Abstract
BACKGROUND AND PURPOSE: The HydroCoil Embolic System (HES) was developed to improve the efficacy of endovascular treatment of cerebral aneurysms. The purpose of this study is to study the periprocedural results in patients with cerebral aneurysms treated with HES.
METHODS: We report the initial periprocedural results in 191 cerebral aneurysms treated with HES in the HydroCoil for Endovascular Aneurysm Occlusion, or HEAL, study. Initial aneurysm occlusion and periprocedural complication rates were evaluated and compared with historical control data regarding aneurysms treated with platinum coils.
RESULTS: An initial occlusion result of “complete” or “near-complete” was achieved in 91.8% of aneurysms. Periprocedural thromboembolic events occurred in 8.1% of aneurysms treated with neurologic deficits related to thromboemboli occurring in 2.1% of aneurysms treated. Intraprocedural aneurysm perforations occurred in 2.8% of previously ruptured aneurysms, and in 0% of previously unruptured aneurysms.
CLUSION: The initial occlusion success and complication rate when HES is used to treat cerebral aneurysms is not significantly different from platinum coils. Follow-up angiography is currently being collected and will be evaluated to determine if use of the HES reduces the rate of aneurysm recurrence.
A principal disadvantage of endovascular therapy of cerebral aneurysms with endovascular coils as compared with surgical clipping is aneurysm recurrence.1–3 Aneurysm recurrence following endovascular therapy is likely related, at least in part, to the attenuation of packing of coils into the aneurysm, defined as volumetric percentage occlusion of the aneurysm cavity with the endovascular device.4–7 An embolic agent that improves packing attenuation might reduce the rate of aneurysm recurrence. The HydroCoil Embolic System (HES; MicroVention, Inc.; Aliso Viejo, Calif) is designed for improved packing attenuation, with an expansile hydrogel that should fill more of the aneurysm lumen than standard platinum coils.8 The HES is constructed as a hybrid hydrogel-platinum coil device (Fig 1). The initial diameter of these devices is 0.009 inch, and the expanded diameter is 0.027 inch. In blood, the hydrogel swells to its maximum diameter (3 times the original coil diameter) in approximately 20 minutes. HES received the CE Mark on May 27, 2002 and US Food and Drug Administration 510(k) clearance on July 29, 2002.
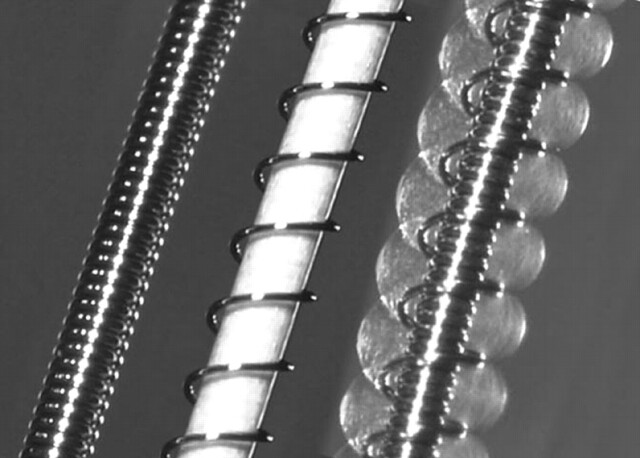
Fig 1.
Hybrid hydrogel-platinum coil device. Left, Bare platinum coil. Middle, Prehydration image shows initial profile of the device. Highly compact hydrogel material is wrapped around a platinum coil. An outer “overcoil” is wrapped around the hydrogel-covered coil. The outer diameter of the coil is 0.008 inch. The thickness of the hydrogel is approximately 0.0005 inch, such that the outer diameter of the gel covering is 0.009 inch. With the “overcoil,” the outer diameter is 0.013 inch. Right, Posthydration image of the device shows marked expansion of the hydrogel material, which has become translucent. The radial thickness of the expanded hydrogel is approximately 0.009 inch, such that the total outer diameter of the hydrated device is 0.027 inch.
The HydroCoil for Embolic Aneurysm Occlusion (HEAL) registry is a prospective registry of patients with cerebral aneurysms treated with the HES. The HEAL trial is sponsored by MicroVention, Inc. We report the results of initial treatment of cerebral aneurysms with the HES in the HEAL registry, with particular attention to the degree of aneurysm filling with embolic coils, and safety.
