Abstract
BACKGROUND AND PURPOSE: Discrimination between enhancing mass lesions in acquired immunodeficiency syndrome (AIDS) patients with conventional CT and MR imaging remains difficult. We determined the effect of lesion size on thallium-201 brain single-photon emission tomography (SPECT) imaging in differentiating primary brain lymphoma from cerebral toxoplasmosis.
METHODS: We retrospectively identified 35 AIDS patients with a total of 48 focal enhancing mass lesions on contrast-enhanced brain CT and/or MR images who subsequently underwent thallium-201 brain SPECT imaging. The thallium index of each lesion was evaluated on the basis of the ratio of mean uptake in the lesion compared with the corresponding contralateral side. Receiver operator curves were drawn to determine the optimal thallium index threshold. The effect of lesion size on scan accuracy was evaluated.
RESULTS: Malignant lesions in 20 patients had a mean thallium index of 2.4 (range, 1–11). Infectious lesions in 15 patients had a mean thallium index of 1.6 (range, 1–3.6). Twenty-five lesions were <2 cm (14 malignant, 11 nonmalignant) and 23 lesions were ≥2 cm (14 malignant, 9 nonmalignant). Thallium index was not a significant predictor of malignancy in the lesions <2 cm by using the logistic regression (P = .27). Receiver operator curve analysis by using thallium index of 2 in small lesions yielded 50% sensitivity and 82% specificity. In contrast, thallium index was a significant predictor of malignancy in lesions ≥2 cm (P < .01), yielding 100% sensitivity and 89% specificity.
CONCLUSION: Lesion size is a significant determinant of the accuracy of thallium-201 brain SPECT imaging, which should be the initial diagnostic tool for lesions ≥2 cm.
Toxoplasmosis and lymphoma are the 2 most common central nervous system (CNS) mass lesions in patients with acquired immunodeficiency syndrome (AIDS; 1). A definitive diagnosis of either malignancy (predominantly primary brain lymphoma) or infection (predominantly toxoplasma encephalitis) remains difficult on the basis of anatomic imaging alone, because they share overlapping features on contrast-enhanced CT and MR imaging. Clinicians often use empiric therapeutic trials with antitoxoplasmosis medications for 10–14 days to differentiate toxoplasma encephalitis from other entities (2, 3). This delay in correct diagnosis was previously thought to be insignificant (4). Aggressive cerebral lymphomas, however, may progress very rapidly, with fatal results (5). Prompt radiation therapy for cerebral lymphoma has been shown to more than triple life expectancy, from 42 to 134 days (6). We previously evaluated a group of 27 patients and found that thallium-201 brain single-photon emission tomography (SPECT), performed early in the course of empiric therapy, was a useful technique to make a definitive diagnosis more rapidly (7) To the best of our knowledge, the effect of lesion size on the accuracy of thallium SPECT in AIDS patients has not been explicitly studied. We postulate that cerebral lesions greater than a critical size can be evaluated by thallium-201 brain SPECT with high sensitivity and specificity to differentiate malignant from infectious etiologies.
Methods
Patient Population
We retrospectively identified 43 consecutive AIDS patients who, during 4 years, underwent thallium-201 brain SPECT after contrast-enhanced CT or MR brain imaging demonstrated one or more enhancing mass lesions. Eight patients were excluded because no final diagnosis was available or they were lost to follow-up. The study group consisted of 35 patients, 29 male and 6 female, with a mean age of 42 years (range, 27–58 years). Twenty-two patients had contrast-enhanced MR and 13 patients had contrast-enhanced CT scans. With regard to a pre-existing institutional protocol, patients were started on empiric steroids and antibiotics and SPECT imaging was performed soon afterward, in most cases ≤24–48 hours to minimize the effects of therapy on thallium uptake. Seven patients had more than 1 lesion, with 5 in the malignancy group (range, 2 to 4 lesions) and 2 in the nonmalignancy group (range, 3 to 4 lesions), making a total of 48 lesions available for final analysis. In 22 patients, the final diagnosis was made by histopathologic evidence at biopsy (18 patients) or autopsy (4 patients). In 13 patients, the presumptive diagnosis was made on the basis of clinical course after radiation therapy (2 patients) or antibiotic therapy (11 patients). These clinical diagnoses were confirmed by follow-up CT and/or MR imaging, which showed marked improvement to complete resolution of all lesions as compared with the pretherapy scans. The mean follow-up period for these patients was 14.4 months (range, 0.9–57.4 months).
