Abstract
SUMMARY: Vein of Galen aneurysmal malformations (VGAM) are characterized by multiple arteriovenous connections draining into a markedly enlarged median draining vein. This ectatic vein is not the vein of Galen, but its embryonic precursor, the median prosencephalic vein of Markowski. During normal development, the posterior portion of the median prosencephalic vein persists as the vein of Galen, while its anterior portion regresses in parallel with the formation of the internal cerebral veins (ICV). It has been traditionally thought that, in children with a VGAM, the deep venous system does not connect to and, a fortiori, does not drain into the ectatic median prosencephalic vein/vein of Galen. This report describes a case of successfully treated VGAM in which the drainage of an ICV into the vein of Galen was only demonstrated by follow-up MR imaging and venography. The potential implications of this finding for the management of VGAMs are discussed.
Vein of Galen aneurysmal malformations (VGAMs) are intracranial vascular lesions typical of the pediatric population. They are characterized by multiple arteriovenous connections draining into a markedly enlarged median draining vein. This ectatic vein is not, however, the vein of Galen per se, but its embryonic precursor, the median prosencephalic vein of Markowski.1 During normal development, the posterior portion of the median prosencephalic vein persists as the vein of Galen, while its anterior portion regresses in parallel with the formation of the left and right internal cerebral veins (ICVs). The ICVs have a paramedian anteroposterior course that normally ends into the vein of Galen (hence the name of Galenic system sometimes given to the deep venous system). It is classically admitted that, in children with a VGAM, the deep venous system does not connect to and, a fortiori, does not drain into the ectatic median prosencephalic vein/vein of Galen. This notion, however, has been recently challenged by a publication documenting 2 cases in which connections between the deep venous system and the draining vein of a VGAM were documented.2 This report describes a case of successfully treated VGAM in which the drainage of an ICV into the vein of Galen was demonstrated only by follow-up MR imaging and venography. The potential implications of this finding for the management of VGAMs are discussed.
Case Report
The patient was a baby boy born vaginally at term to a healthy 21-year-old woman (birth weight 4349 g; Apgar scores of 6 and 7 at 1 and 5 minutes, respectively). The pregnancy had been uncomplicated until prenatal sonography performed at 35 weeks revealed a VGAM, without evidence of other malformations. Prenatal echocardiography demonstrated a structurally normal heart with right atrial dilation and mildly decreased right ventricular function. Immediately after delivery, the infant became tachycardic, tachypneic, and poorly saturated (72% on room air). At admission to the neonatal intensive care unit, physical examination revealed a pale and hypotonic infant with a hyperactive precordium, loud S2, and a II/VI harsh holosystolic murmur at the lower left sternal border. A conventional radiograph of his chest demonstrated an enlarged cardiac silhouette with bilateral interstitial lung disease. Complete blood count was unremarkable. Severe right ventricular (RV) hypertension developed on day 2, with estimated RV pressure of 95 mm Hg. Right-to-left shunt surgery across the patent ductus arteriosus (PDA) and foramen ovale and normal ventricular function were diagnosed. Over the ensuing 2 days, cyanosis and hypotension required intubation, mechanical ventilation, and dopamine infusion. An echocardiogram revealed elevation of the RV pressure to 100 mm Hg and mildly decreased biventricular function. Although the infant received aggressive therapy for pulmonary hypertension (inhaled nitric oxide, volume, vasopressors, high-frequency ventilation, and alkalinazation), he progressively worsened. Echocardiogram on day 3 revealed elevation of the RV pressure to 120–140 mm Hg with decreasing function of a dilated right ventricle.
Because of the known correlation between VGAM and pulmonary hypertension,3 and the lack of success of the conventional therapy, the patient underwent emergent transarterial embolization of his VGAM on day 5. Arterial access was obtained via the umbilical artery. After initial digital subtraction angiography (DSA) (Fig. 1A), superselective embolization of 3 major feeders arising from both anterior choroidal arteries was performed by using n-butyl cyanoacrylate (n-BCA). Following embolization, the infant’s status improved. Dopamine was discontinued within 48 hours, and echocardiography revealed falling RV pressures to subsystemic levels with improved biventricular function. He continued to receive 100% oxygen and was weaned to a conventional ventilator. Cranial sonography demonstrated decreased flow through the VGAM.
