Abstract
BACKGROUND AND PURPOSE: The purpose of this study was to report the incidence, clinical presentation, endovascular treatment, and outcome of aneurysms of the posterior cerebral artery (PCA).
PATIENTS AND METHODS: Among 1880 aneurysms treated between January 1995 and January 2005, 22 aneurysms (1.2%) in 22 patients were located on the PCA. Ten patients presented with subarachnoid hemorrhage (SAH) from the PCA aneurysm: 2 of these patients had additional visual field deficits and 2 had additional occulomotor palsy. One patient presented with acute occulomotor palsy only. Eleven PCA aneurysms were unruptured: 9 were additional to another ruptured aneurysm and 2 were incidentally discovered. Three aneurysms were >15 mm and the other 19 aneurysms were ≤8 mm. Eighteen aneurysms were saccular, 2 were fusiform, one was dissecting, and one was mycotic.
RESULTS: All aneurysms were successfully treated, 17 with selective occlusion of the aneurysm with coils and 5 with simultaneous occlusion of the aneurysm and parent PCA with coils. There were no complications of treatment. Two patients died of sequelae of SAH shortly after treatment. One patient died 2 months after coiling of an unruptured P1 aneurysm with intramural thrombus of SAH from the same aneurysm. One patient had persistent hemianopsia. In 2 patients with intact visual field in which the parent PCA was occluded, no hemianopsia developed due to sufficient leptomeningeal collateral circulation.
CONCLUSION: Aneurysms of the PCA are rare with an incidence in our practice of 1.2% of all types of aneurysms. Clinical presentation is variable with SAH, occulomotor palsy, visual field deficit or a combination. Endovascular treatment with either selective occlusion of the aneurysm or occlusion of the aneurysm together with the parent artery with coils is safe and effective with good clinical results.
Aneurysms of the posterior cerebral artery (PCA) are rare.1–4 The PCA supplies part of the temporal cortex, the calcarine and occipital cortex as well as parts of the brain stem and thalamus. PCA aneurysms may be saccular, fusiform, or dissecting and can be located at various segments of the PCA. Aneurysms on the proximal P1 segment may impede on the occulomotor nerve. Dissecting aneurysms may occlude the PCA causing homonymous hemianopsia if collateral circulation is not sufficient.4 Endovascular or surgical treatment consists of selective occlusion of the aneurysm or occlusion of the parent artery. Surgery of PCA aneurysms is technically challenging owing to the complexity of the perforating branches from the PCA and their relationship with cranial nerves and the upper brain stem.1–4 Reports about endovascular treatment of PCA aneurysms are scarce.5,6 In this study, we report our experience with 22 PCA aneurysms treated with selective occlusion with coils or occlusion of the parent PCA together with occlusion of the aneurysm.
Patients and Methods
Between January 1995 and January 2005, 1880 aneurysms were treated at our institution. Surgery was performed in 948 aneurysms and 932 aneurysms were treated by endovascular techniques: selective aneurysm occlusion with detachable coils was performed in 841 aneurysms and parent vessel occlusion in 91 aneurysms (57 occlusions of the internal carotid artery and 34 occlusions of vessels of the posterior circulation). Of 1880 treated aneurysms, 22 (1.2%) aneurysms in 22 patients were located on the PCA (Table 1). There were 14 women and 8 men ranging in age from 27 to 72 years (mean, 49.4 years). All 22 PCA aneurysms were treated endovascularly: selective aneurysm occlusion with coils in 17 patients and occlusion of both the aneurysm and parent PCA in 5 patients. Of the 22 aneurysms, 17 were saccular, 2 were fusiform, one was saccular with dissection of the wall (intramural hematoma), one was mycotic, and one was dissecting. Median size of the aneurysms was 5 mm (mean, 7.3 mm; range 2–35 mm). Of the 22 aneurysms, 19 (86%) were ≤8 mm and 3 (14%) were >15 mm. Clinical presentation was subarachnoid hemorrhage (SAH) in 10 patients (one with additional occulomotor palsy and 2 with additional homonymous hemianopsia), acute occulomotor palsy in one patient. In 9 patients the aneurysm was an additional finding to another ruptured aneurysm and in 2 patients the aneurysm was incidentally discovered. Eleven (50%) patients had multiple aneurysms, and one patient with a giant PCA (flow) aneurysm had an ipsilateral occipital arteriovenous malformation (AVM).