CT Imaging
CT imaging was performed on a helical scanner. Contiguous 5-mm collimated sections were obtained at 5-mm intervals from the skull base to the vertex. Scans were obtained before and after the administration of 100 mL of iodinated ionic or nonionic contrast.
MR Imaging
MR imaging was performed on a 1.5T unit. Sagittal and axial T1-weighted (484–600/15) images (repetition time [TR] millisecond/echo time [TE] milliseconds) and axial T2-weighted (2200–2500/102) images were acquired for each patient. Coronal and axial images T1-weighted images were obtained after intravenous injection of 0.1 mmol/kg bolus of gadopentetate dimeglumine contrast material. Each section was acquired by using a thickness of 5 mm, spacing of 1 mm, field of view of 20 × 20–24 cm, and 256 × 192–256 matrix.
Thallium-201 Brain SPECT
Thallium-201 imaging was performed 0–11 days (mean, 4.3 days) after brain CT or MR imaging. Brain SPECT was obtained 30 minutes after intravenous injection of 5 mCi (185 MBq) of thallium-201 by using a low-energy, general-purpose parallel-hole collimator, 360° circular orbit, and 15 seconds per stop for a total of 128 stops on a single head camera or 64 stops per detector for a dual-head camera. Images were acquired with a 30% window centered at 72 keV and 30% window centered at 167 keV, 64 × 64 acquisition matrix, and 1.6 zoom factor. No attenuation correction was performed. Images were reconstructed with filtered back projection (ramp prefiltering and Butterworth filter: 0.3 Nyquist cutoff and power of 10) and displayed in transaxial, sagittal, and coronal projections. The transaxial images were reoriented to approximate the angle of reconstruction on the CT or MR image.
Interpretation and Correlation of Thallium-201 Brain SPECT Images
The maximum diameter of the abnormally enhancing mass as measured with calipers on one CT or MR image in any plane was used as the lesion size. The number and location of the lesions were noted to correlate with the thallium-201 SPECT images. A single board-certified nuclear radiology physician, with knowledge of the lesion size and location from CT and/or MR imaging, reviewed the thallium-201 SPECT studies while blinded to the final outcomes. At the time of interpretation, the use of CT or MR was exclusively for anatomic correlation of the lesion(s). Images were evaluated on the computer monitor by using a color and gray-scale display to look for focal uptake corresponding to the contrast-enhancing lesions on CT or MR. Whenever a focus of increased uptake was noted, thallium index was measured: a single transaxial section through the center of the lesion was chosen, and an irregular region of interest was manually drawn around the lesion to yield the value of mean counts per pixel. If there was no focal abnormality on the contralateral side, an identical region of interest was drawn on the corresponding contralateral location to give a control value of counts per pixel. If there was a focal abnormality on the contralateral side, as occurred in 4 patients, the control value was derived from a region of interest placed over the background adjacent and ipsilateral to the lesion. The thallium index of the lesion was defined as the ratio of mean counts per pixel value in the lesion to the mean counts per pixel value in the control region.
Statistical Methods
Logistic regression was then used to determine the degree to which the thallium index differentiated malignant lesions from infectious lesions. Correlation coefficients (c-coefficients) measuring the accuracy of the prediction of the malignant lesions were generated based on the results of the logistic regression. The c-coefficient ranged between 0.5 and 1.0, with 0.5 representing a differentiation no better than chance, and 1.0 reflecting perfect discrimination.
Receiver operator curve analysis was then carried out to determine optimal thresholds for distinguishing malignant from nonmalignant lesions on the basis of the thallium index and lesion size. An optimal threshold was defined as the level of thallium index that maximized sensitivity while keeping specificity ≥0.80. All analyses were performed by using SAS 8.0 (SAS, Cary, NC). The alpha level for all statistical tests was 0.05.
To assess interobserver variation in the placement of regions of interest over the lesions and background, as well as its impact on the eventual calculation of thallium index, 3 observers independently calculated thallium index in 9 lesions. The reviewers, aware of anatomic locations of the lesions but unaware of thallium index as calculated by other reviewers, reviewed brain thallium SPECT images on the workstation and placed regions of interest over lesions and over background. Thallium index was calculated, and mixed model analysis of variance was used to evaluate for differences between readers.