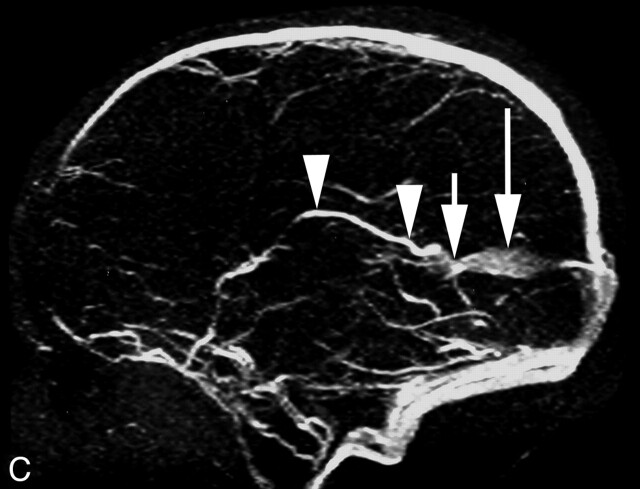
Fig 1.
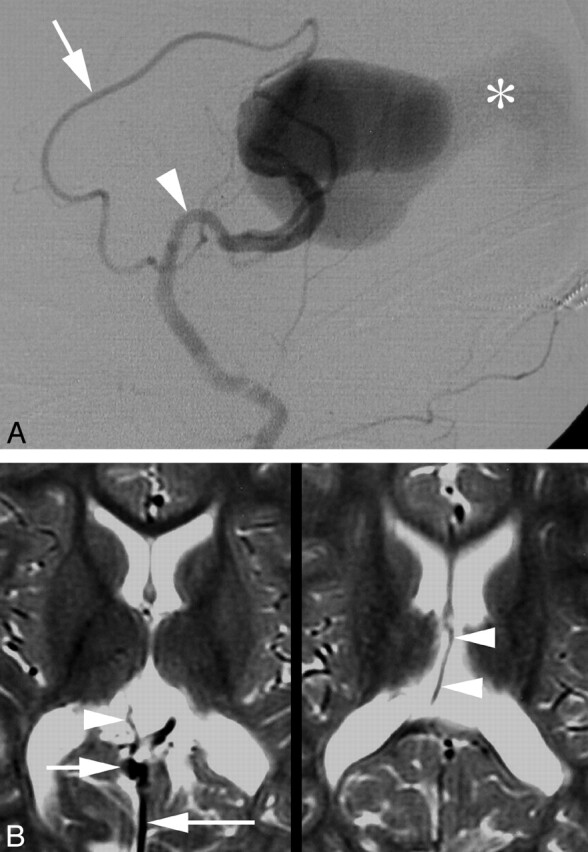
Five-day-old child with cardiorespiratory failure.
A, Digital subtraction angiography (DSA), left common carotoid artery, lateral view, showing enlarged anterior cerebral (arrow) and anterior choroidal (arrowhead) arteries feeding a vein of Galen aneurysmal malformations (VGAM). Note the drainage of the malformation through a falcine sinus (asterisk).
B, Follow-up magnetic MR imaging 2 years after endovascular therapy; axial T2-weighted images, showing the flow void of a right internal cerebral vein (ICV) (arrowheads) draining into the shrunken vein of Galen (arrow). Note the falcine sinus (long arrow).
C, Two-year follow-up MR venography, sagittal view, showing the course of the right ICV (arrowheads), its termination into the small vein of Galen (arrow), and the falcine sinus (long arrow).
The infant remained stable for 5 days but then had repeated desaturations. Echocardiography again documented severe pulmonary hypertension (right ventricular pressures of 110–155 mm Hg) with moderate right ventricular dysfunction despite the use of inhaled nitric oxide and high-frequency ventilation. The infant developed hypertension and irregular, tonic-clonic movements. Because of suspected seizure activity, phenobarbital was started. Repeat cranial sonography showed no evidence of intracranial bleed. The patient underwent a second endovascular procedure, this time through a femoral access. Z additional feeders were embolized with n-BCA. Following the procedure, the infant made a dramatic recovery. The right ventricular pressures fell to systemic levels within 3 days of the embolization, and he was extubated to nasal cannula shortly thereafter. He steadily improved until discharge to home on day of life 46. Follow-up MR imaging obtained two years after treatment confirmed regression of the lesion and showed a normal right ICV draining into the shrunken vein of Galen (Fig 1B, -C).
