Abstract
BACKGROUND AND PURPOSE: Inadvertent foreign body embolization is a rarely diagnosed and neglected complication of cerebral angiography that has not been studied systematically.
METHODS: We undertook a comprehensive 5-year retrospective study of all available postmortem cases of postangiographic neurologic complications, as well as a comprehensive histologic examination of all surgically resected central nervous system arteriovenous malformations, at our institution.
RESULTS: Among the autopsy series, we found 3 patients for whom cerebral infarction, sometimes catastrophic, is attributable to inadvertent cotton fiber, Gelfoam, or polyvinyl alcohol particulate emboli during cerebral angiography. All cases described had concurrent atherosclerotic vascular disease. Particulate embolization, which is usually cotton fiber, is present in as many as 25% of resected arteriovenous malformations: the risk of finding such emboli is in part dependent on a history of prior interventional (as opposed to diagnostic) angiographic procedures. It is not surprising that the amount of tissue examined also increases the risk of finding such emboli.
CONCLUSIONS: Unintentional foreign body emboli remain common in modern angiographic practice and are probably underappreciated clinically. Although such emboli are usually asymptomatic, they can be clinically devastating, and a high index of suspicion is required for diagnosis. Foreign body emboli should be included in the differential diagnosis of postangiographic ischemia or infarction.
Inadvertent foreign body embolization was first identified as a complication of diagnostic cerebral angiography in 19601 and has been described in occasional reports since then.2–7 However, since the advent of digital subtraction angiography and interventional vascular radiology, there has been little comment and no systematic study of the phenomenon. Many studies of postangiographic ischemia and infarction, including many major reviews, completely fail to mention the phenomenon at all,8–13 though the variety of embolizations from cardiovascular procedures is well described.14 Evidence from past studies6 would suggest, however, that in some settings it might be frequent. In this article, we describe a series of postmortem cases demonstrating inadvertent and clinically unsuspected foreign body embolization following diagnostic and therapeutic angiography and, by systematic review of resected arteriovenous malformations, attempt to identify risk factors for finding such emboli.
Patients and Techniques
Instances of cerebral infarction associated with with foreign body emboli were retrieved from the autopsy service at a large tertiary referral center during the previous 5 years. This sampling period includes the last 4 years of the large comprehensive analysis of all complications in cerebral angiography performed at our institution15 that has recently been published. All autopsy reports with diagnoses of intracranial hemorrhage, aneurysm, vascular malformation, or multiple infarctions were reviewed. This required a review of 841 autopsy reports. In these cases, 35 had a recorded history of cerebral angiography, and in 2 cases pathologic confirmation of foreign body embolization was made. In a third case, the angiography was performed at this institution, but after angiography the patient was transferred back to the referring hospital and the postmortem performed there (case 2). Postmortem examination of brain and spinal cord with histologic examination of formalin fixed, paraffin-embedded tissue was performed according to standard protocols, and sections were reviewed by using standard bright field and polarized light microscopy.
We also retrieved slides and paraffin blocks of all arteriovenous malformations (AVMs) resected at out institution during the 5 previous calendar years. Tissue blocks were handled according to routine protocols, and tissue sections were stained with either hematoxylin/eosin or trichrome stains and were viewed by using both standard bright field and polarized light optics. For initial review, one section per tissue block in each specimen was microscopically examined to assess the presence or absence of foreign material, other than embolic material deliberately introduced in therapeutic angiograms. If foreign material was found, an additional section immediately adjacent in the block to the section reviewed was then examined.
To minimize the possibility that foreign material was due to contamination, foreign material was only accepted as embolic in origin if it met 5 criteria, which were set prospectively. The foreign material had to (1) lie within the plane of section and (2) show evidence of sectioning by the microtome. (3) It had to be accompanied by a tissue reaction, either of neutrophil adhesion, foreign body type reaction, or attenuated fibrosis with evidence (ie, iron deposition, vascular recanalization) of previous organizing thrombosis. (4) The foreign material had to lie within the lumen or wall of a vessel or within the profile of a fully or partially thrombosed and organized vessel. Finally, (5) we required it to be present in the same location in an adjacent microscopic section from the same block.