Results
Thirty-five patients with 48 intracranial mass lesions had complete data sets with clinical and/or radiographic follow-up available for analysis. The final diagnosis was established by brain biopsy, autopsy, or clinical course and improvement of lesion(s) on follow-up imaging (18, 4, and 13 patients, respectively). Twenty patients had malignant lesions (19 lymphoma and 1 hemangiopericytoma). The diagnosis in 18 patients was established by biopsy (14 patients; Fig 1) or autopsy (4 patients). The diagnosis in 2 patients was established by favorable responses to radiation therapy (marked decrease in lesion size on follow-up CT and/or MR); these lesions were presumed to be lymphoma, the most common primary cerebral malignancy in AIDS patients. The malignant lesions in these 20 patients had a mean thallium index of 2.4 (range, 1–11). Fifteen patients had nonmalignant lesions. The diagnosis in 4 patients was established by biopsy due to nonimproving or deteroriating clinical courses (1 toxoplasma encephalitis; 1 tubercular abscess; and 2 cases of nonspecific inflammation without an identifiable organism, possibly attributable to ongoing antitoxoplasmosis therapy; Fig 2). The diagnosis in the remaining 11 patients was established by significant clinical response to antitoxoplasmosis therapy (presumed toxoplasma encephalitis due to decrease in lesion size or complete resolution on follow-up CT and/or MR imaging). The infectious lesions in these 12 patients with biopsy-proven or clinically confirmed toxoplasmosis had a mean thallium index of 1.6 (range, 1–3.6).
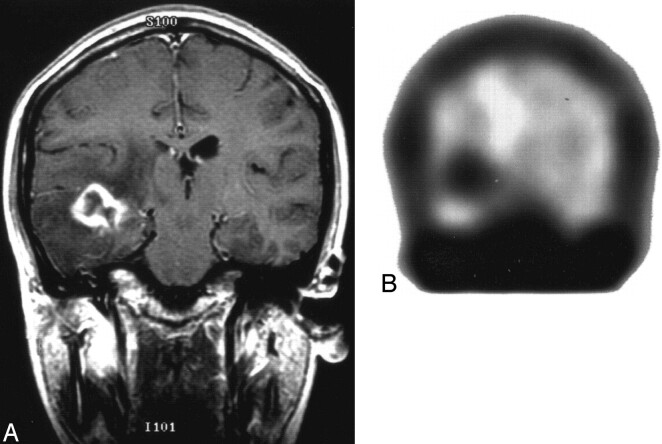
Fig 1.
Primary brain lymphoma.
A, Coronal T1-weighted image with contrast shows ring-enhancing mass lesion in right temporal lobe with surrounding edema changes.
B, Coronal thallium-201 SPECT shows focal uptake in right temporal region with thallium index of 4.8, which is consistent with malignancy and was confirmed by brain biopsy to represent primary brain lymphoma.
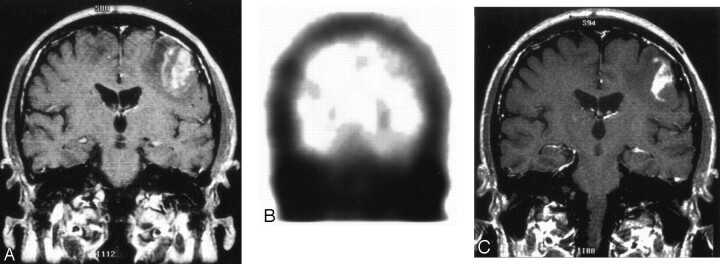
Fig 2.
Toxoplasma encephalitis.
A, Coronal T1-weighted image with contrast shows heterogeneously enhancing mass in left frontoparietal region with surrounding edema. There is also edema in the right frontoparietal region related to an additional enhancing lesion (not shown).
B, Coronal thallium-201 SPECT shows no corresponding area of focally increased radiotracer uptake. This is most consistent with a nonmalignant, infectious etiology, which was proved to be toxoplasmosis by brain biopsy.
C, Follow-up coronal T1-weighted image, 10 days after antitoxoplasma therapy, shows the size of the enhancing left frontoparietal mass to have decreased and improved surrounding edema.
The Table displays the characteristics of all patients with and without malignant lesions. Only the thallium index showed any significant difference between the groups. This result was confirmed by the logistic regression for all patients. Thallium index was a significant predictor of whether the patient had a malignant lesion (P < .004). The c-coefficient was 0.81, which indicated good discrimination between the 2 patient groups. The optimal cut-off for thallium index for all patients was 1.85, which showed a sensitivity of 75% and a specificity of 80%. For the lesions assessed by multiple independent readers, there was no significant difference in the calculated thallium index.