Patients and Techniques
The HEAL registry is a nonrandomized, multicenter, prospective study to investigate the safety and effectiveness of HES in patients with intracranial aneurysms deemed appropriate for endovascular treatment. A total of 15 sites in Europe and the United States participated in the investigation. The study was approved by each participating center’s institutional review board/ethics committee. Written informed consent was obtained for each patient enrolled in the study. The total patient enrollment for the investigation was 184 patients with 191 aneurysms. Patients were enrolled between October 2002 and February 2004.
Physicians were requested to complete training requirements before participation in the study, including placement of the HES coils in 2 aneurysms by using an animal model and completion of 2 human clinical cases with the assistance of MicroVention personnel.
The following criteria were used to determine a patient’s eligibility for enrollment in the investigation:
Inclusion Criteria. (1) The patient has been diagnosed as having an intracerebral aneurysm, either ruptured or unruptured, as demonstrated by arterial angiography. (2) Endovascular coil treatment is judged to be the appropriate treatment as determined by the responsible neuroradiologist and/or neurosurgeon.
Exclusion Criteria. (1) The patient has a cerebral aneurysm considered untreatable by endovascular techniques, which, in the opinion of the investigator, presents an unacceptable risk of morbidity due to the catheterization or embolization process. (2) The patient is contraindicated for a medication, which must be used to conduct this embolization procedure. (3) The patient has a coagulopathy that, in the opinion of the investigator, would make the patient unsuitable for endovascular embolization. (4) The patient has medical, social, or psychological factors that may interfere with his or her ability to comply with the investigational plan requirements or is unwilling to return for all follow-up visits. (5) The patient is pregnant or lactating. (6) The patient is younger than 18 years of age.
Embolization Procedure. Each embolization procedure was performed in a digital angiographic suite by using standard techniques. Anticoagulation with heparin was left to the operating physician’s discretion. A series of coils of appropriate dimensions will be selected and placed into the aneurysm under fluoroscopic control by using an angiographic road map where appropriate. One or more platinum coils (or any other approved 3D platinum coils) were generally used to establish the initial framework in the treatment of the aneurysm and form a multiplanar structure for subsequent HES coil introduction. The HES coils were generally used to provide additional filling of the aneurysm once the initial framework had been established by placement of one or more platinum complex coils. Following placement of the HES coils, additional platinum coils (eg, very soft platinum coils) were used at the discretion of the physician to complete the procedure. Angiograms were taken immediately after the last coil is placed and 15–20 minutes after detachment of the last coil. In each case, the objective of the procedure was angiographic occlusion of the aneurysm.
The following parameters were evaluated at the time of initial therapy with HES: aneurysm location; aneurysm size; aneurysm rupture status; Hunt and Hess classification; number, type and size of coils used in the procedure; and complications/adverse events. All assessments were made by the treating physician.
The core laboratory conducted an analysis of the pre- and posttreatment angiograms, including the degree of aneurysm occlusion. The degree of aneurysm occlusion was classified as complete, near-complete (>95%), or incomplete (<95%). Angiographic evidence of any thromboembolic complication was documented as an adverse event. The core laboratory assessed the occlusion status of each aneurysm. The occlusion status was scored as complete, near-complete, or incomplete.
The aneurysm volume was calculated by assuming that the aneurysms were eliptical, by using the formula:
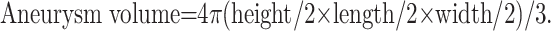 |
Height, length, and width were assessed by each operating physician, on the basis of their own angiographic equipment capabilities. Coil volumes were calculated by the following formula, which assumes that all HydroCoils expanded to their maximum volume:
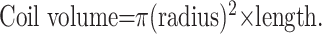 |
The radii of the various coils used were determined by using manufacturer specification data. The coil packing attenuation was expressed by using the following formula:
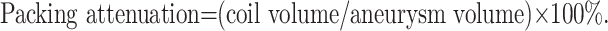 |
Results
Aneurysms included were located in a wide variety of locations in both the anterior and posterior circulation. The mean aneurysm size was 8.0 mm, and the median was 6.5 mm. The mean neck diameter was 4.3 mm, and the median was 3.9 mm. The mean dome-to-neck ratio was 1.7, and the median was 1.6. There were 71 ruptured aneurysms (37%) and 120 unruptured aneurysms (63%). Balloon remodeling was used in 27 cases, and adjunctive stent placement was used in 9 cases. Twenty aneurysms had been treated previously with platinum coils and thus were recurrences that were being treated with HES.