For each specimen, the dates of prior angiography, the presence or absence of clinical postangiographic complications, the date of resection of the specimen, and the number of tissue blocks submitted for microscopic sectioning were recorded. After initial histologic review, a randomly selected subset in which foreign material was not detected was serially sectioned and examined in toto at 20-μ intervals. Differences between numbers of tissue blocks between observed groups were assessed by using Mann-Whitney U test, and the significance of observed odds ratios was calculated by using z score transformation.
Diagnostic cerebral angiography in the patients reported was performed according to a standard protocol, which involved the introduction of a 5F sheath, which was infused with a continuous drip of normal saline containing 1000 U of heparin per 1 L of saline. Catheterization was then performed with standard 4F or 5F catheters and 028 or 032 hydrophilic guidewires. When catheters or wires were not used, they were kept in a bowl with heparinized normal saline. For interventional procedures a similar standard protocol was used regarding the sheath. The guiding catheter and microcatheters used for the interventional procedure were attached to continuous drip infusions of normal saline (closed systems). Patients were heparinized at the time of the procedure by using an intravenous bolus of heparin followed by hourly doses maintaining an ACT between 250 and 300 seconds. Microcatheters and microwires were placed in a bowl of heparinized saline when not in use. Standard available cotton gauze (non-lint-free) was used to clean guidewires before their introduction into the guiding catheters. Patients were draped with disposable drapes and resterilized surgical towels were used when needed.
Results
Case 1
A 62-year-old woman presented with a grade 3 subarachnoid hemorrhage. Diagnostic angiography was made difficult by tortuous proximal vessels and revealed bilateral middle cerebral artery bifurcation aneurysms. Following angiography, the patient developed an attenuated left hemiplegia. A large cerebral infarct involving the right middle cerebral artery (MCA) and anterior cerebral artery territories evolved on subsequent CT scans (Fig 1A). She died of complications of increased intracranial pressure 3 days later.
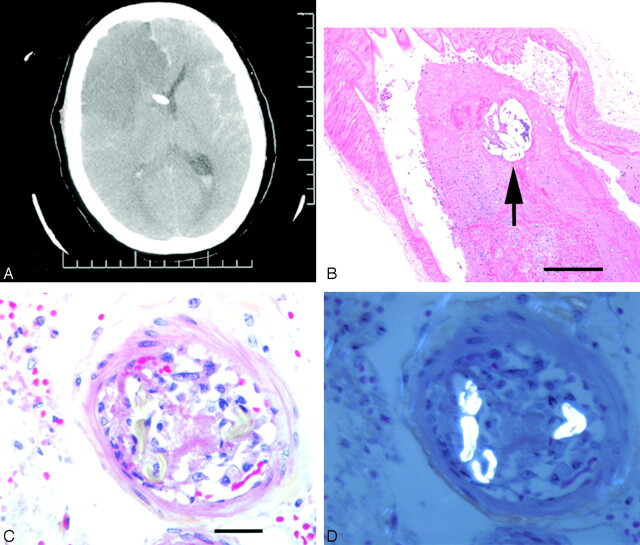
Fig 1.
A, CT scan of patient 1, 2 days after angiography, demonstrating right anterior and middle cerebral artery territory infarcts, as well as a ventricular drain in situ. B, Section of middle cerebral artery containing recent thrombus and a particle of polyvinyl alcohol (arrow, hematoxylin phloxine saffron, ×200). C, Small leptomeningeal artery demonstrating acute cellular reaction and thrombosis and (D) under polarized light, strongly birefringent, hollow fibers characteristic of cotton (C and D, hematoxylin phloxine saffron, ×630).
