Abstract
Summary: We describe a case of glioblastoma multiforme of the conus medullaris in a child. MR imaging showed at the T12-L1 level an intramedullary mass with signal alteration, with only two nodules of contrast enhancement. The finding was consistent with astrocytoma. Pathologic evaluation was consistent with glioblastoma multiforme. Nine months after the surgical treatment, the patient showed leptomeningeal recurrence. This case appears to be unusual for both the hysto-type and the nonspecific MR imaging features.
Spinal neoplasms in the adult population are represented mostly by extradural (55%) and intradural extramedullary tumors (40%), whereas intramedullary tumors are rare (5%) (1). In contrast, the rate of intramedullary tumors in children is higher, up to 35% of all spinal neoplasms (2). According to literature data (3), most spinal cord tumors are malignant, mostly represented by gliomas (90%–95%) (ependymoma and astrocytoma), whereas nonglial tumors (ie, hemangioblastomas, primitive neuroectodermal tumors) are less frequently found. Moreover, the most frequent histologic type of tumor differs in adults and children, being ependymoma in adults and astrocytoma in children. Clinical presentation is related to the region of spinal cord involved, irrespective of tumor type.
In an overall evaluation, the more reported symptoms in children are pain (42%), motor regression (36%), gait abnormality (27%), torticollis (27%), and progressive kyphoscoliosis (24%) (2).
To the best of our knowledge, glioblastoma multiforme is only rarely seen in children. We describe a rare case of glioblastoma multiforme of the conus medullaris that occurred in a child.
Case Report
A 14-year-old boy had slowly progressing motor weakness in his legs lasting for 2 years, which was exacerbated 15 days before presentation, after a fall from a bike. He was admitted to the hospital because of a right-sided paraparesis, right-directed right-foot dorsal and plantar flexion deficit, right sciatalgy (Lasegue test, 30°), a painful abnormal gait, urinary retention alternating with occasional urinary incontinence, and right perineal hypoesthesia. Patellar reflexes were increased, whereas the Achilles’ reflexes were totally absent.
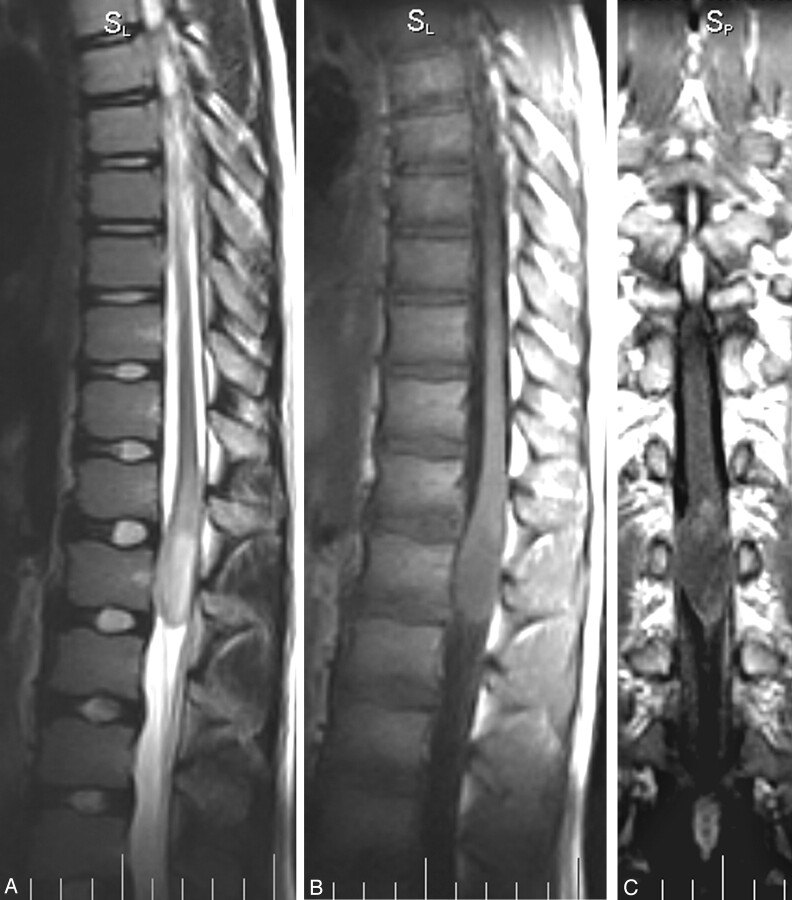
The patient underwent brain and spine MR imaging. Findings in the brain and cervical spine were negative for masses and signal intensity alterations, whereas at the T12 level, there was evidence of an intramedullary signal intensity alteration, extending down to the L1 level. The finding was 3.5 × 2 cm, with ovoid morphology, showing homogeneous hyperintense signal intensity on spin-echo T2-weighted images (Fig 1A), hypointense signal intensity on spin-echo T1-weighted images (Fig 1B), and well-demarcated circumferential cord expansion. The mass was defined as intramedullary and localized at the conus level.