Table 1:
Clinical and aneurysm characteristics of 22 patients with posterior cerebral artery aneurysms
| Patient No./Age (y)/Sex | Clinical Presentation | Site | Aneurysm Type | Size (mm) | Treatment | Outcome and Duration of Follow-up | Associated Disease | |
|---|---|---|---|---|---|---|---|---|
| 1/F/41 | SAH other aneurysm | P1–P2 | Saccular | 6 | Coil occlusion | GOS 5 | 12 mo | 1 other aneurysm |
| 2/F/72 | SAH other aneurysm | P1–P2 | Saccular | 6 | Coil occlusion | GOS 5 | 24 mo | 1 other aneurysm |
| 3/F/59 | SAH other aneurysm | P3–P4 | Saccular | 4 | Coil occlusion | GOS 5 | 8 mo | 3 other aneurysms |
| 4/F/49 | SAH other aneurysm | P1–P2 | Saccular | 3 | Coil occlusion | GOS 5 | 10 mo | 3 other aneurysms |
| 5/F/47 | SAH other aneurysm | P1–P2 | Saccular | 3 | Coil occlusion | GOS 5 | 12 mo | 5 other aneurysms |
| 6/F/45 | SAH other aneurysm | P1–P2 | Saccular | 4 | Coil occlusion | GOS 5 | 6 mo | 7 other aneurysms |
| 7/F/55 | SAH other aneurysm | P1–P2 | Saccular | 4 | Coil occlusion | GOS 5 | 8 mo | 3 other aneurysms |
| 8/F/51 | SAH other aneurysm | P1–P2 | Saccular | 2 | Coil occlusion | GOS 5 | 6 mo | 2 other aneurysms |
| 9/M/56 | SAH other aneurysm | P1–P2 | Saccular | 4 | Coil occlusion | GOS 5 | 7 mo | 1 other aneurysm |
| 10/M/49 | Incidental | P1–P2 | Saccular | 7 | Coil occlusion | No deficit | 6 mo | |
| 11/F/43 | Incidental | P2 | Fusiform | 8 | Coil PVO | No deficit | 6 mo | |
| 12/M/35 | CN III palsy | P1–P2 | Saccular/wall dissection | 8 | Coil occlusion | Died 2 months later of SAH | ||
| 13/M/64 | SAH HH I and CN III palsy | P2 | Fusiform dissection | 20 | Coil PVO | GOS 5 no deficit | 12 mo | |
| 14/M/52 | SAH HH II and CN III palsy | P1 | Saccular | 6 | Coil occlusion (X2) | GOS 5 no deficit | 18 mo | Occluded left internal carotid artery |
| 15/F/44 | SAH HH I | P1–P2 | Saccular | 3 | Coil occlusion | GOS 5 | 60 mo | 1 other aneurysm |
| 16/M/47 | SAH HH I | P1–P2 | Saccular | 3 | Coil occlusion | GOS 5 | 6 mo | |
| 17/F/52 | SAH HH II | P1–P2 | Saccular | 35 | Coil occlusion (X2) | GOS 5 | 34 mo | Ipsilateral occipital AVM |
| 18/F/62 | SAH HH I | P1–P2 | Saccular | 6 | Coil occlusion | GOS 5 | 20 mo | 1 other aneurysm |
| 19/F/41 | SAH HHI | P1–P2 | Saccular | 4 | Coil occlusion | GOS 5 | 6 mo | |
| 20/M/32 | SAH HH III, hemianopsia, after rebleeding HH V | P2–P3 | Dissecting | 16 | Coil PVO | Death | ||
| 21/M/27 | SAH HH IV | P4 | Mycotic | 8 | Coil PVO | Death | AIDS, endocarditis | |
| 22/F/64 | SAH HH II, hemianopsia | P4 | Saccular | 2 | Coil PVO | GOS 4 (hemianopsia) | 6 mo | |
Note.—SAH indicates subarachnoid hemorrhage; GOS, Glasgow Outcome Score; HH, Hunt and Hess grade; CN III, occulomotor nerve; AVM, arteriovenous malformation; PVO, parent vessel occlusion.