At autopsy, the right MCA adjacent to the aneurysm was occluded by thrombus of recent origin. Step sections through the thrombus revealed that it was centered on a 250-μ-diameter particle of foreign material, with a microscopic appearance consistent with polyvinyl alcohol (PVA; Fig 1B). In other sections taken from the frontal convexities, step sections of multiple vessels showed partial to complete occlusion by foreign material having the structure and brightly refractile, birefringent properties of cotton fibers (Figs 1C, -D). All the foreign material was associated with a brisk inflammatory and endothelial response.
Case 2
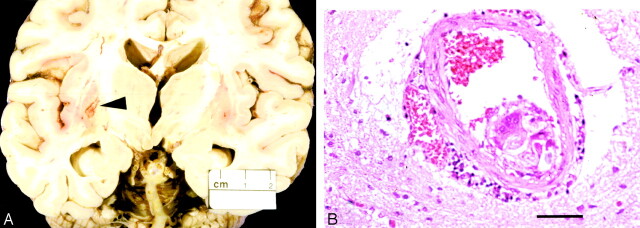
A 62 year-old man with a history of diabetes and hypertension presented with an acute grade 2 subarachnoid hemorrhage. Angiography revealed a basilar tip aneurysm. Endovascular therapy with platinum coils was attempted, but satisfactory placement of the coils could not be obtained, and they were withdrawn. Following the procedure, the patient deteriorated rapidly and became comatose. He failed to improve and died of multiple pulmonary emboli and a myocardial infarction 23 days after admission. Autopsy revealed multiple infarcts involving the cerebellar hemispheres, the right paramedian pons, and the basal ganglia (Fig 2A). Atherosclerosis of the circle of Willis and posterior circulation was noted. All infarcts were of similar histologic age, and diligent search revealed intraarterial foreign body giant reaction containing cotton fibers adjacent to a basal ganglia infarct (Fig 2B).
Fig 2.
A, Brain of patient 2 with cavitating infarct (arrowhead) in left lentiform nucleus and (B) adjacent perforating artery containing foreign body reaction and cotton fibers (hematoxylin and eosin, ×200).
Case 3
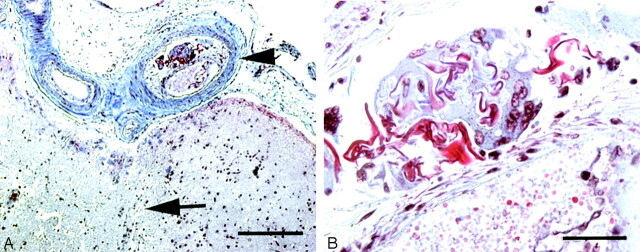
A 50-year-old woman with a history of poorly controlled type I diabetes mellitus, hypertension, and multiple cerebral infarcts presented with recurrent intracerebral hemorrhages. Diagnostic cerebral angiography was performed and did not reveal a focal lesion. Angiographic access was recorded as being difficult because of extensive atherosclerosis. Four months later, she died of a recurrent intracranial hemorrhage. Examination of the brain revealed diffuse severe atherosclerosis and arteriosclerosis. Her recurrent hemorrhages were attributed to severe hypertensive and diabetic vasculopathy. In addition, a cortical infarct was demonstrated in the right parietooccipital cortex (Fig 3A). The associated cortical artery showed evidence of thrombosis and recanalization, with partial occlusion by loose fibrous tissue and an endothelial-lined neolumen. Within the organized thrombus was a foreign body giant cell reaction with associated acellular material. This material was neither birefringent nor refractile, did not stain with saffron, Congo red, or Van Gieson’s elastic, was weakly PAS positive, stained red on a Martius Scarlet blue, was not reactive on immunohistochemistry for either low molecular weight or high molecular weight cytokeratins, and was consistent with Gelfoam (Fig 3B).
Fig 3.
A, Leptomeningeal artery in patient 3, demonstrating recanalized artery (arrowhead) immediately adjacent to infarcted brain (arrow, Martius Scarlet blue, ×100). B, Subintimal foreign body reaction containing foreign material (Martius Scarlet blue, ×1000).