The primary pediatrician for this patient reports that he is a happy, healthy, and developmentally normal 4-year-old boy.
Discussion
The management of VGAMs, once associated with high mortality and morbidity rates, has been revolutionized by the introduction of minimally invasive endovascular techniques and by the progresses made in the intensive care management of neonates and infants.4–6 From the endovascular perspective, 2 main options are available for the treatment of a VGAM. The first option involves the superselective catheterization and embolization of the VGAM arterial feeders.7 The embolic material of choice is a liquid acrylic polymer, n-BCA, often simply referred to as glue. Other embolic agents, such as pushable, liquid, or detachable coils, can be used as an alternative to n-BCA. The second option is a transvenous approach, either with distant access (femoral or jugular vein) or through direct puncture of the torcula.8–10 The aneurysmal vein is retrogradely catheterized and occluded by using detachable microcoils. Indications for a transvenous approach have significantly decreased with the improvement in quality and availability of microcatheters and embolic materials that allow for easier transarterial access and delivery of the therapeutic agent at the site of the fistula. Both techniques have partisans and detractors. Among the advantages mentioned in favor of transarterial n-BCA embolization are rapidity of execution and the ability to finely tune the degree of devascularization, an ability essential to successful staging of the embolization procedure. NBCA embolization requires, however, that the operator be experienced and comfortable with the use of glue. The venous approach to a VGAM, by contrast, is technically less demanding and can be performed in situations where a superselective arterial embolization is not feasible (eg, when arterial stenoses prevent distal catheterizations or when a normal cortical branch arises in the immediate proximity of the arteriovenous shunt). One of the drawbacks of the transvenous approach is that the degree of embolization cannot be accurately controlled once the thrombotic process within the aneurysmal vein has been induced. Rapid closure of the venous drainage of the VGAM can lead to the development of “normal perfusion breakthrough syndrome,” with disastrous consequences, including malignant brain swelling and intracranial hemorrhages.7,11 Incomplete transvenous embolization can also result in the formation of an extensive varicose collateral venous network.
It is generally assumed, as a premise making its endovascular occlusion acceptable, that the venous aneurysm of a VGAM does not participate to the drainage of normal cerebral structures. Indeed, it is classically believed that venous structures normally constituting the deep or Galenic venous system, such as the ICV and the basal vein of Rosenthal, are not connected to the vein of Galen or its aneurysmal equivalent in patients with VGAMs.7 This concept, however, has been questioned in a recent publication unequivocally documenting the existence of connections between the deep venous system and the aneurysmal draining vein in 2 patients with VGAMs.2 In their seminal publication discussing the embryology of VGAMs, Raybaud et al had already reported the existence of connections between the ICV and the aneurysmal collector of the VGAM.1 These connections, observed in 6 of 12 cases with adequate angiographic documentation, always involved a unilateral, nondilated ICV.
There was no evidence of connection between the normal deep venous system and the VGAM in either of the 2 angiographic procedures our patient underwent. In particular, the normal ICV was not opacified retrogradely by blood coming from the arteriovenous shunts. This finding is important, because it implies that the ICV was not involved in the drainage of the VGAM, but was most likely draining normal cerebral tissue in an antegrade fashion despite the increase in venous pressure. It is only via the follow-up MR imaging and venography (Fig 1B, -C), after shrinking of the aneurysmal vein, that a single, nondilated ICV draining into the vein of Galen (ie, into the posterior portion of the initial malformation) became apparent. It is reasonable to think that this normal ICV had been present from the beginning but was not opacified during the angiographic studies, either because the flow, and therefore the contrast agent, was preferentially directed toward high velocity arteriovenous shunts, or because elevated venous pressure prevented ICV drainage into the aneurysmal sac at the time of the angiogram. Our observation indicates that normal deep veins can drain into the venous component of a VGAM, even though they may not be angiographically detectable, in particular on studies obtained before treatment of the lesion. The potential existence of normal deep veins draining into a VGAM is, in our opinion, another argument in favor of transarterial embolization as the technique of choice for VGAM management. Some of the complications reported after transvenous embolization of VGAMs, such as basal ganglia stroke and hemorrhages, may indeed result from flow impairment in deep veins that were connected to the venous component of the lesion. Transarterial embolization often allows keeping patent the venous pathways formerly draining the VGAM. In cases where total obliteration of the venous drainage eventually occurs, transarterial embolization may minimize the potential repercussion on normal cerebral tissue by producing a less abrupt alteration of the drainage pattern. Although occlusion of the vein of Galen in patients with no evidence of impaired venous drainage before treatment is likely to be well tolerated, the functional significance of vein of Galen occlusion in patients with a normal ICV connected to the aneurysmal sac remains unclear.