Treatment
Endovascular treatment was performed under general anesthesia with systemic heparinization on a biplane angiographic unit (Philips Integra V3000 Neuro; Philips Medical Systems, Best, the Netherlands). Heparinization was continued for 48 hours after treatment, followed by 80 mg aspirin daily for 3 months. Aneurysm occlusion was performed with Guglielmi detachable coils (Boston Scientific, Fremont, Calif), and one giant PCA aneurysm was occluded with very long mechanically detachable coils (Detach 18; Cook, Copenhagen, Denmark). Aneurysms with a well-defined neck were selectively occluded with coils and aneurysms without a defined neck were occluded together with the parent PCA. Test occlusion before permanent PCA occlusion was not performed. Complications of treatment were recorded. Surviving patients with aneurysms who were selectively occluded had a follow-up angiogram after 6 months. Surviving patients with aneurysms who were occluded with deliberate occlusion of the parent PCA had follow-up MR imaging after 6 weeks.
Results
Selectively Occluded Aneurysms
Of 22 aneurysms, 17 were selectively occluded with coils. Initial occlusion was complete in 16 and near-complete in one aneurysm. One patient (12), who initially presented with acute occulomotor palsy based on wall dissection of an unruptured P1 aneurysm, died 2 months later of rupture of the coiled aneurysm. All other patients were clinically unremarkable at follow-up. Of the remaining 16 aneurysms, follow-up angiography at 6 months showed partial reopening by compaction in 2 (14 and 17), and both aneurysms were additionally coiled with stable complete occlusion at extended angiographic follow-up.
Aneurysms Occluded Together with the Parent PCA
Of 22 aneurysms, 5 were occluded with coils together with the parent artery. Two of these 5 patients presented with SAH and homonymous hemianopsia: one of these 2 patients (20) died of sequelae of SAH, and the other patient (22) survived with persistent hemianopsia. One patient with AIDS and endocarditis with a ruptured P4 mycotic aneurysm died several days after occlusion of the aneurysm and parent artery. One patient (13) who presented with Hunt and Hess (HH) grade I SAH and occulomotor palsy recovered completely after occlusion of the aneurysm and the parent PCA with coils without hemianopsia, and one patient (11) with an incidentally discovered fusiform P2 aneurysm remained neurologically intact after occlusion of the aneurysm and parent PCA.
Complications of Treatment
In 24 treatments of 22 aneurysms, no complications occurred (0%; 97.5% confidence interval, 0%–18%).
Illustrative Cases
Patient 11.
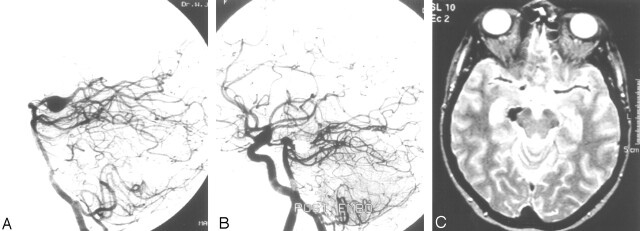
This 43-year-old woman had an incidentally discovered fusiform P2 aneurysm on MR imaging performed for chronic headaches (Fig 1). The aneurysm was occluded with coils together with the parent PCA. Angiography after occlusion showed good collateral supply to the distal PCA territory. She remained neurologically intact with normal MR imaging 6 weeks later.
Fig 1.
Patient 11, a 43-year-old woman with an incidentally discovered fusiform P2 aneurysm.
A, Lateral projection of vertebral aneurysm shows an 8-mm fusiform P2 aneurysm.
B, Simultaneous angiogram of vertebral artery and right internal carotid artery shows complete occlusion of the aneurysm including the parent PCA and good filling of distal PCA branches through leptomeningeal collateral vessels.
C, MR imaging 6 weeks after PCA occlusion shows no infarction in PCA territory.
Patient 20.