Only thallium index shows statistical significance between malignant and nonmalignant brain lesions
| Characteristic | Infectious Lesions(n = 20) | Malignant Lesions(n = 28) |
|---|---|---|
| Age (y) | 42.5 ± 7.8 | 41.1 ± 7.6 |
| Gender (% male) | 70 | 89 |
| Average size (cm) | 1.98 ± 0.79 | 2.49 ± 1.47 |
| Thallium index | 1.59 ± 0.92 | 2.41 ± 2.14* |
P < .004.
The data were analyzed separately for lesions with average size <2 cm (15 malignant, 9 nonmalignant) and lesions with average size ≥2 cm (17 malignant, 7 nonmalignant). For lesions <2 cm, thallium index was not a significant predictor of the presence of a malignant lesion based on the logistic regression (P = .27) (Fig 3). The c-coefficient was 0.68, whereas the optimal threshold for discriminating malignant lesions, thallium index of 2, yielded 50% sensitivity with 82% specificity. In contrast, for lesions ≥2 cm in size, thallium index was a significant predictor of the presence of a malignant lesion (P < .01), with a c-coefficient of 0.94. For lesions ≥2 cm, the optimal threshold was 2, which yielded 100% sensitivity and 89% specificity.
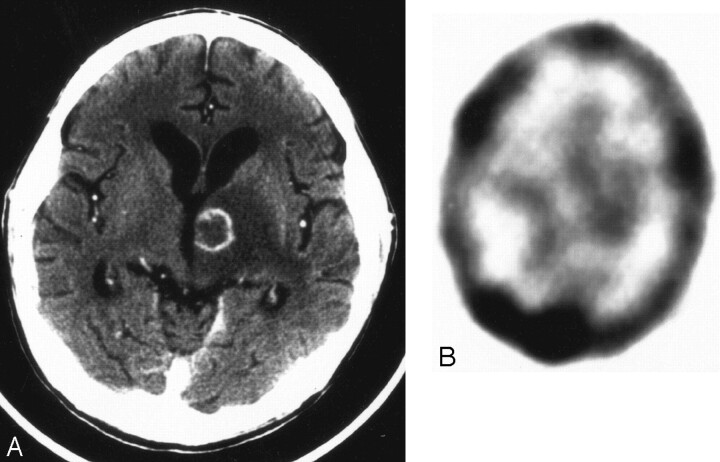
Fig 3.
False-negative thallium-201 SPECT of lymphoma.
A, Axial CT image with contrast shows a 1.9-cm ring-enhancing mass lesion in the left thalamus with surrounding edema.
B, Axial thallium-201 SPECT shows no corresponding area of focally increased tracer uptake. The slight asymmetry in basal ganglia activity (greater on the left) is due to patient angulation (note also asymmetry of the transverse sinus activity, greater on the right). Although suggestive for an infectious etiology, this lesion falls below our size threshold and, by brain biopsy, was due to lymphoma.
The diagnosis for 30 lesions in 22 patients was established by brain biopsy or autopsy. Five of these patients had more than one lesion (mean, 2.6; range, 2–4). One or more of the lesions was sampled based on the preference of the clinical service and neurosurgeon. All visible lesions (range, 2–3) were sampled in 3 patients, with coexisting lesions in these patients showing the same pathology. In 2 patients with 2 and 4 lesions, respectively, only the dominant mass was sampled and the remaining lesions were assumed to represent the same pathology. For histopathologically confirmed lesions ≥2 cm, a thallium index of 2 demonstrated 100% sensitivity and 93% positive predictive value for malignancy.
Discussion
Other investigators have studied the usefulness of thallium brain SPECT imaging in differentiating malignant from nonmalignant intracranial lesions in AIDS patients. O’Malley et al (8) found 100% sensitivity and 85% specificity for lymphoma in 13 patients, with thallium index of 2.95–4.30 (mean, 3.65) representing CNS lymphoma and thallium index of 0.77–1.95 (mean, 1.45) representing infectious lesions; Miller et al (9) applied O’Malley’s criteria to their 22 patients and found thallium ratio ≥2.9 to have 55% sensitivity and 100% specificity for lymphoma. Ruiz et al (10) achieved 100% sensitivity and 100% specificity for CNS lymphoma in 37 patients, though they did not quantify their thallium index.
