, C-reactive protein, electrocardiogram, and lumbar puncture were normal. She was admitted with a working diagnosis of ischemic optic neuropathy (ION). Comorbid factors on admission included controlled hypertension for 5 years on angiotensin converting enzyme inhibitor, heavy smoking, “social” drinking, controlled hyperlipidmia on simvastatin, hypothyroidism on L-thyroxine during a 10-year period, 29% stenosis of the internal carotid arteries bilaterally, osteoporosis, and (maternal) family history of diabetes mellitus type II.
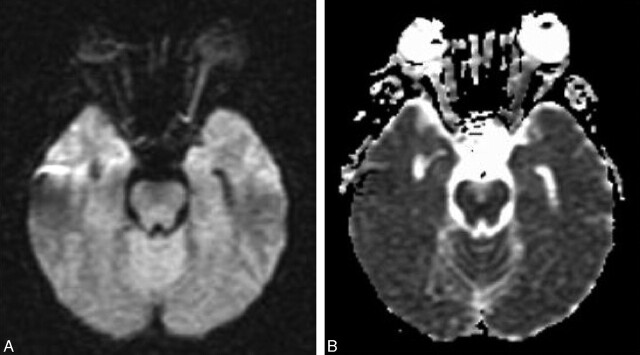
MR imaging of the brain preformed on day 2 after admission showed high signal intensity in the left optic nerve on diffusion imaging (Fig 1A) with decreased apparent diffusion coefficient (ADC; Fig 1B) indicating ischemic injury. Diffusion-weighted imaging (DWI) parameters were axial plane, TR = 10,000, TE = 94, b = 1000, section thickness = 5 mm with 0.5-mm gap, matrix = 128 × 128, field of view = 30 cm, standard quadrature head coil, and 1.5T Echospeed MR imaging (GE Healthcare, Milwaukee, Wis). ADC in the left optic nerve was decreased by 46% compared with the right optic nerve. Fluid-attenuated inversion recovery images of the brain revealed a few small foci of increased T2 signal in the hemispheric white matter, which indicated the presence of mild microangiopathic disease.
Fig 1.
A, Diffusion-weighted image (TR, 10 seconds; TE, 94.5 milliseconds; b, 1000 s/mm2) shows much higher signal intensity in the left optic nerve compared with the right. B, ADC image shows decreased signal intensity in the left optic nerve proving that the higher signal intensity in the left optic nerve on DWI is due to restriction in water diffusion.
Parenteral prednisolone was started, and her headache improved within 24 hours after admission. Temporal artery biopsy (3 cm in length) showed marked subintimal proliferation with no inflammation. The patient was discharged with stable vision and resolution of headache. During the following 2 weeks, steroids were tapered and eventually discontinued. The patient was followed for a period of 19 months at the neuro-ophthalmology clinic. Her vision improved gradually, reaching 20/40 OS on the left side with full visual field at 19 months. Orbital sonography at 12 months showed peripapillary Drusen, especially noticeable on the left retina in addition to pallor of the left optic disk. Ishihara color plate was 17/17 on the right and 3–4/17 OU on the left, which indicated unilateral color blindness. Visual evoked potentials were delayed on the left compared with right and slightly above normal limits with P 100 : 125.50 on the left versus 104.50 on the right (lab normal range, 92–115). Brain stem, auditory, and upper and lower limb somatosensory evoked potentials were normal.
Discussion
ION is one of the most prevalent and visually crippling diseases in the middle-aged and elderly population. It is an acute ischemic condition caused by interruption of blood flow in the ophthalmic artery and or its branches resulting in varying degrees of blindness. ION is classified into anterior (AION) and posterior (PION) according to the affected part of the optic nerve.1 AION is manifested by pale edema of the optic disk and peripapillary hemorrhage. PION is retrobulbar involving the optic nerve and or optic chiasm, but the optic disk is not initially swollen.2
AION is characterized by a sudden, painless, monocular loss of vision, a RAPD, and monocular visual field defects.3 There are 2 forms, the arteritic (0.36 per 100,000 in United States) and nonarteritic (2.3–10.3 per 100,000). In general, the arteritic form has a poorer prognosis.4
Arteritic AION is secondary to blood vessel inflammation (ophthalmic, central retinal) and SPCA (short posterior ciliary arteries), most commonly from giant cell arteritis. On the other hand, nonarteritic AION is secondary to noninflammatory diseases and is seen in association with hypertension, diabetes mellitus, myocardial infarction, and elevated cholesterol. The role of smoking in this disease is unclear.4
PION was first reported in 1981 and has a much lower incidence than AION.1 The diagnosis depends on excluding other causes of acute visual loss such as stroke and neoplasms. The blood supply to the optic nerve is different from the optic disk in that the optic nerve is not supplied by the SPCAs, but rather central retinal arteries and their multiple pial vessels arising from the ophthalmic artery.3 Associated diseases are the same as described in nonarteritic AION. From a clinical perspective, acute onset of color blindness is usually a feature of PION and not AION.
