Abstract
BACKGROUND AND PURPOSE: The mechanisms by which the glucocorticoid dexamethasone produces its therapeutic action in patients with intracranial tumors still remain unclear. The purpose of this study was to investigate whether dexamethasone affects cerebral perfusion and water molecule diffusion by using quantitative dynamic susceptibility contrast perfusion MR imaging (DSC-MR imaging) and diffusion tensor MR imaging (DT-MR imaging).
METHODS: Ten consecutive patients with glioblastoma multiforme underwent DSC-MR imaging and DT-MR imaging before and 48–72 hours after dexamethasone treatment (16 mg/day). Cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT), and water mean diffusivity (<D>) were measured for enhancing tumor, nonenhancing peritumoral edematous brain, and normal-appearing contralateral white matter before and after steroid therapy. The percentage change in CBF, CBV, MTT, and <D> for the 3 tissue types was calculated for each patient, a mean value obtained for the population, and the statistical significance determined by using a paired-samples Student t test.
RESULTS: After dexamethasone treatment, there was no significant change in tumor CBF, CBV, or MTT. Edematous brain CBV and MTT were also unchanged. There was, however, an increase in edematous brain CBF (11.6%; P = .05). <D> was reduced in both enhancing tumor (−5.8%; P = .001) and edematous brain (−6.0%; P < .001). There was no significant change in CBF, CBV, MTT, or <D> for normal-appearing contralateral white matter after treatment.
CONCLUSION: These data suggest that dexamethasone does not significantly affect tumor blood flow but may, by reducing peritumoral water content and local tissue pressure, subtly increase perfusion in the edematous brain.
Because of its rapid and beneficial clinical effects, the synthetic glucocorticoid dexamethasone has been routinely used in the management of patients with intracranial tumors for the past 3 decades.1,2 Its precise mode of action, however, remains unclear. Studies in both humans and animal models suggest that its principal effect is to reduce edema formation, hence lowering intracranial pressure.3,4 This edema resolution is achieved by either decreasing the blood-tumor barrier permeability to small solutes5 or increasing parenchymal resistance to fluid transport.3 Alternatively, dexamethasone may act directly on the cerebral vasculature. Unfortunately, those studies performed to date that have investigated how steroids affect cerebral perfusion have produced contradictory results, both in terms of the magnitude and direction of perfusion changes.5–9 This is because these studies have not acquired baseline data in the absence of steroid treatment,9 or have imaged subjects over a wide and variable range of times after treatment initiation,6,8,9 have been performed in heterogeneous groups of tumors5–7 and in patients who have already undergone operative procedures.8
In view of the conflicting data and these methodologic problems, a pilot study was performed in which dynamic susceptibility contrast perfusion MR imaging (DSC-MR imaging) was used to measure quantitative values of cerebral blood flow (CBF), cerebral blood volume (CBV), and mean transit time (MTT) for enhancing tumor, nonenhancing peritumoral edematous brain, and normal-appearing contralateral white matter in a group of patients with high-grade glioma before and 48–72 hours after dexamethasone treatment. By measuring these parameters before and after treatment in the 3 regions, it is possible to determine whether and by how much cerebral perfusion is altered by dexamethasone and whether the changes are localized to the tumor region or are more global in nature. In addition, to determine how changes in cerebral perfusion parameters compare with alterations in water molecule mobility, which has been shown to decrease significantly in peritumoral edematous brain after dexamethasone treatment,4 the mean diffusivity of water (<D>) was also obtained pre- and poststeroid treatment for the 3 tissue types.
Methods
Patients
Ten consecutive patients (8 men and 2 women; age range, 40–76 years; mean age, 58.1 ± 14.3 years) with newly diagnosed glioblastoma mutliforme (GBM) were included in this prospective study. Their radiologic data did not suggest any neurologic disorders other than the primary neoplasm. The tumor type was histologically confirmed in all patients following MR imaging. At the time of imaging, none of the patients had (1) begun steroid treatment, (2) had any prior radiation therapy or chemotherapy, or (3) undergone any prior cranial surgery. They also had no contraindications to MR imaging. The local ethics committee approved the study and informed consent was obtained from each patient.
MR Imaging Protocol
All MR imaging data were obtained by using a GE Signa LX 1.5T (General Electric, Milwaukee, Wisc) clinical scanner, equipped with a self-shielding gradient set (22 mT/m maximum gradient strength) and manufacturer-supplied “birdcage” quadrature head coil. The MR imaging examination consisted of a standard fast spin-echo T2-weighted sequence, the DT-MR imaging and DSC-MR imaging protocols described below, and a contrast-enhanced T2-weighted volume sequence. The DT-MR imaging and DSC-MR imaging protocols shared the same field of view (FOV) and section locations. The examination was repeated 48–72 hours after dexamethasone treatment had been initiated (16 mg/day).