Several coexisting illnesses may be encountered in AIDS patients. It is possible that 1 or more of our 7 patients with multiple lesions (range, 2–4) may have had coexisting malignant and infectious lesions, or even mixed malignant and infectious disease within the same lesion (9, 11). Three patients had all of their lesions (total of 7) sampled by biopsy or at autopsy and demonstrated the same pathology (lymphoma). In the cases of 2 patients, only the dominant lesion was biopsied. In view of the uncommon occurrence of coexisting infectious and malignant pathology in the same lesion or coexisting lesions in the same patient, we felt that it was unnecessary to subject every lesion to biopsy. Instead, after multidisciplinary consultation, the most amenable lesion was chosen for biopsy. Two patients improved clinically after empiric treatment. All lesions in these 4 patients improved or resolved with subsequent follow-up CT or MR imaging. Nineteen of the 20 patients with malignant lesions had primary brain lymphoma. One patient with a multiloculated parietal enhancing mass underwent surgical resection with pathology, which revealed hemangiopericytoma, a malignant mesenchymal tumor according to the World Health Organization classification (12).
False-positive results have occurred with aggressive infectious processes (13, 14). We observed 2 false-positive events in this study. One patient had a 2.4-cm hyperattenuated mass in the right cerebellar vermis with thallium index of 15 that was initially thought to represent lymphoma on the basis of CT and SPECT imaging. Pathology revealed only nonspecific inflammation, and no organism was isolated. The indeterminate pathology result was thought secondary to sampling error at biopsy. Although we suspected lymphoma because of the very high thallium index, the lesion was considered false-positive in our analysis. Another patient with toxoplasmosis had a 4-cm right parietal mass with thallium index of 3.6 that satisfied our criteria for malignancy; the other 2 lesions (3.5 and 3.9 cm) in this patient were accurately predicted as nonmalignant, with thallium indices of 1.5 and 1.7, respectively. False-negative results have also occurred (15, 16) and are likely related to small lesion size and marked central tumor necrosis occurring in aggressive malignancies such as primary brain lymphoma in AIDS patients (15, 17). False-negative results occurred in 4 lymphoma patients, 2 of whom had multiple lesions—2 and 4 lesions, respectively. The larger lesion (3 cm) in the patient with 2 lesions had a thallium ratio of 4.8, whereas the smaller lesion (0.8 cm) had a thallium ratio of 1.0. The 4 lesions (1.0–1.8 cm) in the other patient had thallium ratios of 1.2–1.4.
Thallium Index
Various methods can be used to derive the thallium index. We calculated the mean count per pixel in an irregular region of interest manually drawn around the lesion with an identical control region of interest drawn on the corresponding contralateral side; this is one of the most commonly used methods (8, 9, 13, 18–21). Two other methods are ratio of maximum counts per pixel (peak activity) in the lesion and corresponding contralateral side (19) and ratio of activity in the lesion to that in the scalp (10, 22, 23). Calculation of the mean lesion activity provides a measure of global uptake and the ability of the lesion to concentrate thallium. We believe that this is the best way to characterize the lesion. When using the ratio of mean thallium activity in the lesion over the background, there is concern that the ratio may be lowered because of necrosis within the tumor. It has been suggested that using the ratio of maximal activity may therefore be more accurate. Despite use of mean activity instead of maximal activity for determination of the thallium index, all lymphoma lesions ≥2 cm in our series had a thallium index greater than the threshold value. This means that potential decreases in mean thallium index did not reduce our sensitivity for lesion detection.
In lymphoma lesions <2 cm in size, potential sites of necrosis may have influenced sensitivity. In addition, it is well known that lesions smaller than gamma camera system resolution may not be detected with thallium SPECT imaging. Region-of-interest placement in the smaller lesions may not necessarily sample tumor alone, and inclusion of adjacent background may lower the thallium index, thereby influencing sensitivity. Gamma cameras generally have lower resolution than anatomic imaging techniques. Detectability of lesions is closely related to system resolution, with larger lesions more conspicuous to visual inspection and more amenable to quantitative analysis. Low count attenuation is a major problem in metabolic imaging and a frequent confounder in AIDS patients with smaller, heterogeneous or frankly necrotic lesions. In our experience, the use of 2 cm as a critical size for differentiating malignant from infectious cerebral mass lesions has been extremely useful. This permits the upper thallium index cutoff for suspecting malignancy to be 2, which is considerably less than that described by other authors.
Positron-emission tomography (PET) has been shown to differentiate malignant from nonmalignant lesions accurately. In AIDS patients with intracranial mass lesions, 18f-fluoro-2-deoxyglucose (FDG) PET has been used with 89%–100% accuracy to distinguish primary brain lymphoma from other etiologies (24–26). Much of the PET experience came from C-11 tracers that require an on-site cyclotron, which is not routinely available and significantly limits the utility of PET imaging (27). FDG PET continues to be more expensive than thallium brain SPECT, however, and is further confounded by the high background gray matter FDG uptake (due to preferential glucose utilization by gray matter) that may limit lesion detectability. MR spectroscopy cannot differentiate benign from malignant lesions, because the spectra show only nonspecific findings due to lesion heterogeneity and marked necrosis (27, 28).