Fig 1.
A–C, Fast spin-echo T2-weighted sagittal acquisition (A) shows a hyperintense and homogeneous mass at the T12 level, involving the conus and the epicomus, with a diffuse aspect and without a clear cleavability plane. Spin-echo T1-weighted sagittal (B) and coronal (postgadolinium) (C) acquisitions show a hypointense lesion with a mild patchy nodular enhancement of the upper portion, associated with a faint enhancement along the pial surface.
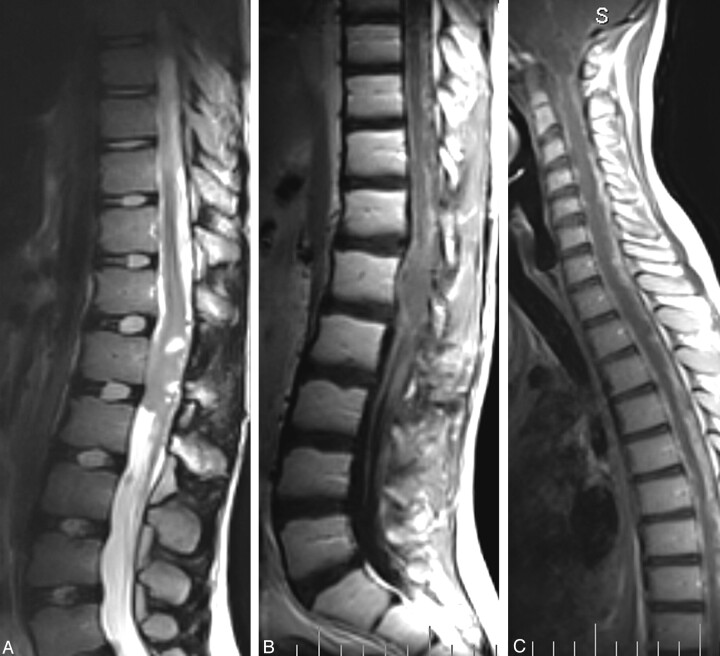
After an intravenous injection of gadolinium, there was a faint enhancement, mainly localized in 2 nodules in the upper cranial portion of the conus alteration, better shown in the coronal plane (Fig 1C); the remaining part of the lesion showed no enhancement. The finding was consistent with a spinal cord astrocytoma of low grade or, with less probability, an epidermoid tumor. The patient was surgically treated with ablation of the mass, resulting in a postoperative course with moderate progressive improvement of leg strength but also with a neurologic impairment of bladder function. At the 9-month follow-up, the patient showed a leptomeningeal endocanalar spread, up to posterior fossa (Fig 2A–C), indicating recurrent disease.
Fig 2.
A–C, Nine-month follow-up after surgery. Fast spin-echo T2-weighted (A) and spin-echo T1-weighted (postgadolinium) (B) sagittal lumbar spine acquisitions show a local recurrence of the disease. Note the leptomeningeal spread (B, C) along the entire spinal canal, up to the posterior cerebral fossa (C).
Pathologic Findings
Small fragments of the surgical specimen were fixed in buffered formalin for 24 hours and embedded in paraffin. We obtained 4-μm-thick sections and stained them with hematoxylin and eosin. We took further sections for immunohistochemistry, using antibodies against glial fibrillary acidic protein, synaptophysin, p53, Ki67 (MIB-one clone), and S100 protein.