An additional interesting feature present in our patient was the occurrence of severe pulmonary hypertension. Postnatal manifestations of VGAMs vary with age of presentation. Neonates classically develop congestive heart failure because of tremendous left-to-right shunt surgery through the low-resistance arteriovenous connections of the VGAM.12 Symptomatic infants with intracranial arteriovenous malformations may develop both volume and pressure overload on the right ventricle, leading to cyanosis from shunt surgery across the PDA and the atrial septum.3,5,13–15 Suprasystemic pulmonary artery pressures may develop and portend a poor prognosis.3 In this setting, echocardiography has a primary role for the assessment of ventricular function and shunt surgery across the PDA and atrial septum. Echocardiography also provides pulmonary artery pressure estimations and can identify associated congenital cardiac anomalies.3,5,16
Medical stabilization of the symptomatic neonate with VGAM can be exceedingly difficult in the presence of cardiac failure with pulmonary hypertension. Optimal strategies have not yet been defined. For infants with acyanotic congestive heart failure, diuretic and inotropic therapy may be sufficient to stabilize the infant until embolization of the VGAM is performed. Infants with coexisting pulmonary hypertension present a more challenging task. The presence of the right-to-left shunt at the atrial and ductal levels is exacerbated by the low total systemic vascular resistance largely due to the VGAM. Resistance through the VGAM may be low enough to induce a steal phenomenon manifesting as reversal of aortic flow during diastole, causing subendocardial and peripheral ischemia.5 Nitric oxide, the most-effective therapy for traditional persistent pulmonary hypertension of the neonate, will likely play a wider role in the future, but only a limited numbers of infants with VGAM and pulmonary hypertension have been treated with nitric oxide and its effectiveness remains unclear. Alternate therapies have included beta agonists (dopamine, dobutamine), prostaglandin infusions, phosphodiesterase inhibitors, digoxin, and the combination of vasodilation with low-dose beta agonist (sodium nitroprusside plus low-dose dopamine).3,5 No trials have been performed and no consensus has arisen for treatment strategies in the neonates with the worst cardiac failure. Older infants and children tend to present with hydrocephalus, developmental delay, megalencephaly, seizures, and/or dilated facial veins. Cardiac failure, when present in infants, is milder and more amenable to treatment. Pulmonary hypertension is uncommon among older children, though one case of prominent and ultimately fatal pulmonary hypertension at age 2 months has been reported.17 In our patient, the endovascular correction of the anatomic anomaly (ie, the transarterial embolization of the high-flow vascular shunts of the VGAM) seemed to have been instrumental in the hemodynamic recovery of the patient and, ultimately, in the excellent clinical outcome.
Conclusion
A case of VGAM is reported, in which a normal ICV was connected to the aneurysmal component of the lesion. The normal ICV was not visible on 2 initial angiographic studies. It only became apparent after endovascular treatment of the lesion and shrinkage of the venous aneurysm. Our case emphasizes that, though they may not be angiographically detectable, normal deep veins can be connected to the venous component of a VGAM. This particular anatomy represents a pitfall for transvenous embolization of VGAMs and may potentially result in complications such as venous infarcts and hemorrhages associated with the transvenous approach. In addition, our case documents the occurrence of severe pulmonary hypertension in association with a VGAM and its disappearance after successful transarterial embolization of the lesion.