Limitations
Thallium-201 SPECT imaging was performed within 24–48 hours after the diagnosis of enhancing mass lesion(s), which we believe is before the effects of empiric antibiotic therapy can be observed. Thallium uptake is minimally affected by concomitant steroid therapy (29). Elevated serum IgG antibody titers may have limited sensitivity (representing prior or recent infection) and may not be detectable in some AIDS patients with toxoplasma encephalitis (30). Negative antibody titers are very suggestive, however, for a diagnosis other than toxoplasmosis. When brain biopsy was not performed, we made clinical determinations by integrating thallium-201 SPECT and toxoplasma serology results, a technique shown to improve diagnostic accuracy (23). We did not routinely obtain CSF access in all our patients, and other authors have agreed that lumbar puncture or direct ventricular sampling may be difficult or contraindicated and performed in only 25%–40% patients (9, 31). Although CSF evaluation for toxoplasma is not helpful, polymerase chain reaction assay for Epstein-Barr virus (EBV) DNA in CSF can indicate primary brain lymphoma with 85% sensitivity and 98% specificity (31). Combined thallium-201 SPECT and CSF EBV results have shown 100% positive and 88% negative predictive value (32). EBV viral load measurements provide valuable information but may be available only on certain days of the week and usually require a minimum of 2–4 days to obtain, in contrast to the near ubiquitous availability of thallium-201 imaging.
Histopathologic evidence was obtained for nearly two-thirds of our patients. Brain biopsy yields a definitive diagnosis in 88%–93% but entails 8%–12% morbidity and 2%–3% mortality (31, 33). As many as 20% of brain biopsies may be nondiagnostic, particularly in nonenhancing lesions likely to have greater amounts of necrosis (34). Stereotactic biopsy is subject to sampling errors because of areas of necrosis or lower grade tumor that are not representative of the true nature of the mass. No anatomic or metabolic imaging method has been able to achieve a similar diagnostic rate, however, and only biopsy can reliably diagnose coexisting lymphoma and toxoplasmosis or other infection in the same patient. In the subgroup of patients for whom biopsy or autopsy correlation of the lesions was not available, we believe that clinical and imaging improvement permitted reasonably confident diagnoses short of histopathologic analysis. When all lesions in a patient were examined, either by multiple brain biopsies or at autopsy, only one underlying disease was found for each patient.
Lesions ≥2 cm can be accurately evaluated with 100% sensitivity and 89% specificity. The results can then direct further testing and/or therapy. A thallium index ≥2 is strong evidence for malignancy and should expedite brain biopsy and/or empiric radiation therapy/chemotherapy. Thallium index <2 indicates nonmalignant infectious disease and should initiate appropriate antibiotic therapy with clinical and radiologic follow-up to monitor lesion size by contrast-enhanced CT and/or MR studies. Thallium-201 brain SPECT of lesions <2 cm has relatively limited utility, with only 50% sensitivity and 82% specificity. The limited sensitivity in these smaller lesions is likely due to resolution limits of the gamma camera systems.
Conclusion
Lesion size is a very important determinant of the accuracy of thallium-201 brain SPECT. This is an effective noninvasive examination to differentiate between malignant and infectious enhancing cerebral mass lesions ≥2 cm in size in AIDS patients. In lesions <2 cm, SPECT is not a useful examination, because only half the lesions were correctly differentiated.
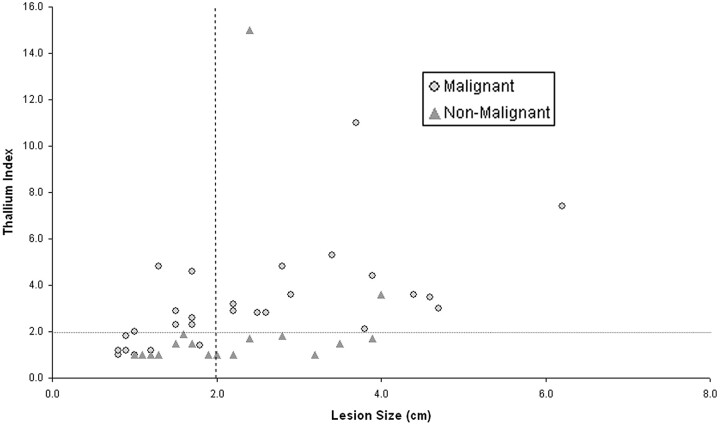
Fig 4.