To ensure that the section locations used in the second examination corresponded as closely as possible to those in the first, the subject’s head position and tilt in the first scan were recorded and the patient repositioned in exactly the same manner for the second scan. At least one of the sections was taken through a prominent anatomic landmark so as to minimize any deviation in section location in the second scan. Computational image realignment techniques were then used to warp the images in the second examination to the first, thereby minimizing any small remaining positioning differences.
In the DT-MR imaging protocol,4 sets of axial single-shot spin-echo echo-planar (EP) images (b = 0 and 1000 s/mm2) were collected with diffusion gradients applied sequentially along 6 noncollinear directions. Five acquisitions consisting of a baseline T2-weighted echo-planar (EP) image and 6 diffusion-weighted EP images, a total of 35 EP images, were collected per section position. The acquisition parameters for the EP imaging sequence were 15 axial sections of 5-mm thickness and 1.0-mm section gap, a FOV of 240 × 240 mm, an acquisition matrix of 128 × 128 (zero filled to 256 × 256), a repetition time (TR) of 10 seconds, and an echo time (TE) of 98.8 milliseconds.
Cerebral perfusion was measured by imaging the dynamic signal intensity change following a bolus injection of a gadolinium-based contrast agent. Thirty-four volumes of 15 axial sections of 5-mm thickness and 1.0-mm section gap were acquired over a period of 85 seconds by using a single-shot gradient-echo EP imaging sequence with a FOV of 240 × 240 mm, an acquisition matrix of 128 × 128 (zero filled to 256 × 256), a TR of 2.5 seconds, a TE of 30 milliseconds, and a flip angle of 90°. Four baseline volumes were collected before 20 mL of gadopentetate dimeglumine (Magnevist, Berlex Laboratories, Wayne, NJ) was administered intravenously at a rate of 5 mL/s by using an MR-compatible pump (Medrad, Indianola, Pa).
Following the DSC-MR imaging protocol, a contrast-enhanced T1-weighted volume sequence was also collected by using the following acquisition parameters: 110 contiguous axial sections of 1.5-mm thickness, a FOV of 240 × 240 mm, an acquisition matrix of 256 × 256, a TR of 7.3 milliseconds, a TE of 3.2 milliseconds, and an inversion time of 400 milliseconds.
Image Analysis
Quantitative coregistered maps of CBF, CBV, and MTT for the pre- and poststeroid treatment examinations were obtained in the following manner. By using a 3D computational image alignment program (FLIRT; www.fmrib.ox.ac.uk/fsl),10 intra- and interscan bulk patient motion artifacts were removed from the DSC-MR imaging data by registering the component gradient-echo EP imaging volumes to the T2-weighted EP imaging volume acquired as part of the first examination DT-MR imaging protocol.4
Quantitative perfusion measurements were made by using the methods described by Østergaard et al.11,12 Tissue and arterial concentration-time curves were determined on a voxel-by-voxel basis from the dynamic signal intensity change following injection of the contrast agent. The concentration-time curves were fitted by using a gamma-variate function to estimate and remove the difference in bolus arrival time and recirculation artifacts.13 The arterial input function (AIF) was determined from the average concentration-time curve taken from small 3 × 3 voxel (2.8 × 2.8 × 5 mm volume) regions of interest covering the terminal portion of the internal carotid arteries just inferior to the circle of Willis. Singular value decomposition was used to perform the deconvolution of the AIF and tissue concentration-time curves. Values of CBV (the ratio of the areas under the tissue and AIF concentration-time curves), CBF (the maximum value of the residue function obtained from the deconvolution operation), and MTT (αCBV/CBF, where α is the macrovascular to microvascular hematocrit ratio of 2/3) were calculated on a voxel-by-voxel basis and converted into Analyze format (Mayo Foundation, Rochester, Minn).12 Maps of <D> were also calculated from the DT-MR imaging data registered to the DSC-MR imaging data as described elsewhere.4
To allow regions of enhancing tumor to be accurately positioned on the cerebral perfusion and water diffusion parametric maps for both scans, the contrast-enhanced T1-weighted volume images from the presteroid treatment scan were registered directly onto the corresponding T2-weighted EP images by using image boundary and internal landmark information in SPM (www.fil.ion.ucl.ac.uk/spm).