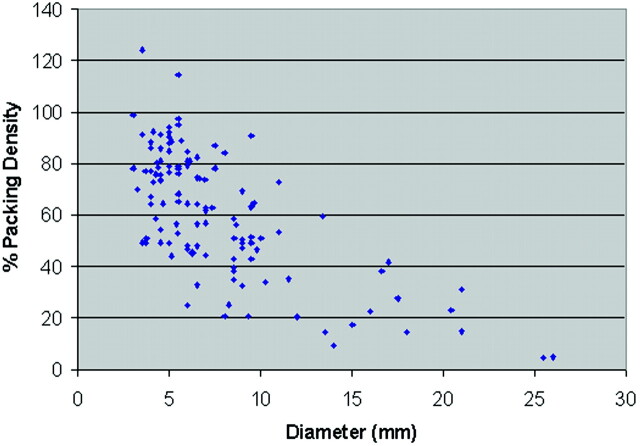
The mean number of HES coils per case was 4.2, and the median was 3.0. The HES provided a relatively high packing attenuation, with a mean of 60% and a median of 57%. Packing attenuation decreased with increasing aneurysm size (Fig 2). The initial outcome of endovascular therapy was complete in 93 cases (48.7%), near-complete in 79 cases (41.4%), and incomplete in 19 cases (9.9%).
Fig 2.
Scatter graph of packing attenuation versus aneurysm diameter in aneurysms treated with HydroCoil Embolic System in the HEAL registry.
Thromboembolic events occurred in 15 cases (8.1%). Permanent symptoms related to thromboembolic events occurred in 4 cases (2.1%). All thromboembolic events were noted at the time of treatment (ie, none began in the postprocedural period). Intraprocedural perforation occurred in 2 cases of ruptured aneurysm (2.8%) and in no cases of unruptured aneurysm (0%).
Discussion
The HEAL data demonstrate 90.1% of aneurysms treated in HEAL received complete or near-complete treatment. This suggests that HES provides an initial treatment success rate comparable to that reported for platinum coils (range, 75%–98%).3,9–17 This is an important finding, because it demonstrates that the physical properties of the HES do not hinder physicians from obtaining adequate initial filling of aneurysms.
The overall safety of aneurysm treatment with HES also compares favorably to treatment with platinum coils. The overall thromboembolic complication rate of 8.1% is quite similar to the rate of 8.2% reported by Qureshi et al18 in their review of literature. In patients treated with platinum coils,18 the permanent ischemic deficit rate was 4.6%, which is higher than the rate of 2.1% in HEAL patients. The lower rate of permanent ischemic deficit in HEAL patients may reflect recent advances in management of intraprocedural thromboembolic complications, including abciximab administration.19 The intraprocedural rupture rates for HEAL were 2.8% in previously ruptured aneurysms and 0% in unruptured aneurysms, which are similar to the rates of 4.1% for ruptured aneurysms and 0.5% for unruptured aneurysms reported in a meta-analysis by Cloft and Kallmes.20
Because HEAL is an uncontrolled study of the use of HES for treatment of cerebral aneurysms, platinum historical controls must be used to assess the relative procedural success and safety. Because of potential differences between HEAL patients and historical controls with regard to aneurysm size, neck width, rupture status, and other factors, such comparisons might be misleading. Future direct comparison of HES to platinum coils for treatment of cerebral aneurysms in a randomized, prospective study will provide more definitive information.
For HES to represent a significant advance in the treatment of cerebral aneurysms, it must offer a reduced aneurysm recurrence rate relative to platinum coils. Cerebral aneurysms treated with endovascular coil packing are only partially filled with coils, with most the intraluminal volume being filled initially with thrombus.21 Natural thrombolytic processes acting on this unstable thrombus may, in part, cause recurrence of aneurysms following endovascular coil therapy. An aneurysm filled with fewer coils and more thrombus could reasonably be expected to have a greater likelihood of recurrence. Indeed, low aneurysm coil packing attenuation is associated with an increased risk of aneurysm recurrence, especially if <25%.4–7
The expanding hydrogel on the HES displaces blood from the aneurysm lumen, resulting in a better packing attenuation with embolic agent. The outer polymer layer of the HES coils is designed to expand after exposure to blood (approximately 90% expansion after 20 minutes). Although it is not expected that the HES coils will fill 100% of the space, the amount of space filling is expected to be significantly greater than that of platinum coils alone. The increased aneurysm filling is intended to reduce the risk of coil compaction and aneurysm recanalization and improve long-term patient outcomes. The hydrogel polymer might also reduce the risk of aneurysm recurrence by providing a scaffold for neointima to traverse the aneurysm neck.8
The HES in HEAL provided a mean packing attenuation that is higher than is achievable with platinum coils. Early experience demonstrated that the HES allows substantially improved packing of the aneurysm lumen relative to standard platinum coils (72% vs 32%; P = .0001).22 It is possible to improve aneurysm packing significantly, even in cases in which only a single HES was used.