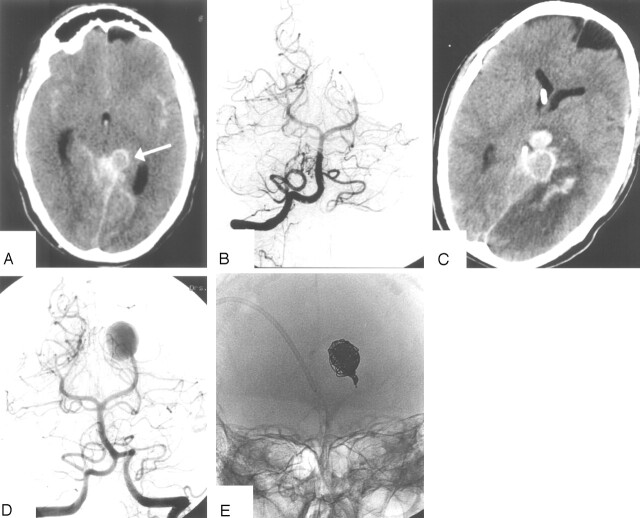
This 32-year-old man presented with HH grade III SAH and hemianopsia. A CT scan showed subarachnoid blood in the basal cisterns, infarction in the left PCA territory, and a hyperattenuating aneurysm in the left ambient cistern (Fig 2). Vertebral angiography revealed an occlusion of the left PCA beyond the P2 segment, apparently by a thrombosed dissecting aneurysm. Coil occlusion of the afferent P2 was considered but was judged not necessary. In retrospect, this was an incorrect decision: 4 days later, the patient suddenly deteriorated to HH grade V. CT scan showed a recurrent SAH and a left thalamic hematoma. The aneurysm had enlarged and the infarction in the left PCA territory had become hemorrhagic. Repeated angiography now showed filling of a large dissecting aneurysm, and this aneurysm was occluded with coils including the afferent P2. The patient died 3 days later.
Fig 2.
Patient 20, a 32-year-old man presenting with HH grade III SAH and hemianopsia.
A, CT scan on the day of admission shows SAH and aneurysm in the left ambient cistern (arrow).
B, Vertebral angiogram shows occluded left PCA beyond the P2, presumably by a dissecting aneurysm. Endovascular therapy was judged not necessary.
C, CT scan after sudden clinical detoriation 4 days after admission shows enlargement of the aneurysm, recurrent SAH with thalamic hematoma and hemorrhagic infarction in the PCA territory.
D, Angiogram after recurrent SAH shows filling of large dissecting aneurysm.
E, Occlusion of the aneurysm with coils including the afferent P2. The patient died 3 days later.
Patient 13.
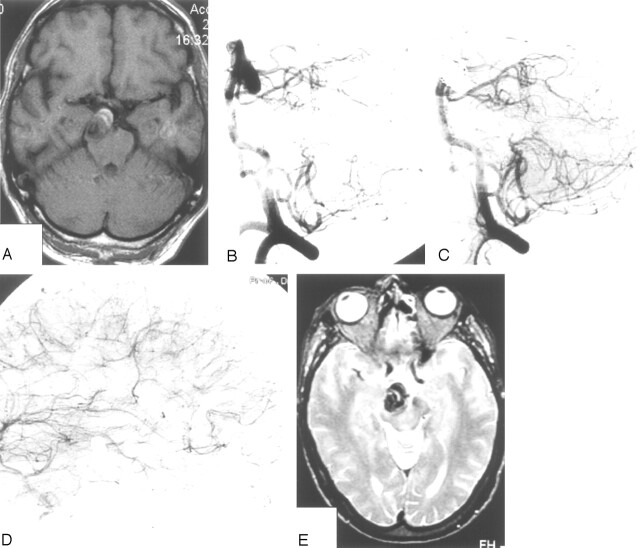
This 64-year-old man presented with an HH grade I SAH and right occulomotor palsy. MR imaging and angiography revealed a large fusiform dissecting aneurysm on the right P2 with intramural thrombus (Fig 3). The aneurysm was occluded with coils including the parent PCA. Angiography demonstrated good leptomeningeal collateral supply to the distal PCA territory. The patient’s visual field remained intact. The occulomotor palsy resolved completely in the following months.
Fig 3.
Patient 13, a 64-year-old man presenting with HH grade I SAH and right occulomotor palsy.
A and B, MR imaging and angiography show dissecting fusiform P2 aneurysm with intramural thrombus.
C and D, Vertebral (C) and right internal carotid (D) angiogram after occlusion of the aneurysm including the parent PCA show good collateral supply to the occipital lobe through leptomeningeal collaterals.
E, MR imaging 6 weeks after PCA occlusion demonstrates no infarction in right PCA territory.
Patient 22.