Region of Interest Analysis
Enhancing tumor and surrounding nonenhancing peritumoral edematous brain boundaries on the pre- and poststeroid treatment CBF, CBV, MTT, and <D> parametric maps were determined by using the contrast-enhanced T1-weighted volume and T2-weighted EP MR imaging data acquired in the first examination. The effects of dexamethasone on cerebral perfusion and water diffusion in these tissue types were quantified by using the following region of interest analysis.4,14 Regions of enhancing tumor were first drawn on the coregistered T1-weighted volume images and overlaid on the presteroid treatment T2-weighted EP images. Nonenhancing peritumoral edematous brain was then defined for each section as the largest region of signal intensity hyperintensity on the T2-weighted EP images, which extended beyond enhancing tumor (Fig 1). Next, these tissue boundaries were transferred to the CBF, CBV, MTT, and <D> parametric maps for both pre- and poststeroid treatment scans in Analyze. Finally, for each appropriate section, values of CBF, CBV, MTT, and <D> for enhancing tumor, peritumoral edematous brain, and normal-appearing contralateral white matter (centrum semiovale) were measured. Overall mean cerebral perfusion and water diffusion values for the entire enhancing tumor and peritumoral edematous brain volumes in each patient were then calculated from this multisection data. These volume measurements were typically obtained from thousands of voxels in 5–12 sections for enhancing tumor and peritumoral edematous brain and hundreds of voxels in a single section for normal-appearing contralateral white matter in centrum semiovale.
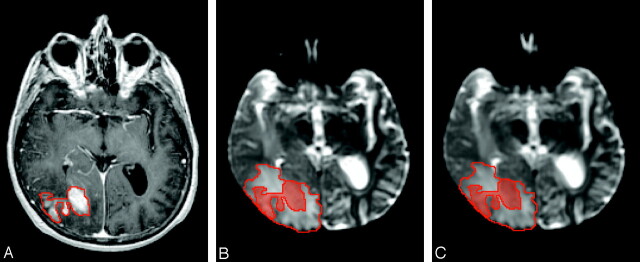
Fig 1.
Images obtained from patient 4. A, Presteroid treatment contrast-enhanced T1-weighted volume image with region of interest indicating enhancing tumor. Pre- (B) and 72 hours (C) poststeroid treatment T2-weighted EP images with shaded and unshaded region of interest indicating enhancing tumor and nonenhancing peritumoral edematous brain.
Statistical Analysis
All data were reported as a mean ± 1 SD. For each patient, the percentage change in CBF, CBV, MTT, and <D> for the 3 tissue types following steroid treatment was determined as follows:
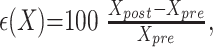 |
1) |
where X = CBF, CBV, MTT, or <D>. The percentage change (ε) in these parameters for the 3 tissue types was calculated for each patient, and a mean value (<ε>) was obtained for the patient population. To assess whether changes in cerebral perfusion and <D> were significant, the mean pre- and poststeroid treatment values for each patient were compared by using a paired-samples Student t test.5 All statistical tests were performed by using SPSS 10.0 (SPSS Inc., Chicago, Ill), with P < .05 being considered statistically significant.
Results
Figure 1 shows presteroid treatment contrast-enhanced T2-weighted volume and pre- and poststeroid treatment T2-weighted EP images for a representative section location acquired from a 61-year-old woman (patient 4). Regions corresponding to enhancing tumor and nonenhancing peritumoral edematous brain are indicated by shaded and unshaded regions of interest in the T2-weighted EP images. Note the very subtle reduction in signal intensity of peritumoral edematous brain between the pre- and poststeroid treatment T2-weighted EP images. This subtle reduction in edematous brain T2-weighted signal intensity was also seen in several other patients.
Figure 2 shows maps of <D>, CBF, CBV, and MTT pre- and poststeroid treatment for the same patient. The reduction in <D> for peritumoral edematous brain is evident (red arrow). No gross change in cerebral perfusion pre- and poststeroid treatment is visible in these maps, though there is a subtle increase in CBF for cortical/subcortical tissue within the edematous brain region (pink arrow).
Fig 2.