Unfortunately, no treatment of known value is available for nonarteritic ION. About 35% of patients will eventually develop progressive visual loss in the contralateral eye. Trials of systemic corticosteroids used in the early stages helped to improve vision in a minority of patients.1
Our patient presented with findings that support the diagnosis of nonarteritic AION and PION. She had risk factors for both types. Her funduscopic findings support the diagnosis of nonarteritic AION as did the biopsy of the superficial temporal artery. The presence of color blindness and retrobulbar optic neuropathy on MR imaging indicate PION. Her painful visual loss and mild temporal tenderness were atypical presentations of ION that led us initially to include arteritic AION. These symptoms have not previously been reported for PION.1,4
Imaging modalities used to assess the orbits and/or regional arteritis include Doppler sonography,1,5 fluorescein fundus angiography,1 CT, and MR imaging. The role of MR imaging in the assessment of acute visual loss is primarily to rule out nonischemic disorders such as demyelination or mass lesions compressing the optic nerve. The application of T1-weighted MR imaging, with and without gadolinium, has previously been reported for assessment of ischemic injury to the optic nerve but did not reveal any abnormalities in nonarteritic AION.6,7 On the other hand, T1-weighted MR imaging with gadolinium-enhanced optic nerves was reported once in PION showing gadolinium enhancement in the injured nerve.8 Despite the efficacy of diffusion imaging in detecting acute ischemia of the CNS,9 there are no reports of the application of DWI in the setting of ION. DWI has played a central role in the assessment of acute ischemic insults to the brain, and there are reports of diffusion positive ischemic lesions in the spinal cord.10 Because the optic nerve is an extension of the CNS as is the spinal cord, the pathophysiology of acute optic nerve ischemia is expected to be similar to that seen in the brain and the spinal cord with cell swelling and restriction of water diffusion leading to reduction in signal intensity on ADC. Our case emphasizes this point and demonstrates the potential of DWI for assessing ischemic injury to the optic nerve, indicating that MR imaging can be used in establishing the diagnosis of acute ischemic injury involving any portion of the CNS.
The usefulness of diffusion imaging in acute brain stroke is that it can predict the presence of significant brain ischemia within minutes of the onset of ischemic injury. In the brain, restriction in water diffusion continues for approximately 7–10 days. The lowest value for ADC is usually reached within 24 hours from the ictal event and then slowly increases reaching normal values at approximately day 7–10.11 It then increases and remains at super-normal values. This process is explained by the conversion of cytotoxic edema to vasogenic edema. Resolution of vasogenic edema occurs, but water diffusion remains elevated due to decreased cellular attenuation and increased water content of the residual infarcted (gliotic) tissue. Our case demonstrates that the ADC value was decreased at day 2 after her acute worsening, in keeping with the expected ADC pattern as seen in acute ischemic injury to the brain. The temporal evolution of ADC changes in the optic nerve was not studied and remains unknown. DWI in spinal cord ischemia reveals clear detection of ischemic injury at an early stage, showing signal intensity conversion comparable to that in acute cerebral stroke.4 Despite the absence of gray matter, the optic nerve is similar to the spinal cord in that it is composed of parallel axonal tracts. The fact that it shows low ADC is in keeping with similar findings of white matter ischemia in the brain and spinal cord. Our ability to image the optic nerve successfully in this patient was somewhat fortuitous, in that the applied diffusion sequence was designed for brain and not orbital imaging. In the future, we would recommend using thinner sections—3 mm instead of 5 mm—for this purpose.
Finally, DWI is effective in distinguishing acute demyelination from acute ischemia in the brain, because there are only rare reports in the literature of low ADC in acute multiple sclerosis plaques.12 This is a point that will need confirmation through future assessment of patients presenting with optic neuritis.
References
- 1.Hayreh SS. Ischaemic optic neuropathy. Indian J Opthamol 2000;48:171–94. [PubMed] [Google Scholar]
- 2.Paul B, McElvanney AM, Agarwal S, et al. Two rare causes of posterior ischaemic optic neuropathy: eosinophilic fasciitis and Wegener’s granulomatosis. Br J Ophthalmol 2002;86:1066–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biousse V, Schaison M, Touboul PJ, et al. Ischemic optic neuropathy associated with internal carotid artery dissection. Arch Neurol 1998;55:715–19 [DOI] [PubMed] [Google Scholar]
- 4.Younge MD. Anterior ischemic optic neuropathy. eMedicine; http://www.emedicine.com/oph/topic161.htm
- 5.Lee AG. Non-arteritic ischemic optic neuropathy. Ophthalmic hyperguide; http://www.ophthalmic.hyperguides.com/default.asp?section=/body.asp
- 6.Rizzo JF, Andreoli CM, Rabinov JD. Use of MRI to differentiate optic neuritis and non-arteritic anterior ischemic optic neuropathy. Ophthalmology 2002 Sep;109(9):1679–84. [DOI] [PubMed] [Google Scholar]
- 7.Rizzo JF 3rd, Andreoli CM, Rabinov JD. Use of magnetic resonance imaging to differentiate optic neuritis and nonarteritic anterior ischemic optic neuropathy. Ophthalmology 2002;109:1679–84 [DOI] [PubMed] [Google Scholar]
- 8.Vaphiades MS, Optic nerve enhancement in hypotensive ischemic optic neuropathy. J Neuroophthalmol 2004;24:235–36 [DOI] [PubMed] [Google Scholar]
- 9.Smajlovic D, Sinanovic O. Sensitivity of the neuroimaging techniques in ischemic stroke. Med Arh 2004;58:282–84 [PubMed] [Google Scholar]
- 10.Weidauer S, Dettmann E, Krakow K, et al. Diffusion-weighted MRI of spinal cord infarction: description of two cases and review of the literature. Nervenarzt 2002;73:999–1003 [DOI] [PubMed] [Google Scholar]
- 11.Yamada I, Kuroiwa T, Endo S, et al. Temporal evolution of apparent diffusion coefficient and T2 value following transient focal cerebral ischemia in gerbils. Acta Neurochir Suppl 2003;86:147–51 [DOI] [PubMed] [Google Scholar]
- 12.Werring DJ, Brassat D, Droogan AG, et al. The pathogenesis of lesions and normal-appearing white matter changes in multiple sclerosis: a serial diffusion MRI study. Brain 2000;123:1667–76 [DOI] [PubMed] [Google Scholar]