This 64-year-old woman presented with HH grade II SAH and hemianopsia because of an intracerebral hematoma involving the optic tract. Angiography revealed a 2-mm aneurysm on a distal P4 branching point, and this aneurysm was occluded with coils including the parent artery (Fig 4). She made an uneventful recovery with persistent hemianopsia at follow-up.
Fig 4.
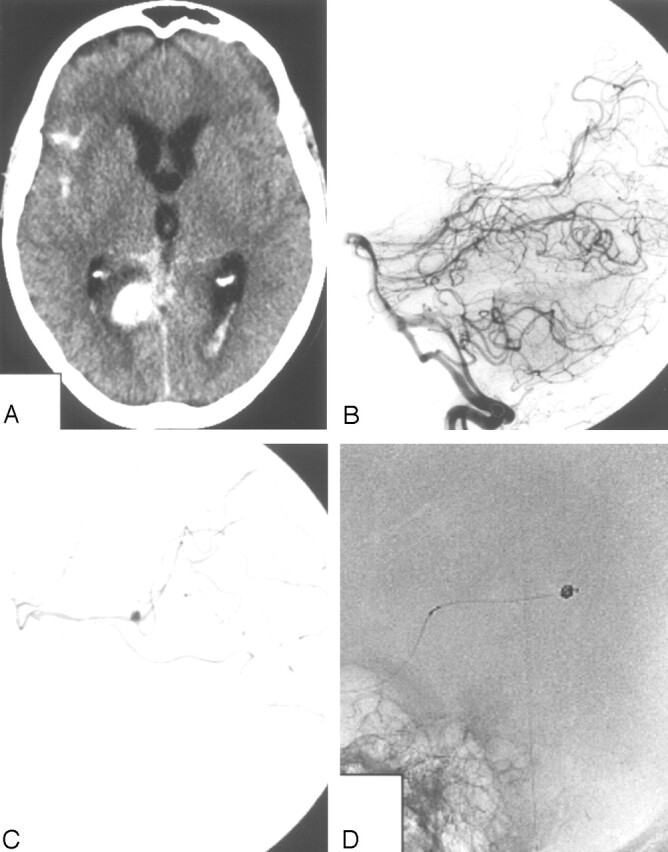
Patient 22, a 64-year-old woman presenting with HH grade I SAH and hemianopsia.
A, CT scan showing subarachnoid and intraventricular blood and a hematoma in the medial occipital lobe.
B, Lateral vertebral angiogram showing a small aneurysm on the P4 (arrow).
C, Superselective angiogram, which better demonstrates the small aneurysm.
D, Occlusion of the aneurysm including the parent artery with coils.
Patient 12.
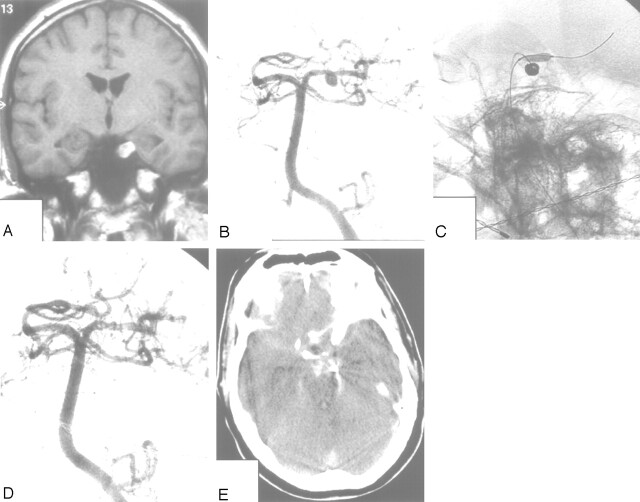
This 35-year-old man presented with acute left occulomotor palsy. MR imaging and angiography demonstrated an 8-mm aneurysm on the left P1–P2 junction pointing downward with intraluminal thrombus caused by wall dissection (Fig 5). The lumen of the aneurysm measured 4-mm and was completely occluded with coils with the aid of a supporting balloon. Two months later, the patient was readmitted with an HH grade V SAH from the previously coiled aneurysm. He died before angiography could be performed. We postulate that the intraluminal thrombus in this aneurysm had resolved in the weeks following coiling causing reopening of the aneurysm with subsequent rupture.
Fig 5.