Arteriovenous Malformations
Sixty arteriovenous malformations were identified and retrieved from our files. On initial examination, 12 (20%) contained foreign material other than the methacrylate glue used in therapeutic embolization. In all but one case, this foreign material was composed of birefringent cotton fiber. In one case a diagnostic angiogram had been performed 4 months before therapeutic embolization, and the AVM was resected 1 day after therapeutic embolization. The AVM contained a synthetic fiber associated with a foreign body giant cell reaction, as well as cyanoacrylate and a cotton fiber with an associated neutrophilic response. We interpret this histology as demonstrating 2 embolic events: one compatible with the timing of the patient’s first angiogram and the other very recent.
In 11 of 12 cases, the patient had a history of therapeutic angiogram, with embolization using cyanoacrylate. In one of these 11 cases, there was a postangiographic venous infarct, apparently due to migration of embolic material, but no other complications were noted. Among the 11 patients who had undergone therapeutic embolization, in 9 the fibers were present in or directly adjacent to the methylmethacrylate glue. In 2, the fibers were present within the vessel walls with an associated foreign body giant cell reaction, or within the occluded and organized lumen of a thrombosed vessel. In 19 of the AVMs that had undergone previous embolization, 11 contained refractile cotton fibers. In the 41 AVMs that had not undergone therapeutic embolization, cotton fiber was found in one. Angiography in this case had been performed entirely at a different institution. Therapeutic embolization substantially increases the odds of finding fiber emboli (odds ratio equals 58.0; P < .001).
Fifteen AVMs in which cotton was not initially identified were submitted for serial sectioning and complete examination. Of that subsample, 4 of the 15 were found to contain cotton emboli (27%). Of patients who had not undergone therapeutic embolization, one of 11 (9%) contained cotton fibers, whereas 3 of 4 patients (75%) undergoing embolization contained emboli. Serial sectioning therefore adds slightly to the rates of detection of embolic material and confirms that therapeutic embolization is a strong risk factor for finding cotton fibers.
When cotton fibers were present, they were usually in small numbers and in a minority percentage of blocks of each case (mean, 30% of blocks; range, 55%–5%). Cotton fibers were rarely found in malformations, in which only one or 2 tissue blocks were available for examination (8%; n = 37), but were present in 60% of specimens from which more than 5 blocks were examined (n = 10). Among AVMs with a history of therapeutic embolization, cases in which cotton emboli were detected comprised a significantly larger number of blocks than cases that did not (median number of blocks, 3 and 2 respectively; Mann Whitney U = 13; n = 16.5; P = .021). The amount of tissue available for examination therefore independently predicts detection of inadvertent foreign body embolization.
Discussion
The case reports illustrate unequivocally that inadvertent foreign body embolization remains a potentially lethal cause of postangiographic ischemia and infarction. Detection of the embolic material in our series of postmortems took careful examination, serial sections, and multiple blocks from the affected areas. Cerebral ischemia and infarction from foreign body embolism is a recognized complication of cardiovascular surgery and angiography but is rarely histopathologically documented following cerebral angiography, though subsequent surgical intervention and histopathologic examination can enable antemortem identification.7 Retinal embolization has been diagnosed on ophthalmoscopy.3,4 Careful examination, at least subserial sections, and a high index of suspicion may be required to make the diagnosis at autopsy. Radiographic differentiation from other causes of cerebral infarction or ischemia might be difficult: embolic infarctions involved both penetrating arteries of the cerebrum (case 3) as well as large basal arteries (case 1) and cortical arteries of the convexities (cases 1 and 3). The infarcts may also be adjacent to arterial border zones (case 3), and certainly distal arterial territories are frequently affected.1,2,7
The origin of the foreign material cannot always be ascertained and the variety of material suggests that it is not all due to a single source. In case 1, the presence of a PVA particle prompted careful examination of the angiography suite logs. None of the prior angiograms that day used PVA. Contamination may have occurred by inadvertent transfer from an unidentified contaminated surface, or perhaps due to inadequate laundering of sterile drapes. The cotton fiber emboli are common, but cotton lint or synthetic fibers may originate from many sources, including sterile drapes, airborne dust, or gauze. In light of numerous potential sources of contact, it is not possible to identify the source of these fibers unequivocally, though it would seem prudent to reduce the contact of angiographic equipment with sources of cotton lint, including gauze, as well as limiting use of laundered drapes.