When using thallium index threshold of 2 for all lesions ≥2 cm, no malignant lesions were missed and only two false-positive events occurred.
Acknowledgments
We are grateful for the generous contributions made by Drs. Sanjaya Viswamitra, Patricia H. Choi, Jay M. Rosenberg, Peeyush Bhargava, and Peter Homel.
References
- 1.Rosenblum ML, Levy RM, Bredesen DR. Neurosurgical implications of the acquired immunodeficiency syndrome (AIDS). Clin Neurosurg 1988;34:419–445 [PubMed] [Google Scholar]
- 2.Cohn JA, McMeeking A, Cohen W, et al. Evaluation of the policy of empiric treatment of suspected toxoplasma encephalitis in patients with the acquired immunodeficiency syndrome. Am J Med 1989;86:521–527 [DOI] [PubMed] [Google Scholar]
- 3.Luft BJ, Hafner R, Korzun AH, et al. Toxoplasmic encephalitis in patients with the acquired immunodeficiency syndrome. N Engl J Med 1993;329:995–1000 [DOI] [PubMed] [Google Scholar]
- 4.Iglesias-Rozas JR, Bantz B, Adler T, et al. Cerebral lymphoma in AIDS: clinical, radiological, neuropathological and immunopathological study. Clin Neuropathol 1991;10:65–72 [PubMed] [Google Scholar]
- 5.Chiang FL, Miller BL, Chang L, et al. Fulminant cerebral lymphoma in AIDS. AJNR Am J Neuroradiol 1996;17:157–160 [PMC free article] [PubMed] [Google Scholar]
- 6.Baumgartner JE, Rachlin JR, Beckstead JH, et al. Primary central nervous system lymphomas: natural history and response to radiation therapy in 55 patients with acquired immunodeficiency syndrome. J Neurosurg 1990;73:206–211 [DOI] [PubMed] [Google Scholar]
- 7.Ghesani M, Viswamitra S, Kagetsu NL, et al. Lesion size is an important determinant of the accuracy of TL-201 brain SPECT in the evaluation of intracranial lesions in AIDS patients [Abstract]. J Nucl Med 1999;40:199P [Google Scholar]
- 8.O’Malley JP, Ziessman HA, Kumar PN, et al. Diagnosis of intracranial lymphoma in patients with AIDS: value of P201P Tl single-photon emission computed tomography. AJR Am J Roentgenol 1994;163:417–421 [DOI] [PubMed] [Google Scholar]
- 9.Miller RF, Hall-Craggs MA, Costa DC, et al. Magnetic resonance imaging, thallium-201 SPET scanning, and laboratory analyses for discrimination of cerebral lymphoma and toxoplasmosis in AIDS. Sex Transm Infect 1998;74:258–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruiz A, Ganz WI, Post MJD, et al. Use of thallium-201 brain SPECT to differentiate cerebral lymphoma from toxoplasma encephalitis in AIDS patients. AJNR Am J Neuroradiol 1994;15:1885–1894 [PMC free article] [PubMed] [Google Scholar]
- 11.Chang L, Cornford ME, Chiang FL. Radiologic-pathologic correlation: cerebral toxoplasmosis and lymphoma in AIDS. AJNR Am J Neuroradiol 1995;16:1653–1663 [PMC free article] [PubMed] [Google Scholar]
- 12.Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumours. Brain Pathol 1993;3:255–268 [DOI] [PubMed] [Google Scholar]
- 13.Krishna L, Slizofski WJ, Katsetos CD, et al. Abnormal intracerebral thallium localization in a bacterial brain abscess. J Nucl Med 1992;33:2017–2019 [PubMed] [Google Scholar]
- 14.Gorniak RJT, Kramer EL, McMeeking AA, et al. Thallium-201 uptake in cytomegalovirus encephalitis. J Nucl Med 1997;38:1386–1388 [PubMed] [Google Scholar]
- 15.Fisher DC, Chason DP, Mathews D, et al. Central nervous system lymphoma not detectable on single-photon emission CT with thallium 201. AJNR Am J Neuroradiol 1996;17:1687–1690 [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell BG, Hurley J, Zimmerman RD. False-negative single-photon emission CT in AIDS lymphoma: lack of effect of steroids. AJNR Am J Neuroradiol 1996;17:1000–1002 [PMC free article] [PubMed] [Google Scholar]