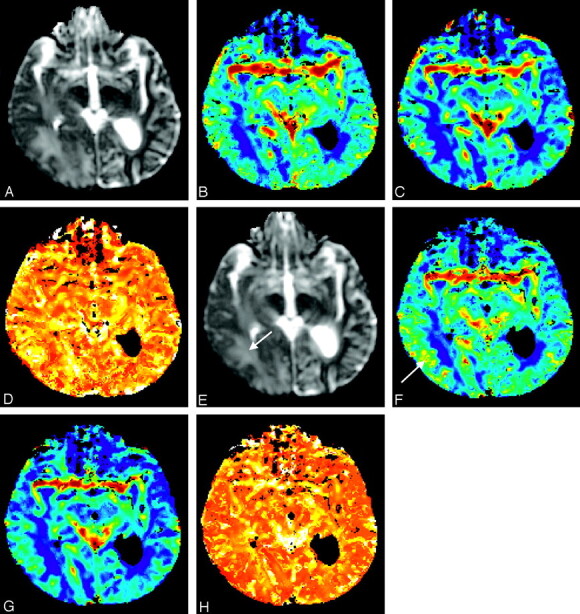
Images obtained from patient 4. [<D>] (A and E), CBF (B and F), CBV (C and G), and MTT (D and H) maps obtained pre- (first row) and 72 hours poststeroid treatment. Note the reduction in [<D>] (red arrow) and the subtle increase in CBF for cortical/subcortical tissue within the edematous brain region (pink arrow). The maps are scaled to a maximum of 2500 × 10−6 mm2/s for [<D>], 75 mL/100 g/min for CBF, 7 mL/100 g for CBV, and 5 seconds for MTT.
Pre- and poststeroid treatment values of CBF, CBV, MTT, and <D> for enhancing tumor and nonenhancing peritumoral edematous brain are shown in Tables 1 and 2 for the 10 patients studied. In 3 patients (2, 5, and 10), there was no evidence of edema on T2-weighted EP imaging. For this patient group, average enhancing tumor CBF was practically unchanged (53.0 ± 12.7–53.2 ± 12.4 mL/100 g/min; P = .95), whereas tumor CBV (4.8 ± 1.1–4.6 ± 1.4 mL/100 g; P = .49) and MTT (3.7 ± 0.6–3.5 ± 0.8 seconds; P = .28) were slightly reduced after dexamethasone treatment. There was an increase in edema CBF (18.4 ± 2.5–20.6 ± 4.1 mL/100 g/min; P = .05) at the level of significance, but no significant change in CBV (1.5 ± 0.3–1.6 ± 0.5 mL/100 g; P = .53) or MTT (3.4 ± 0.4–3.2 ± 0.7 seconds; P = .67). <D> was significantly reduced for both enhancing tumor (1245 ± 236–1172 ± 221 × 10−6 mm2/s; P = .001) and edematous brain (1448 ± 169–1361 ± 159 × 10−6 mm2/s; P < .001).
Table 1:
Values of cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT), and mean diffusivity 〈D〉 for the entire volume of enhancing tumor pre- and postdexamethasone treatment for the 10 patients participating in this study
| Patient No. | Enhancing Tumor |
|||||||
|---|---|---|---|---|---|---|---|---|
| CBF (mL/100 g/min) |
CBV (mL/100 g) |
MTT (s) |
<D> (×10−6 mm2/s) |
|||||
| Presteroids | Poststeroids | Presteroids | Poststeroids | Presteroids | Poststeroids | Presteroids | Poststeroids | |
| 1 | 45.4 (±18.1) | 42.6 (±20.3) | 4.1 (±2.1) | 2.6 (±1.3) | 3.6 (±0.9) | 2.4 (±0.6) | 1516 (±206) | 1458 (±222) |
| 2 | 41.9 (±13.9) | 49.4 (±19.2) | 5.3 (±1.8) | 5.9 (±2.1) | 5.1 (±0.8) | 4.9 (±1.1) | 1133 (±143) | 1135 (±136) |
| 3 | 56.8 (±34.0) | 58.0 (±32.1) | 6.2 (±4.2) | 5.7 (±3.6) | 4.2 (±1.3) | 3.8 (±1.5) | 1684 (±361) | 1560 (±381) |
| 4 | 29.3 (±8.2) | 29.4 (±9.6) | 2.5 (±0.9) | 2.4 (±0.8) | 3.5 (±0.8) | 3.4 (±0.8) | 1060 (±145) | 951 (±109) |
| 5 | 68.5 (±24.5) | 68.8 (±24.9) | 5.5 (±2.0) | 4.5 (±1.5) | 3.2 (±0.4) | 2.6 (±0.4) | 994 (±96) | 917 (±51) |
| 6 | 61.5 (±20.9) | 70.2 (±31.6) | 5.1 (±1.8) | 6.7 (±3.1) | 3.4 (±0.8) | 3.9 (±0.9) | 1268 (±209) | 1201 (±240) |
| 7 | 56.9 (±25.2) | 62.6 (±28.7) | 4.8 (±2.1) | 4.5 (±1.9) | 3.4 (±0.7) | 2.9 (±0.6) | 1300 (±246) | 1155 (±225) |
| 8 | 71.4 (±35.5) | 51.9 (±27.4) | 6.4 (±3.5) | 3.8 (±2.0) | 3.6 (±1.0) | 3.0 (±0.5) | 1229 (±226) | 1136 (±215) |
| 9 | 49.3 (±21.3) | 52.4 (±21.9) | 4.3 (±1.9) | 5.1 (±2.2) | 3.6 (±0.6) | 3.9 (±1.0) | 1353 (±268) | 1302 (±277) |
| 10 | 49.3 (±22.2) | 46.5 (±22.7) | 4.3 (±2.2) | 4.6 (±2.7) | 3.5 (±1.3) | 4.1 (±1.9) | 914 (±295) | 907 (±258) |
| Mean | 53.0 (±12.7) | 53.2 (±12.4) | 4.8 (±1.1) | 4.6 (±1.4) | 3.7 (±0.6) | 3.5 (±0.8) | 1245 (±236) | 1172 (±221) |
| ε (%); P | 1.2 (±12.8); .95 | −4.6 (±22.9); .49 | −5.6 (±16.0); .28 | −5.8 (±3.8); .001 | ||||
All values are reported as mean (±SD).
