Abstract
SUMMARY: Sacral insufficiency fractures frequently cause significant pain and limit activities of daily living in patients with osteoporosis. Percutaneous vertebroplasty is a common procedure to alleviate the pain associated with thoracic and lumbar vertebral compression fractures. The sacral percutaneous vertebroplasty procedure (sacroplasty) has recently been introduced as an alternative to medical management of osteoporotic sacral insufficiency fractures. We describe our CT fluoroscopy technique in performing percutaneous sacroplasty.
The techniques and outcomes of percutaneous vertebroplasty for thoracolumbar compression fractures have been widely documented.1–4 In general, thoracolumbar vertebroplasty is performed entirely under fluoroscopic guidance. With the use of biplane fluoroscopy, vertebroplasty of the thoracic and lumbar vertebral bodies can be safely performed without the need for CT assistance; however, treatment of sacral insufficiency fractures involves additional technical challenges. Previous reports of the sacral percutaneous vertebroplasty procedure (sacroplasty) technique have described the use of CT needle placement followed by injection of cement under standard fluoroscopy or performance of the entire procedure with fluoroscopy alone.5–7 This technique requires the transfer of the patient from CT to fluoroscopy areas or the cumbersome use of a C-arm in the CT suite. Precise cement placement is still limited by this technique, because cement encroachment on the sacral foramina or soft tissue extravasation can occur. We describe a technique using CT fluoroscopy that eliminates the need for transferring the patient or using a mobile C-arm during the procedure.
Technical Note
Patient Information
An 86-year-old woman with osteoporosis was admitted to the hospital 3 weeks after a fall. She had worsening severe pain in the buttocks with radiation to the bilateral thighs. A pelvic CT demonstrated bilateral superior pubic rami fractures and bilateral sacral insufficiency fractures, which were also apparent on bone scintigraphy (Fig 1A, -B). The patient was unable to ambulate or perform basic self-care activities because of the severe pain. Before the procedure, she used a fentanyl patch and a patient-controlled morphine pump. The patient required significant doses of narcotics for pain control, which resulted in unresponsiveness requiring naloxone administration. The patient was therefore referred to neuroradiology to be evaluated for percutaneous sacroplasty. Her pain before the procedure was reported as 9 of 10 in severity.
Fig 1.
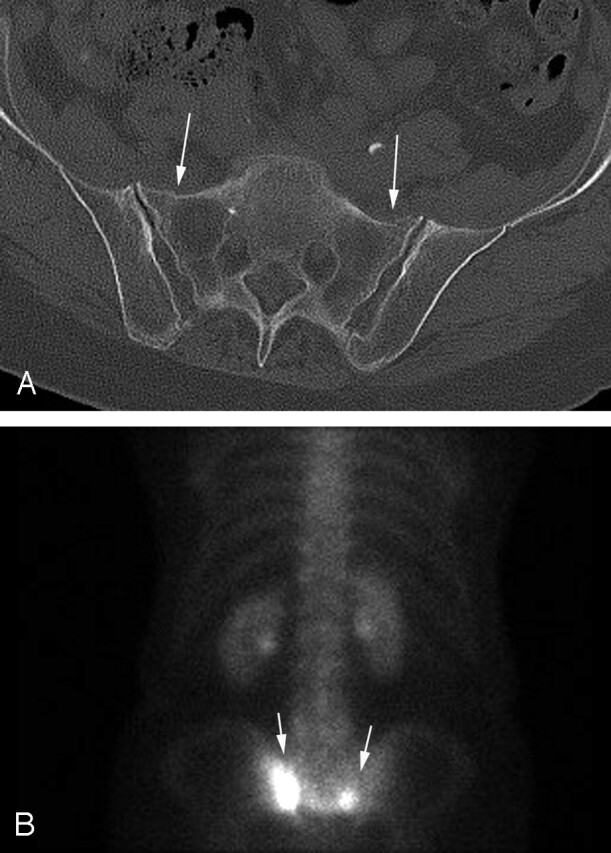
A, Axial pelvic CT demonstrates subtle bilateral sacral insufficiency fractures (arrows).