Patient 12, a 35-year-old man presenting with acute left occulomotor palsy.
A and B, MR imaging and angiography show a left P1–P2 aneurysm pointing downward with an intramural thrombus.
C and D, Coiling of the aneurysm with the aid of a supporting balloon results in complete occlusion of the lumen.
E, CT scan 2 months later shows SAH from the coiled aneurysm. The patient died the next day.
Discussion
Anatomic Considerations
The PCA can anatomically be subdivided into 4 segments.7 The P1 segment extends from the tip of the basilar artery to the origin of the posterior communicating artery. The occulomotor nerve has an intimate relationship with the P1 and courses between the P1 and the proximal superior cerebellar artery. The P2 segment extends from the posterior communicating artery to the dorsal aspect of the midbrain. From the P1 and P2 segment perforating arteries to the thalamus (thalamo-perforating and posterior choroidal arteries) and brain stem (pedunculo-perforating and long circumflex arteries) arise. The P3 and P4 segments extend distally from the dorsal aspect of the midbrain and supply the calcarine and parieto-occipital cortex. A rich collateral network exists between both the deep and superficial territories of the PCA and other arteries: the anterior choroidal artery and the superior cerebellar artery for the deep territory and leptomeningeal collaterals from the anterior and middle cerebral arteries for the superficial territory.
Clinical Considerations
According to previous reports, compared with aneurysms located at other sites, PCA aneurysms tend to be larger, present with symptoms of mass-effect or SAH and appear at an earlier age.4,8–12 The present series does not support these previous observations: 19 of 22 (86%) PCA aneurysms were small and mean patient age (49.4 years) was not significantly less than for patients with aneurysms at other locations. Only 10 of 22 patients (45%) presented with SAH, 9 aneurysms (41%) were additional to another ruptured aneurysm, 2 aneurysms (9%) were an incidental finding, and one aneurysm (5%) presented with mass effect on CN III.
In our 22 patients with PCA aneurysms, we observed a relatively high incidence of coexisting vascular lesions: 11 patients (50%) had multiple aneurysms (≤8 aneurysms) and one patient (5%) had an occipital AVM supplied by the ipsilateral PCA. This has also been observed by Ciceri et al.6
Sixteen of 22 PCA aneurysms (73%) were saccular and located on the P1 or P1–P2 junction, and these aneurysms were selectively occluded with coils with sparing of the parent PCA. This therapy was straightforward and effective in all but one patient, who died 2 months later of SAH from the coiled aneurysm. This patient presented with acute occulomotor palsy and MR imaging showed intramural dissection with thrombus of the P1–P2 aneurysm (Fig 5). Possibly the intramural thrombus resolved soon after treatment and the aneurysm lumen reopened, thereby exposing the patient to a risk of hemorrhage. Very early follow-up angiography should have been performed to detect reopening of this partially thrombosed aneurysm.
Two aneurysms were fusiform (dissecting) aneurysms located on the P2 segment in patients with intact visual fields. Both aneurysms were occluded with coils including the PCA and both patients experienced no visual field deficits after occlusion. We did not consider test occlusion before permanent occlusion in these patients, because occlusion of the PCA at the level of the P2 is generally well tolerated by virtue of a rich collateral arterial network (Figs 1 and 3).1,5,10 Moreover, although revascularization to the PCA is technically possible, these procedures are technically challenging, with largely unknown chance of success and unknown risk of complications.13–15 Three other patients were treated with occlusion of the parent PCA, but these aneurysms were located more distally on a P2–P3 junction in one patient and on P4 in 2 patients. Two of these patients presented with hemianopsia from the onset, and the other patient was in HH grade V and died soon after treatment. The patient with the P2–P3 aneurysm had a remarkable clinical course: a dissection occluded the PCA and the dissecting aneurysm was only visible on repeated angiography after recurrent SAH (Fig 2). In retrospect the occluded PCA at initial angiography should have been “plugged” with coils to prevent opening of the aneurysm.