The mean volume of enhancing tumor was 24.8 ± 21.4 × 10 mm.
Table 2:
Values of cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT), and mean diffusivity 〈D〉 for the entire volume of nonenhancing peritumoral edematous brain pre- and postdexamethasone treatment for the 7 patients who had regions of edema on T2-weighted EPI
| Patient No. | Peritumoral Edematous Brain |
|||||||
|---|---|---|---|---|---|---|---|---|
| CBF (mL/100 g/min) |
CBV (mL/100 g) |
MTT (s) |
<D> (×10−6 mm2/s) |
|||||
| Presteroids | Poststeroids | Presteroids | Poststeroids | Presteroids | Poststeroids | Presteroids | Poststeroids | |
| 1 | 16.3 ± 6.7 | 18.1 ± 7.7 | 1.5 ± 0.7 | 1.0 ± 0.4 | 3.7 ± 1.1 | 2.3 ± 0.6 | 1465 ± 231 | 1401 ± 196 |
| 3 | 19.8 ± 11.7 | 24.1 ± 13.9 | 1.9 ± 1.4 | 2.0 ± 1.4 | 3.7 ± 1.1 | 3.2 ± 0.9 | 1484 ± 275 | 1407 ± 262 |
| 4 | 20.8 ± 7.2 | 22.3 ± 7.9 | 1.6 ± 0.6 | 1.7 ± 0.6 | 3.1 ± 0.7 | 3.1 ± 0.5 | 1153 ± 167 | 1081 ± 146 |
| 6 | 19.8 ± 11.6 | 24.4 ± 15.3 | 1.4 ± 1.0 | 2.3 ± 1.5 | 2.9 ± 0.8 | 4.2 ± 2.9 | 1537 ± 237 | 1418 ± 232 |
| 7 | 20.7 ± 15.8 | 24.2 ± 19.1 | 1.8 ± 1.4 | 1.8 ± 1.4 | 3.7 ± 1.6 | 3.0 ± 0.8 | 1578 ± 268 | 1466 ± 262 |
| 8 | 17.1 ± 5.1 | 14.6 ± 3.5 | 1.2 ± 0.4 | 1.0 ± 0.3 | 3.6 ± 1.3 | 2.6 ± 0.3 | 1627 ± 106 | 1542 ± 136 |
| 9 | 14.4 ± 9.5 | 16.7 ± 9.4 | 1.3 ± 0.9 | 1.7 ± 1.0 | 3.7 ± 0.6 | 4.2 ± 1.3 | 1290 ± 193 | 1209 ± 197 |
| Mean | 18.4 ± 2.5 | 20.6 ± 4.1 | 1.5 ± 0.3 | 1.6 ± 0.5 | 3.4 ± 0.4 | 3.2 ± 0.7 | 1448 ± 169 | 1361 ± 159 |
| ε (%); P | 11.6 ± 12.8; .05 | 7.2 ± 30.5; .53 | −2.8 ± 25.8; .67 | −6.0 ± 1.2; <.001 | ||||
All values are reported as mean (±SD). The mean volume of edematous brain was 62.4 ± 52.7 × 103 mm3.
For normal-appearing contralateral white matter in these patients, the average pretreatment values of CBF, CBV, MTT, and <D> were 16.0 ± 3.0 mL/100 g/min, 1.2 ± 0.2 mL/100 g, 3.2 ± 0.3 seconds, and 757 ± 48 × 10−6 mm2/s, respectively. There was no significant change in any of these parameters after dexamethasone treatment, with CBF, CBV, MTT, and <D> being 17.1 ± 3.6 mL/100 g/min (P = .20), 1.3 ± 0.3 mL/100 g (P = .59), 3.0 ± 0.4 seconds (P = .33), and 764 ± 57 × 10−6 mm2/s (P = .30), respectively.