The most recent systematic investigation of inadvertent foreign body embolization dates from 1983,6 in which 3 of 84 resected AVMs were noted to contain cotton or talc emboli: none in that series had undergone therapeutic angiogram, and all emboli were apparently asymptomatic. Serial sectioning was not performed. In our series, embolic material attributable to diagnostic angiography alone was detected in 3 of 61 AVMS and was only detected following serial sectioning in 1 of the 3. The rate in this series is therefore similar to that estimated 20 years ago. Asymptomatic foreign body embolus remains common in cerebral angiography, whether therapeutic or diagnostic, and the likelihood of detection is to some degree proportional to the diligence with which it is sought. At our institution, the overall number of neurologic complications among 2900 consecutive diagnostic cerebral angiograms was 39 (1.3%), of which 20 (0.7%) were transient, 5 (0.2%) were reversible, and 14 (0.5%) were permanent. These rates are not higher than those in other institutions,8,9,11,15 and indeed, in one resected AVM, foreign body emboli were detected where all angiography had been performed elsewhere. The observation that our institutional complication rate is not elevated, and the observation of foreign body emboli having occurred at other institutions, suggest that this complication is neither confined to our institution nor causes injury in excess of that found at other institutions.
The strong association between therapeutic embolization and inadvertent cotton fiber embolization may reflect that the cotton fibers deposit within the AVM due to the same hemodynamic factors that favor deposition of the cyanoacrylate material. It is possible that they might be carried in with the glue. Alternatively, the prolonged procedural complexity of therapeutic embolization as opposed to purely diagnostic angiography may provide greater opportunity for catheter or guidewire contamination. It is notable that in this series of autopsy cases, all patients had both proximal atherosclerosis and difficult angiographic access, which have been identified as risk factors for ischemia after angiography.8,10,15,16
In some studies, serial MR imaging following angiography not infrequently detects transient ischemic lesions on diffusion-weighted sequences.10,15 Such ischemia is typically attributed to microthrombi, or air bubbles. Air filters have been demonstrated to reduce the showers of microemboli that occur consequent to contrast injection, which in turn reduces but does not eliminate postangiographic changes in MR imaging.17 However, air filters did not eliminate episodic, single microembolic phenomena unrelated to contrast injection. We suggest that foreign debris might contribute to such microembolic events, the consequences of which might also be amenable to heparin therapy.
We conclude that unintentional foreign body embolization is common in modern cerebral angiography and, though usually asymptomatic, has lethal potential. Such emboli should be included in the differential diagnosis of postangiographic complications and must be sought diligently to be found.
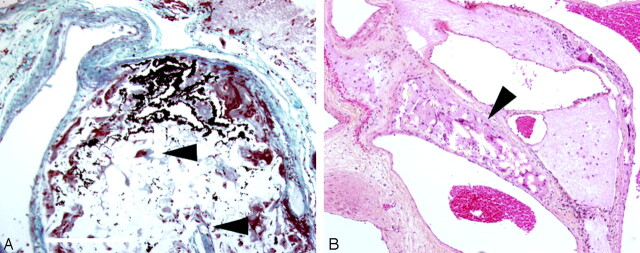
Fig 4.
Resected arteriovenous malformations with (A) glue admixed with birefringent cotton fibers (arrowheads, Elastic Masson Trichrome, ×200) or (B) within medial foreign body giant cells (hematoxylin phloxine saffron PS, ×100).