In conclusion, aneurysms of the PCA are rare, with an incidence in our practice of 1.2% of all types of aneurysms, not just saccular. Clinical presentation is variable with SAH, occulomotor palsy, visual field deficit, or a combination. PCA aneurysms do not differ in type or size from aneurysms located at other sites. Saccular aneurysms can safely and effectively be treated with selective occlusion with coils and sparing the parent PCA with good long-term anatomic results. Occlusion of the aneurysm together with the parent PCA with coils is an effective treatment for fusiform and dissecting aneurysms that is usually tolerated well as a result of adequate collateral circulation via the anterior choroidal artery, the superior cerebellar artery, and leptomeningeal collaterals from middle and anterior cerebral artery. Dissecting aneurysms that occlude the PCA and saccular aneurysms with intramural thrombus and dissection may reopen exposing the patient to the risk of (recurrent) SAH.
References
- 1.Drake CG, Peerless SJ, Hernesniemi JA. Surgery of vertebrobasilar aneurysms: London, Ontario experience in 1767 patients. New York: Springer-Verlag;1996. :221–48
- 2.Hernesniemi JA, Vapalahti MP, Niskanen M, et al. One-year outcome in early aneurysm surgery: a 14 year experience. Acta Neurochir (Wien) 1993;122:1–10 [DOI] [PubMed] [Google Scholar]
- 3.Peerless SJ, Hernesniemi JA, Gutman FB, et al. Early surgery for ruptured posterior circulation aneurysms. J Neurosurg 1994;80:643–49 [DOI] [PubMed] [Google Scholar]
- 4.Ferrante L, Acqui M, Trillo G, et al. Aneurysms of the posterior cerebral artery: do they present specific characteristics? Acta Neurochir (Wien) 1996;138:840–52 [DOI] [PubMed] [Google Scholar]
- 5.Hallacq P, Piotin M, Moret J. Endovascular occlusion of the posterior cerebral artery for the treatment of P2 segment aneurysms: retrospective review of a 10-year series. AJNR Am J Neuroradiol 2002;23:1128–36 [PMC free article] [PubMed] [Google Scholar]
- 6.Ciceri EF, Klucznik RP, Grossman RG, et al. Aneurysms of the posterior cerebral artery: classification and endovascular treatment. AJNR Am J Neuroradiol 2001;22:27–34 [PMC free article] [PubMed] [Google Scholar]
- 7.Zeal AA, Rhoton AL. Microsurgical anatomy of the posterior cerebral artery. J Neurosurg 1978;48:534–59 [DOI] [PubMed] [Google Scholar]
- 8.Chang HS, Fukushima T, Takakura K, et al. Aneurysms of the posterior cerebral artery: report of ten cases. Neurosurgery 1986;19:1006–11 [DOI] [PubMed] [Google Scholar]
- 9.Drake CG, Amacher AL. Aneurysms of the posterior cerebral artery. J Neurosurg 1969;30:468–74 [DOI] [PubMed] [Google Scholar]
- 10.Drake CG, Peerless SJ. Giant fusiform intracranial aneurysms: review of 120 patients treated surgically from 1965 to 1992. J Neurosurg 1997;87:141–62 [DOI] [PubMed] [Google Scholar]
- 11.Sakata S, Fujii K, Matsushima T, et al. Aneurysms of the posterior cerebral artery: report of eleven cases—surgical approaches and procedures. Neurosurgery 1993;32:163–67 [DOI] [PubMed] [Google Scholar]
- 12.Hamada J, Morioka M, Yano S, et al. Clinical features of aneurysms of the posterior cerebral artery: a 15-year experience with 21 cases. Neurosurgery 2005;56:662–70 [DOI] [PubMed] [Google Scholar]
- 13.Tulleken CA, van der Zwan A, van Rooij WJ, et al. High-flow bypass using nonocclusive excimer laser-assisted end-to-side anastomosis of the external carotid artery to the P1 segment of the posterior cerebral artery via the Sylvian route: technical note. J Neurosurg 1998;88:925–27 [DOI] [PubMed] [Google Scholar]
- 14.Vishteh AG, Smith KA, McDougall CG, et al. Distal posterior cerebral artery revascularization in multimodality management of complex peripheral posterior cerebral artery aneurysms: technical case report. Neurosurgery 1998;43:166–70 [DOI] [PubMed] [Google Scholar]
- 15.Nagasawa S, Sakaguchi I, Ohta T. The posterior temporal artery as the recipient in superficial temporal artery to posterior cerebral artery bypass: technical note. Surg Neurol 1999;52:73–77 [DOI] [PubMed] [Google Scholar]