Discussion
Several previous imaging studies have investigated whether dexamethasone affects cerebral perfusion in intracranial tumors. Unfortunately, the results of these studies are contradictory possibly because of methodologic differences and heterogeneous tumor patient populations. Here, in the largest MR imaging study to date, quantitative cerebral perfusion parameters were measured in a group of patients with high-grade glioma to address further this question.
After 48–72 hours of steroid treatment, tumor CBF was practically unchanged, whereas edematous brain CBF increased on average by 11.6%. Several other studies produced results that are consistent with this observation. Van Roost et al8 used xenon-enhanced CT (Xe-CT) to assess the effects of daily dose (0–32 mg), cumulative dose (0 to 1000 mg), and duration of dexamethasone treatment (0–36 days) on cerebral perfusion in 26 patients with GBM. They found that, although CBF was inversely correlated with daily dose of dexamethasone in several gray and white matter regions within the contralateral hemisphere, CBF of solid and necrotic tumor was not correlated with any of the dexamethasone treatment parameters. Furthermore, they found that edematous brain CBF was positively correlated with both duration and total dose of dexamethasone treatment. Apart from a reduction in CBF in some normal tissue regions, these findings of no alterations in tumor CBF and an increase in edematous brain CBF are similar to those presented above. Such data, combined with the observation that dexamethasone produces a significant localized reduction in the magnitude of extracellular water molecule mobility (<D>) and hence extracellular water content in edematous brain, provides support for the views of Reulen et al,7 who suggest that steroids may act by reversing localized hypoaemia in peritumoral edematous brain. By using the 133Xe inhalation method, they reported a significant increase in ipsilateral hemispheric CBF of 38% after 5–7 days of dexamethasone treatment (24 mg/day) in 6 patients with a range of tumors. In brief, they argue that the raised extracellular water content of edematous brain produces an increase in the local tissue extravascular pressure, which in turn reduces peritumoral blood flow by collapsing the capillaries and raising the local cerebrovascular resistance. As dexamethasone reduces edematous brain water content, the local tissue pressure is also reduced leading to an increase in peritumoral blood flow. This may be one of the mechanisms responsible for the increase in edematous brain perfusion seen in the current study.
The view that dexamethasone produces no change in tumor perfusion and an increase in edematous brain perfusion is contradicted by results from the studies by Leenders et al6 and Behrens et al.9 In a 15O-positron emission tomography (PET) study of 10 patients with glioma (n = 4) and metastatic carcinoma, Leenders et al6 found that dexamethasone produced a significant reduction in both CBF and CBV in tumor and contralateral tissue, but not edematous regions, 1–5 days after treatment (intravenous dose of 20 mg followed by an oral regimen of 16 mg/day). In that study, it was hypothesized that dexamethasone causes vasoconstriction by inhibiting the release of prostacyclin, a powerful vasodilator, from vascular endothelial cells. Behrens et al9 reported a 32% decrease in peritumoral edema CBF compared with contralateral white matter in 11 patients with malignant glioma treated with dexamethasone (12–24 mg/day) for a least 6 days by using Xe-CT. They also found that CBF in contralateral cortex and white matter was significantly reduced compared with values measured in a control group of 10 patients with Parkinson disease; however, because all the patients were undergoing steroid treatment at the time of imaging and no baseline scans were acquired, it is not possible to determine completely the effects of dexamethasone on cerebral perfusion from this study.
In the only other study that has used MR imaging to measure the effects of dexamethasone on cerebral perfusion, Østergaard et al5 measured changes in CBF and CBV relative to contralateral white matter (rCBF and rCBV) and blood-tumor barrier permeability 1–6 hours after steroid therapy (intravenous dose of 20 mg) in 3 patients with astrocytoma, 2 with oligodendroglioma, and one with central nervous system (CNS) lymphoma. They reported a dramatic decrease in blood-tumor barrier permeability, a significant 15% reduction in rCBV for peritumoral gray matter (directly adjacent to edema), and a nonsignificant 6.5% increase in rCBV for peritumoral white matter (white matter within edema). In addition, except for the patient with CNS lymphoma, they found that both peritumoral gray and white matter rCBF did not show any systematic change after steroid treatment (data not shown).