B, Bone scintigraphy examination demonstrates increased radiotracer uptake (arrows) in the sacral insufficiency fractures.
Procedure
The procedure was performed under conscious sedation administered by the anesthesia department. A 4-section CT scanner equipped with CT fluoroscopy was used for the entire case. The patient was placed prone on the CT table and a preprocedure CT scan was performed through the entire sacrum. The preliminary CT scan was then used to plan the location/path and number of needles. A total of 4 needles were deemed necessary to distribute the cement adequately and portend the entire extent of the bilateral fractures.
Small metallic BB markers were placed on the skin at the anticipated entry point of each of the 4 needles. Limited CT sections were then obtained through the markers to confirm correct position and the 4 needle entry sites were marked on the skin. The subcutaneous tissues were anesthetized with 1% lidocaine and the periosteum over S1 was anesthetized bilaterally at the expected needle insertion sites with 0.25% Marcaine (bupivacaine). Under CT fluoroscopy guidance, 10-cm 11-gauge biopsy needles were placed bilaterally at the S1 level. Needle tips were positioned in the ventral aspect of the sacrum in anticipation of staged needle repositioning more posteriorly during the cement injection. Polymethylmethacrylate vertebroplasty cement containing barium was then intermittently injected simultaneously through each needle. Before the procedure, the number of turns on the injector required to fill the needle space was determined. CT fluoroscopy was then used to obtain a near immediate series of 3 images following controlled injection of each 