- 17.Berry I, Gaillard JF, Guo Z, et al. Cerebral lesion in AIDS: what can be expected from scintigraphy? Cerebral tomographic scintigraphy using thallium-201: a contribution to the differential diagnosis of lymphomas and infectious lesions. J Neuroradiol 1995;22:218–228 [PubMed] [Google Scholar]
- 18.Lorberboym M, Estok L, Machac J, et al. Rapid differential diagnosis of cerebral toxoplasmosis and primary central nervous system lymphoma by thallium-201 SPECT. J Nucl Med 1996;37:1150–1154 [PubMed] [Google Scholar]
- 19.Kim KT, Black KL, Marciano D, et al. Thallium-201 SPECT imaging of brain tumors: methods and results. J Nucl Med 1990;31:965–969 [PubMed] [Google Scholar]
- 20.D’Amico A, Mesa C, Castagna A, et al. Diagnostic accuracy and predictive value of 201Tl SPET for the differential diagnosis of cerebral lesions in AIDS patients. Nucl Med Commun 1997;18:741–750 [DOI] [PubMed] [Google Scholar]
- 21.Black KL, Hawkins RA, Kim KT, et al. Use of thallium-201 SPECT to quantitate malignancy of gliomas. J Neurosurg 1989;71:342–346 [DOI] [PubMed] [Google Scholar]
- 22.Kessler LS, Ruiz A, Post MJD, et al. Thallium-201 brain SPECT of lymphoma in AIDS patients: pitfalls and technique optimization. AJNR Am J Neuroradiol 1998;19:1105–1109 [PMC free article] [PubMed] [Google Scholar]
- 23.Skiest DJ, Erdman W, Chang WE, et al. SPECT thallium-201 combined with toxoplasma serology for the presumptive diagnosis of focal central nervous system mass lesions in patients with AIDS. J Infect 2000;40:274–281 [DOI] [PubMed] [Google Scholar]
- 24.Heald AE, Hoffman JM, Bartlett JA, Waskin HA. Differentiation of central nervous system lesions in AIDS patients using positron emission tomography (PET). Int J STD AIDS 1996;7:337–346 [DOI] [PubMed] [Google Scholar]
- 25.Pierce MA, Johnson MD, Maciunas RJ, et al. Evaluating contrast-enhancing brain lesions in patients with AIDS by using positron emission tomography. Ann Int Med 1995;123:594–598 [DOI] [PubMed] [Google Scholar]
- 26.Hoffman JM, Waskin HA, Schifter T, et al. FDG-PET in differentiating lymphoma from nonmalignant central nervous system lesions in patients with AIDS. J Nucl Med 1993;34:567–575 [PubMed] [Google Scholar]
- 27.Pomper MG, Constantinides CD, Barker PB, et al. Quantitative MR spectroscopic imaging of brain lesions in patients with AIDS: correlation with [11C-methyl]thymidine PET and thallium-201 SPECT. Acta Radiol 2002;9:396–409 [DOI] [PubMed] [Google Scholar]
- 28.Chinn RJS, Wilkinson ID, Hall-Craggs MA, et al. Toxoplasmosis and primary central nervous system lymphoma in HIV infection: diagnosis with MR spectroscopy. Radiology 1995;197:649–654 [DOI] [PubMed] [Google Scholar]
- 29.Kaplan WD, Takvorian T, Morris JH, et al. Thallium-201 brain tumor imaging: a comparative study with pathologic correlation. J Nucl Med 1987;28:47–52 [PubMed] [Google Scholar]
- 30.Porter SB, Sande MA. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med 1993;23:1643–1648 [DOI] [PubMed] [Google Scholar]
- 31.Antinori A, Ammassari A, De Luca A, et al. Diagnosis of AIDS-related focal brain lesions: a decision-making analysis based on clinical and neuroradiologic characteristics combined with polymerase chain reaction assays in CSF. Neurology 1997;48:687–694 [DOI] [PubMed] [Google Scholar]
- 32.Castagna A, Cinque P, D’Amico A, et al. Evaluation of contrast-enhancing brain lesions in AIDS patients by means of Epstein-Barr virus detection in cerebrospinal fluid and 201-thallium single photon emission tomography. AIDS 1997;11:1522–1523 [PubMed] [Google Scholar]
- 33.Skolasky RL, Dal Pan GJ, Olivi A, et al. HIV-associated primary CNS morbidity and utility of brain biopsy. J Neurol Sci 1999;163:32–38 [DOI] [PubMed] [Google Scholar]
- 34.Chappell ET, Guthrie BL, Orenstein J. The role of stereotactic biopsy in the management of HIV-related focal brain lesions. Neurosurg 1992;30:825–829 [DOI] [PubMed] [Google Scholar]