References
- 1.Silberman J, Cravioto H, Feigin I. Foreign body emboli following carotid angiography. Arch Neurol 1960;3:711–17 [PubMed] [Google Scholar]
- 2.Chason JL, Landers JW, Swanson RE. Cotton fiber embolism, a frequent complication of cerebral angiography. Neurology 1963;13:558–60 [DOI] [PubMed] [Google Scholar]
- 3.Hansen PE, Damgard-Jensen L, Nehen AM. [Retinal embolism following carotid arteriography. 2 cases of exogenous foreign bodies] Ugeskr Laeger 1978;140:3059–61 [in Danish] [PubMed] [Google Scholar]
- 4.Nehen AM, Damgaard-Jensen L, Hansen PE. Foreign body embolism of retinal arteries as a complication of carotid angiography. Neuroradiology 1978;15:85–88 [DOI] [PubMed] [Google Scholar]
- 5.Watanabe T, Suzuki M, Hori S, et al. [Cotton fiber cerebral emboli following carotid angiography: a case report] No Shinkei Geka 1983;11:211–14 [in Japanese] [PubMed] [Google Scholar]
- 6.Vinters HV, Kaufmann JC, Drake CG. “Foreign” particles in encephalic vascular malformations. Arch Neurol 1983;40:221–25 [DOI] [PubMed] [Google Scholar]
- 7.Shiino A, Suda K, Matsuda M, et al. [Cotton fiber embolization during angiography: report of a case with histological confirmation]. Neurol Med Chir (Tokyo) 1987;27:443–46 [in Japanese] [DOI] [PubMed] [Google Scholar]
- 8.Dion JE, Gates PC, Fox AJ, et al. Clinical events following neuroangiography: a prospective study. Stroke 1987;18:997–1004 [DOI] [PubMed] [Google Scholar]
- 9.Grzyska U, Freitag J, Zeumer H. Selective cerebral intraarterial DSA: complication rate and control of risk factors. Neuroradiology 1990;32:296–69 [DOI] [PubMed] [Google Scholar]
- 10.Gerraty RP, Bowser DN, Infeld B, et al. Microemboli during carotid angiography: association with stroke risk factors or subsequent magnetic resonance imaging changes? Stroke 1996;27:1543–47 [DOI] [PubMed] [Google Scholar]
- 11.Hankey GJ, Warlow CP, Sellar RJ. Cerebral angiographic risk in mild cerebrovascular disease. Stroke 1990;21:209–22 [DOI] [PubMed] [Google Scholar]
- 12.Qureshi AI, Luft AR, Sharma M, et al. Prevention and treatment of thromboembolic and ischemic complications associated with endovascular procedures. Part I. Pathophysiological and pharmacological features. Neurosurgery 2000;46:1344–59 [DOI] [PubMed] [Google Scholar]
- 13.Qureshi AI, Luft AR, Sharma M, et al. Prevention and treatment of thromboembolic and ischemic complications associated with endovascular procedures. Part II. Clinical aspects and recommendations. Neurosurgery 2000;46:1360–75 [DOI] [PubMed] [Google Scholar]
- 14.Moody DM, Bell MA, Challa VR, et al. Brain microemboli during cardiac surgery or aortography. Ann Neurol 1990;28:477–86 [DOI] [PubMed] [Google Scholar]
- 15.Willinsky RA, Taylor SM, TerBrugge K, et al. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology 2003;227:522–28 [DOI] [PubMed] [Google Scholar]
- 16.Bendszus M, Koltzenburg M, Burger R, et al. Silent embolism in diagnostic cerebral angiography and neurointerventional procedures: a prospective study. Lancet 1999;354:1594–97 [DOI] [PubMed] [Google Scholar]
- 17.Bendszus M, Koltzenburg M, Bartsch AJ, et al. Heparin and air filters reduce embolic events caused by intra-arterial cerebral angiography: a prospective, randomized trial. Circulation 2004;110:2210–15 [DOI] [PubMed] [Google Scholar]