0.25 mL aliquot of cement. A total of 2.0 mL of cement were injected at each level. After filling S1 bilaterally, the same technique was employed at the S2 level by using 2 additional needles. Imaging parameters included a section thickness of 5 mm and 3 contiguous sections per acquisition were obtained during the cement instillation. The average injection time was 5–7 minutes per needle. Multiple CT fluoroscopic acquisitions were obtained during the course of the cement installation with additional acquisitions obtained in adjacent more superior or inferior table positions to follow the leading edge of the accumulated injected cement. CT fluoroscopy allowed for precise cement delivery without extravasation into the neural foramina or parasacral soft tissues (Fig 2A, -B). The cement was initially injected into the anterior aspect of the sacrum, and the needle was sequentially withdrawn as additional cement was placed. The injectors were depressurized during each needle reposition to avoid brisk unintended cement flow in newly exposed veins or fracture clefts. The needles were removed after cement injection was complete and a final CT through the sacrum was performed (Fig 2C). There were no intraprocedural or postprocedural complications.
Fig 2.
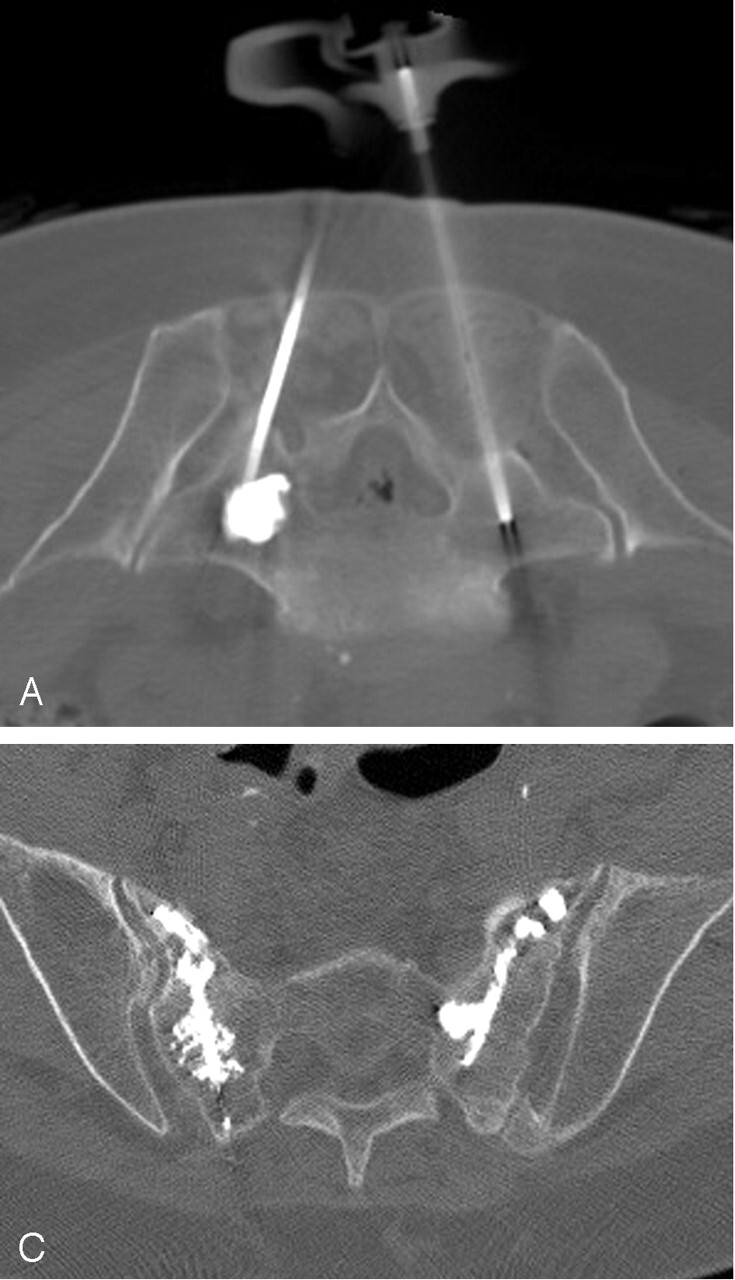
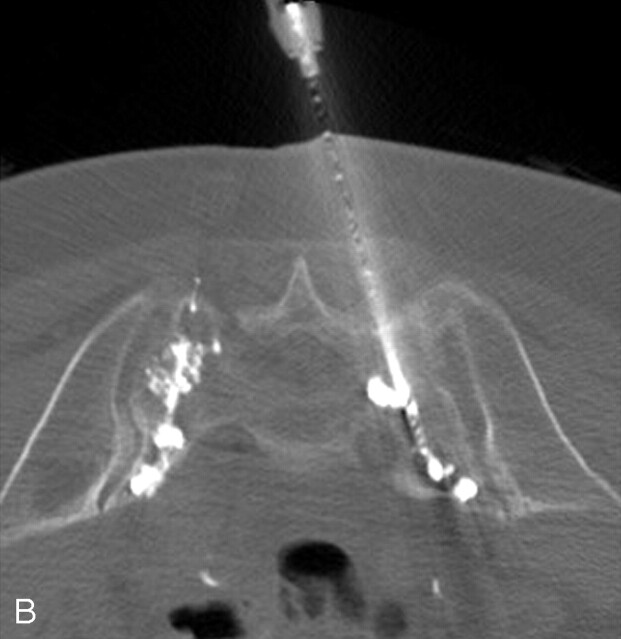
A, Axial CT fluoroscopic image demonstrates clear visualization of the injected cement in the left S1 level and the second needle on the right.
B, CT fluoroscopic image during injection of the right S1 level demonstrates the cement tracts bilaterally.
C, Postprocedure sacral CT demonstrates excellent cement infiltration within the bilateral sacral ala.