The results of this study and those of Van Roost,8 Reulen et al,7 and Østergaard et al5 therefore suggest that dexamethasone does not significantly alter tumor blood flow, but may increase perfusion in peritumoral edematous brain through a marked decline in peritumoral water content, and hence local tissue pressure, caused by the initial reduction in blood-tumor barrier permeability. Differences in imaging methodology, steroid dosage, and time to imaging after steroid administration, however, make direct comparison of these studies difficult. With regard to imaging methodology, although the current study employed a method commonly used to measure quantitative cerebral perfusion parameters from T2*-weighted MR imaging data,11,12 the relationship between measured and true perfusion remains unclear. The main sources of error in perfusion quantification arise from the measurement of the AIF and the proportionality constants used to calculate CBF, CBV, and MTT in the tracer kinetic model.13 Because it is assumed that the AIF measured in a major artery is the exact input to the tissue, any delays or dispersion of the bolus from the measurement site to the tissue of interest will introduce errors in the calculated perfusion parameters. Similarly, the relatively low spatial resolution of EP images may lead to partial-volume averaging of artery and tissue signals, which will affect the characterization of the AIF and hence the measured perfusion parameters. Different tissue characteristics will also affect the accuracy of the perfusion data. For example, κ, the constant of proportionality between signal intensity change and concentration of contrast agent within a voxel, has been shown to be tissue-dependent15 and thus varies between AIF, normal, and pathologic tissue. In view of these problems, several authors have compared DSC-MR imaging with other imaging methods that generate quantitative measures of cerebral perfusion. For example, in a study of 10 healthy volunteers, Wirestam et al16 found that, though the correlation between average CBF measured over an entire section by using DSC-MR imaging and 133Xe single-photon emission CT (SPECT) was linear (r = 0.74–0.83), the CBF value measured by DSC-MR imaging was higher than that provided by SPECT (48 ± 17 vs 33 ± 6 mL/100 g/min) even when account was taken of contaminating signal intensity from large vessels and partial-volume averaging of tissue and artery in voxels used for AIF determination. Conversely, Carroll et al17 measured CBF values in 8 volunteers by using both DSC-MR imaging and PET and found that, though reproducibility was better for PET than MR imaging, there was still reasonably good agreement between the 2 techniques for white matter perfusion (CBFPET-CBFMR imaging = −0.09 ± 7.23 mL/100 g/min). However, in light of the increasing use of DSC-MR imaging to characterize perfusion in a range of different pathologies, further studies are required to investigate the accuracy of perfusion measured by using DSC-MR imaging and its relationship to data obtained from other imaging modalities in both healthy and diseased brain.
In addition to the small number of subjects scanned in this study, a further problem in determining the significance of the current results is demonstrated by Tables 1 and 2, which show how variable tumor and peritumoral edematous brain perfusion is from patient to patient, even in a group of subjects with the same tumor type. High-grade gliomas are characterized by their highly abnormal microvasculature, which gives rise to extremely variable and heterogeneous blood flow patterns,18 so perhaps this is to be expected. However, the response to steroids is also highly variable, with the result that the standard deviations of <ε> are far greater than <ε> itself in all cases except edematous brain CBF. This indicates that the effect size of perfusion changes in peritumoral edematous brain after 48–72 hours of steroid treatment is small. In addition to performing much larger studies, the effect size for edematous brain perfusion changes could be increased by performing imaging at time points beyond 48–72 hours of treatment, whereas the study of Van Roost et al8 suggests that increases in perfusion relative to baseline might become more significant. Future studies aimed at extending the current work should therefore study large homogeneous groups of tumors and map an individual patient’s perfusion patterns at regular and defined time points over perhaps several weeks. Such time-series data on the longer-term effects of steroids on cerebral perfusion will be vital in studies assessing the efficacy of angiogenesis-inhibiting drugs,19 because patients will most likely be taking steroids concurrently with these newer treatments.
Conclusions
In this pilot study, the effects of dexamethasone on cerebral perfusion and [<D>] were measured by using MR imaging in a group of patients with high-grade glioma. After 48–72 hours of treatment, no significant change in CBF, CBV, or MTT was observed in enhancing tumor or normal-appearing contralateral white matter, but nonenhancing peritumoral edematous brain CBF was increased by 11.6%. [<D>] was significantly reduced in both enhancing tumor and edematous brain. These data suggest that dexamethasone does not significantly affect tumor blood flow, but may subtly increase perfusion in edematous brain by reducing peritumoral water content and hence local tissue pressure. Larger studies in homogeneous groups of patients are now required to replicate these findings, and to investigate further the longer-term effects of steroids on cerebral perfusion in intracranial tumors.
Acknowledgments
This work was funded by the Cunningham Trust and undertaken at the SHEFC Brain Imaging Research Centre for Scotland, Edinburgh, United Kingdom (http://www.dcn.ed.ac.uk/bic). Statistical advice was provided by Cat Graham of the Wellcome Trust Clinical Research Facility, University of Edinburgh (http://www.wtcrf.ed.ac.uk).
Footnotes
M.E.B. and T.K.C. contributed equally to this work.