References
- 1.Raybaud CA, Strother CM, Hald JK. Aneurysms of the vein of Galen: embryonic considerations and anatomical features relating to the pathogenesis of the malformation. Neuroradiology 1989;31:109–28 [DOI] [PubMed] [Google Scholar]
- 2.Levrier O, Gailloud P, Souei M, et al. Normal galenic drainage of the deep cerebral venous system in 2 cases of vein of Galen aneurysmal malformation (VGAM). Childs Nerv Syst 2004;20:91–97 [DOI] [PubMed] [Google Scholar]
- 3.Chevret L, Durand P, Alvarez H, et al. Severe cardiac failure in newborns with VGAM: prognosis significance of hemodynamic parameters in neonates presenting with severe heart failure owing to vein of Galen arteriovenous malformation. Intensive Care Med 2002;28:1126–30 [DOI] [PubMed] [Google Scholar]
- 4.Lasjaunias P, Alvarez H, Rodesch G, et al. Aneurysmal malformations of the vein of Galen: follow-up of 120 children treated between 1984 and 1994. Intervent Neuroradiol 1996;2 [DOI] [PubMed] [Google Scholar]
- 5.Frawley GP, Dargaville PA, Mitchell PJ, et al. Clinical course and medical management of neonates with severe cardiac failure related to vein of Galen malformation. Arch Dis Child Fetal Neonatal Ed 2002;87:F144–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell PJ, Rosenfeld JV, Dargaville P, et al. Endovascular management of vein of Galen aneurysmal malformations presenting in the neonatal period. AJNR Am J Neuroradiol 2001;22:1403–409 [PMC free article] [PubMed] [Google Scholar]
- 7.Lasjaunias P. Vein of Galen aneurysmal malformation. In: Vascular diseases in neonates, infants and children. Berlin-Heidelberg: Springer-Verlag;1997. :67–202
- 8.Casasco A, Lylyk P, Hodes JE, et al. Percutaneous transvenous catheterization and embolization of vein of Galen aneurysms. Neurosurgery 1991;28:260–66 [DOI] [PubMed] [Google Scholar]
- 9.Dowd CF, Halbach VV, Barnwell SL, et al. Transfemoral venous embolization of vein of Galen malformations. AJNR Am J Neuroradiol 1990;11:643–48 [PMC free article] [PubMed] [Google Scholar]
- 10.Mickle JP, Quisling RG. The transtorcular embolization of vein of Galen aneurysms. J Neurosurg 1986;64:731–35 [DOI] [PubMed] [Google Scholar]
- 11.Morgan MK, Johnston IH, Sundt TM Jr. Normal perfusion pressure breakthrough complicating surgery for the vein of Galen malformation: report of three cases. Neurosurgery 1989;24:406–10 [DOI] [PubMed] [Google Scholar]
- 12.Gomez M, Whitten C, Nolke A. Aneurysmal malformations of the great vein of Galen causing heart failure in early infancy. Pediatrics 1963;31:400–11 [Google Scholar]
- 13.Hendson L, Emery DJ, Phillipos EZ, et al. Persistent pulmonary hypertension of the newborn presenting as the primary manifestation of intracranial arteriovenous malformation of the vein of Galen. Am J Perinatol 2000;17:405–10 [DOI] [PubMed] [Google Scholar]
- 14.Kilbride HW, Gowdamaran R, Thibeault DW. Neonatal pulmonary vascular and parenchymal changes associated with arteriovenous malformation. Pediatr Pulmonol 1993;16:201–206 [DOI] [PubMed] [Google Scholar]
- 15.Dahdah NS, Alesseh H, Dahms B, et al. Severe pulmonary hypertensive vascular disease in two newborns with aneurysmal vein of Galen. Pediatr Cardiol 2001;22:538–41 [DOI] [PubMed] [Google Scholar]
- 16.McElhinney DB, Halbach VV, Silverman NH, et al. Congenital cardiac anomalies with vein of Galen malformations in infants. Arch Dis Child 1998;78:548–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huh J, Noh CI, Choi JY, et al. Pulmonary hypertensive crisis as an initial manifestation of intracranial arteriovenous malformation with aneurysm of the vein of Galen. Int J Cardiol 1998;66:107–109 [DOI] [PubMed] [Google Scholar]