The HES provided a mean packing attenuation that is higher than is achievable with platinum coils. The packing attenuation for individual cases is prone to much error because the assessment of volumes of aneurysms is prone to much error. Indeed, the volumes of aneurysms determined in HEAL are subject to much error because precise size measurements cannot accurately be determined based on 2D angiographic images and because aneurysms often have irregular shapes that do not allow for simple volume calculations. Also, packing attenuation might be overestimated because our calculation assumes complete expansion of the hydrogel, which may not be possible if the hydrogel is constrained by adjacent coil loops. Nonetheless, packing attenuation measurements are useful for demonstrating an overall decreased packing attenuation in large aneurysms. The lower packing attenuation might be explained by treating physicians being mislead by apparent radiographic packing attenuation and compartmentalization of coils that creates barriers to further deposition and confinement of the microcatheter tip.
The HES was generally not the only type of coil used to treat aneurysms in the HEAL registry. Standard coils were usually used to form a “basket” or “framework” for subsequent deposition of HES 14 coils. The HES 14 coils were then used to fill most the aneurysm volume. The HES 14 coil is stiffer than standard platinum coils, and therefore it is often not generally used as the final, or “finishing,” coil. Rather, the final coil was often a small, soft standard platinum coil. Smaller and softer HES 10 coils suitable for use as “finishing” coils are now available, which allow for improved packing of aneurysms with hydrogel.
The HES represents a new technology that potentially can offer improved outcomes relative to platinum coils. Yet, the introduction of hydrogel to endovascular coil technology with HES might lead to differences in periprocedural complications and initial results relative to platinum coils. Thus, the assessment of periprocedural safety and initial results achieved with HES relative to platinum coils is a key step toward proving its overall safety and efficacy for the treatment of cerebral aneurysms. The HES provides an initial treatment success rate and complication rate comparable to those reported for platinum coils. The HES provided a packing attenuation that is higher than is generally achievable with platinum coils. While we are encouraged by the improvement in aneurysm packing with HES, the effectiveness of HES in preventing aneurysm recurrence remains to be proved. The HEAL trial is currently gathering information regarding aneurysm recurrence following treatment with HES.
Appendix
Principal Investigators at Enrolling Sites
Timothy Malisch, University of Illinois at Chicago; Alejandro Berenstein, Beth Israel Hospital; Emmanual Houdart, Hopital Lariboisier; Isabel Wanke, Universitatsklinik Essen; In Sup Choi, Lahey Clinic; Harish Shownkeen, Loyola University Hospital; Laurent Pierot, Centre Hospitalier Universitaire de Reims; Frank Tong, Emory University Hospital; Soren Bakke, Rikshospitalet; Beverly Aagaard, University of Wisconsin Hospital; Jacques Moret, Foundation Rothschild; Serge Bracard, Centre Hospitaler Universitaire de Nancy; Avery Evans, Tampa General Hospital; Mary Jensen, University of Virginia; Hans Henkes, Alfred Krupp Krankenhaus.