References
- 1.Galicich JH, French LA, Melby JC. Use of dexamethasone in the treatment of cerebral edema associated with brain tumors. Lancet 1961;81:46–53 [PubMed] [Google Scholar]
- 2.Maxwell RE, Long DM, French LA. The clinical effects of a synthetic gluco-corticoid used for brain edema in the practice of neurosurgery. In: Reulen HJ, Schürmann K, eds. Steroids and brain edema. Berlin: Springer-Verlag;1972. :219–32
- 3.Hossmann KA, Hurter T, Oschlies U. The effect of dexamethasone on serum protein extravasation and edema development in experimental brain tumors of cat. Acta Neuropathol 1983;60:223–31 [DOI] [PubMed] [Google Scholar]
- 4.Sinha S, Bastin ME, Wardlaw JM, et al. Effects of dexamethasone on peritumoral oedematous brain: a DT-MRI study. J Neurol Neurosurg Psychiatry 2004;75:1632–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Østergaard L, Hochberg FH, Rabinov JD, et al. Early changes measured by magnetic resonance imaging in cerebral blood flow, blood volume, and blood-brain barrier permeability following dexamethasone treatment in patients with brain tumors. J Neurosurg 1999;90:300–305 [DOI] [PubMed] [Google Scholar]
- 6.Leenders KL, Beaney RP, Brooks DJ, et al. Dexamethasone treatment of brain tumor patients: effects on regional cerebral blood flow, blood volume, and oxygen utilization. Neurology 1985;35:1610–16 [DOI] [PubMed] [Google Scholar]
- 7.Reulen HJ, Hadjidimos A, Schürmann K. The effect of dexamethasone on water and electrolyte content and on rCBF in perifocal brain edema in man. In: Reulen HJ, Schürmann K, eds. Steroids and brain edema. Berlin: Springer-Verlag;1972. :239–52
- 8.Van Roost D, Hartmann A, Quade G. Changes of cerebral blood flow following dexamethasone treatment in brain tumor patients. A Xe/CT study. Acta Neurochir 2001;143:37–43 [DOI] [PubMed] [Google Scholar]
- 9.Behrens PF, Ostertag CB, Warnke PC. Regional cerebral blood flow in peritumoral brain edema during dexamethasone treatment: a xenon-enhanced computed tomographic study. Neurosurgery 1998;43:235–40 [DOI] [PubMed] [Google Scholar]
- 10.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal 2001;5:143–56 [DOI] [PubMed] [Google Scholar]
- 11.Østergaard L, Sorensen AG, Kwong KK, et al. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part II. Experimental comparison and preliminary results. Magn Reson Med 1996;36:726–36 [DOI] [PubMed] [Google Scholar]
- 12.Østergaard L, Weisskoff RM, Chesler DA, et al. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I. Mathematical approach and statistical analysis. Magn Reson Med 1996;36:715–25 [DOI] [PubMed] [Google Scholar]
- 13.Calamante F, Gadian DG, Connelly A. Quantification of perfusion using bolus tracking magnetic resonance imaging in stroke: assumptions, limitations, and potential implications for clinical use. Stroke 2002;33:1146–51 [DOI] [PubMed] [Google Scholar]
- 14.Sinha S, Bastin ME, Whittle IR, et al. Diffusion tensor MR imaging of high-grade cerebral gliomas. AJNR Am J Neuroradiol 2002;23:520–27 [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson KM, Tao JZ, Kennan RP, et al. Intravascular susceptibility agent effects on tissue transverse relaxation rates in vivo. Magn Reson Med 2000;44:909–14 [DOI] [PubMed] [Google Scholar]
- 16.Wirestam R, Ryding E, Lindgren A, et al. Absolute cerebral blood flow measured by dynamic susceptibility contrast MRI: a direct comparison with Xe-133 SPECT. MAGMA 2000;11:96–103 [DOI] [PubMed] [Google Scholar]
- 17.Carroll TJ, Teneggi V, Jobin M, et al. Absolute quantification of cerebral blood flow with magnetic resonance, reproducibility of the method, and comparison with H2(15)O positron emission tomography. J Cereb Blood Flow Metab 2002;22:1149–56 [DOI] [PubMed] [Google Scholar]
- 18.Law M, Yang S, Babb JS, et al. Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol 2004;25:746–55 [PMC free article] [PubMed] [Google Scholar]
- 19.Akella NS, Twieg DB, Mikkelsen T, et al. Assessment of brain tumor angiogenesis inhibitors using perfusion magnetic resonance imaging: quality and analysis results of a phase I trial. J Magn Reson Imaging 2004;20:913–22 [DOI] [PubMed] [Google Scholar]