Postprocedure Follow-Up
The patient experienced significant pain relief the next day and was able to ambulate without assistance. Her pain medication was changed from intravenous narcotics and fentanyl patch to oral oxycodone. Although she did experience persistent pelvic pain, the location and severity was improved and the persistent pain was attributable to her coexistent pubic rami fractures. She was discharged to an assisted living facility 3 days following the sacroplasty for physical therapy. The patient was seen in the clinic 10 weeks after the sacroplasty procedure and no longer had any symptoms related to her sacral or pubic rami fractures. Physical examination elicited no pain on palpation of her iliac crests or lumbosacral spine. The estimated effective dose for the procedure was calculated at 140 mSv. Estimation of the anticipated effective dose from standard fluoroscopy is difficult to extrapolate to the sacroplasty procedure. The estimated effective dose for this patient under routine fluoroscopy would have been approximately 70–100 mSv.
Discussion
At our institution, we have had significant experience with percutaneous vertebroplasty by using fluoroscopic guidance. Our experience with sacroplasty, however, has been limited, and reports describing the technical performance of sacroplasty are limited. Sacral insufficiency fractures generally involve the sacral ala, and there is a limited amount of trabecular bone to accept the vertebroplasty cement. The surface anatomy of the posterior sacrum is widely variable, and it can be difficult to confirm exact needle placement under standard fluoroscopy. During cement injection, the relationship between the cement and sacral foramina can be difficult to ascertain because of the oblique configuration of the sacrum and foramina. Also, fluoroscopic visualization can be difficult in the pelvic region particularly in the lateral projection because of radiography penetration issues. Because the injected cement is superimposed on the needle tip during fluoroscopic injection, it can also be difficult to determine whether cement is extravasating into the soft tissues immediately adjacent to the sacrum.
Because of our success with this case, we have elected to use the CT fluoroscopy technique described above in all of our sacroplasty procedures. The use of CT fluoroscopy allows for combined needle placement and cement injection without the need to move the patient. Furthermore, the precise control of cement placement can be monitored in the axial plane and followed in the cranial caudal direction with swift CT table repositioning and prompt CT fluoroscopic image acquisition. Although some investigators have found CT fluoroscopy makes it difficult to evaluate the injection of cement in the craniocaudal dimension, we have not encountered such limitations.7 By selecting an appropriate section thickness and area of coverage, we are able to confidently assess the distribution of cement in the craniocaudal plane.
Acknowledgments
We wish to thank Beth Schueler, PhD, and Jim Kofler, PhD, for their assistance with the estimated effective doses for fluoroscopic and CT fluoroscopy–guided sacroplasty techniques.
References
- 1.Jensen ME. Percutaneous vertebroplasty: a new therapy for the treatment of painful vertebral body compression fractures. Appl Radiol 2000;6: 7 –11 [Google Scholar]
- 2.Mathis JM, Barr JD, Belkoff SM, et al. Percutaneous vertebroplasty: a developing standard of care for vertebral compression fractures. AJNR Am J Neuroradiol 2001;22:373–81 [PMC free article] [PubMed] [Google Scholar]
- 3.Jensen ME, Evans AJ, Mathis JM, et al. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR Am J Neuroradiol 1997;18:1897–904 [PMC free article] [PubMed] [Google Scholar]
- 4.Deramond H, Depriester C, Galibert P, et al. Percutaneous vertebroplasty with polymethylmethacrylate: technique, indications, and results. Radiol Clin North Am 1998;36:3:533–46 [DOI] [PubMed] [Google Scholar]
- 5.Garant M. Sacroplasty: a new treatment for sacral insufficiency fracture. J Vasc Interv Radiol 2002;13:1265–67 [DOI] [PubMed] [Google Scholar]
- 6.Pommersheim W, Huang-Hellinger F, Baker M, et al. Sacroplasty: a treatment for sacral insufficiency fractures. AJNR Am J Neuroradiol 2003;24:1003–07 [PMC free article] [PubMed] [Google Scholar]
- 7.Butler CL, Given CA 2nd, Michel SJ, et al. Percutaneous sacroplasty for the treatment of sacral insufficiency fractures. AJR Am J Roentgenol 2005;184:1956–59 [DOI] [PubMed] [Google Scholar]