Core Laboratory and Data Analysis: Harry J. Cloft, MD, PhD, Mayo Clinic, Rochester
References
- 1.Cognard C, Weill A, Spelle L, et al. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology 1999;212:348–56 [DOI] [PubMed] [Google Scholar]
- 2.Debrun GM, Aletich VA, Kehrli P, et al. Selection of cerebral aneurysms for treatment using Guglielmi detachable coils: the preliminary University of Illinois at Chicago experience. Neurosurgery 1998;43:1281–95 [DOI] [PubMed] [Google Scholar]
- 3.Kuether TA, Nesbit GM, Barnwell SL. Clinical and angiographic outcomes, with treatment data, for patients with cerebral aneurysms treated with Guglielmi detachable coils: a single-center experience. Neurosurgery 1998;43:1016–25 [DOI] [PubMed] [Google Scholar]
- 4.Satoh K, Matsubara S, Hodoh H, et al. Intracranial aneurysm embolization using interlocking detachable coils: correlation between volume embolization and coil compaction. Intervent Neuroradiol 1997;3(suppl 2):125–29 [DOI] [PubMed] [Google Scholar]
- 5.Tamatani S, Ito Y, Abe H, et al. Evaluation of the stability of aneurysms after embolization using detachable coils: correlation between stability of aneurysms and embolized volume of aneurysms. AJNR Am J Neuroradiol 2002;23:762–67 [PMC free article] [PubMed] [Google Scholar]
- 6.Uchiyama N, Kida S, Nomura M, et al. Significance of volume embolization ratio as a predictor of recanalization on endovascular tratment of cerebral aneurysms treated with Guglielmi detachable coils. Intervent Neuroradiol 2000;6(suppl 1):59–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawanabe Y, Sadato A, Taki W, et al. Endovascular occlusion of intracranial aneurysms with Guglielmi detachable coils: correlation between coil packing density and coil compaction. Acta Neurochir (Wien) 2001;143:451–55 [DOI] [PubMed] [Google Scholar]
- 8.Kallmes DF, Fujiwara NH. New expandable hydrogel-platinum coil hybrid device for aneurysm embolization. AJNR Am J Neuroradiol 2002;23:1580–88 [PMC free article] [PubMed] [Google Scholar]
- 9.Vanninen R, Koivisto T, Saari T, et al. Ruptured intracranial aneurysms: acute endovascular treatment with electrolytically detachable coils: a prospective randomized study. Radiology 1999;211:325–36 [DOI] [PubMed] [Google Scholar]
- 10.Richling B, Gruber A, Bavinzski G, et al. GDC-system embolization for brain aneurysms: location and follow-up. Acta Neurochir (Wien) 1995;134:177–83 [DOI] [PubMed] [Google Scholar]
- 11.Sluzewski M, Bosch JA, van Rooij WJ, et al. Rupture of intracranial aneurysms during treatment with Guglielmi detachable coils: incidence, outcome, and risk factors. J Neurosurg 2001;94:238–40 [DOI] [PubMed] [Google Scholar]
- 12.Murayama Y, Nien YL, Duckwiler G, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years’ experience. J Neurosurg 2003;98:959–66 [DOI] [PubMed] [Google Scholar]
- 13.Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003;34:1398–403 [DOI] [PubMed] [Google Scholar]
- 14.Cognard C, Weill A, Castaings L, et al. Intracranial berry aneurysms: angiographic and clinical results after endovascular treatment. Radiology 1998;206:499–510 [DOI] [PubMed] [Google Scholar]
- 15.Byrne JV, Sohn MJ, Molyneux AJ, et al. Five-year experience in using coil embolization for ruptured intracranial aneurysms: outcomes and incidence of late rebleeding. J Neurosurg 1999;90:656–63 [DOI] [PubMed] [Google Scholar]
- 16.Vallee JN, Aymard A, Vicaut E, et al. Endovascular treatment of basilar tip aneurysms with Guglielmi detachable coils: predictors of immediate and long-term results with multivariate analysis 6-year experience. Radiology 2003;226:867–79 [DOI] [PubMed] [Google Scholar]
- 17.Thornton J, Debrun GM, Aletich VA, et al. Follow-up angiography of intracranial aneurysms treated with endovascular placement of Guglielmi detachable coils. Neurosurgery 2002;50:239–49 [DOI] [PubMed] [Google Scholar]
- 18.Qureshi AI, Luft AR, Sharma M, et al. Prevention and treatment of thromboembolic and ischemic complications associated with endovascular procedures. Part II. Clinical aspects and recommendations. Neurosurgery 2000;46:1360–75 [DOI] [PubMed] [Google Scholar]
- 19.Cloft HJ, Samuels OB, Tong FC, et al. Use of abciximab for mediation of thromboembolic complications of endovascular therapy. AJNR Am J Neuroradiol 2001;22:1764–67 [PMC free article] [PubMed] [Google Scholar]
- 20.Cloft HJ, Kallmes DF. Cerebral aneurysm perforations complicating therapy with Guglielmi detachable coils: a meta-analysis. AJNR Am J Neuroradiol 2002;23:1706–709 [PMC free article] [PubMed] [Google Scholar]
- 21.Gruber A, Killer M, Bavinzski G, et al. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: a 7-year, single-center experience. Neurosurgery 1999;45:793–803 [DOI] [PubMed] [Google Scholar]
- 22.Cloft HJ, Kallmes DF. Aneurysm packing with HydroCoil Embolic System versus platinum coils: initial clinical experience. AJNR Am J Neuroradiol 2004;25:60–62 [PMC free article] [PubMed] [Google Scholar]