On hematoxylin and eosin (Fig 3), the tumor was composed of moderately pleomorphic cells, with hyperchromatic nuclei and prominent nucleoli, focally stained by antiglial fibrillary acidic protein antibody. Occasionally, mucoid degeneration, microcysts, and microvascular proliferation were found. No necrotic areas were identified. The mitotic index was low (2 × 10 high-power field), but growth fraction, as determined by anti-Ki67 antibody indicated high-proliferative tumor cell activity (25%) (Fig 4); p53 was highly expressed (65%). The final pathologic response was consistent with a glial intramedullary neoplasm compatible with glioblastoma multiforme.
Fig 3.

Photomicrograph of histologic findings shows glioblastoma multiforme. Note high cellular attenuation, pleomorphism, and mitosis of neoplastic cells (hematoxylin and eosin, original magnification ×400).
Fig 4.
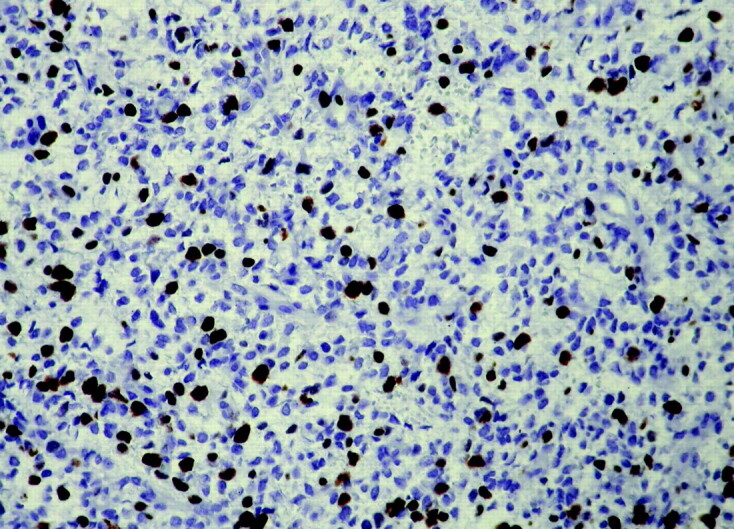
Photomicrograph of histologic findings shows anti-Ki67 (MIB-one) antiserum indicating a high-proliferating index in neoplastic cells (immunoperoxidase, hematoxylin counterstaining, original magnification ×400).
Discussion
As trocytomas are the more frequent glial tumors presenting as intramedullary neoplasms in children. Up to 60% of these cases can show an involvement of the entire spinal cord (holocord presentation) (4), which is more unusual in adults. The single isolated conus medullaris involvement, reported in few cases in the literature (5), is to be considered an infrequent condition (3% of the intramedullary astrocytomas). Astrocytomas in children are mainly low-grade tumors (more than one-third of cases) (6), without infiltration of nervous tissue, but rather with a splaying of this; in adults, the stages are usually III and IV or higher (4).
In a large pediatric intramedullary neoplasms case series (6), high-grade astrocytomas (stages III and IV) accounted for 11% of the cases. In rare cases, there can be a metastatic localization in the conus from a primitive brain glioblastoma multiforme localization, originating elsewhere (7, 8). To the best of our knowledge, there are no other radiologic reports of primitive glioblastoma multiforme of the conus in childhood, none moreover, with a full MR imaging evaluation.
The main aim of the differential diagnosis of astrocytomas in the pediatric population is to exclude gangliogliomas, ependymomas, and epidermoid/dermoid tumors. Typically astrocytomas are represented as eccentric masses, with usual holocord extension in children, most often located in the thoracic spine and usually not involving the conus medullaris, with ill-defined borders and heterogeneous and partial enhancement after gadolinium injection (1). The signal intensity is iso- to hypointense relative to the spinal cord on T1-weighted images and hyperintense on T2-weighted images; rostral and intratumoral cysts are a common finding in this tumor. Intramedullary glioblastoma multiforme also shows a leptomeningeal spread in 60% of cases (3).
Ependymomas are more concentric and have similar T1 and T2 patterns, except for a report that they also can be isointense in T2-weighted images (4). Up to one-third show the “cap” sign, due to a hypointense rim of hemosiderin at both poles. Ependymomas involve fewer vertebral segments than astrocytomas, and the variant myxopapillary ependymoma has a preferential localization in the conus medullaris. Enhancement is present to some degree in all cases but usually is moderate to high and allows a better definition of the margins of the neoplastic mass.
Gangliogliomas represent 1.1% of all spinal neoplasms, are more frequent in children, and involve mainly the cervical and thoracic cord, rarely the conus or the entire cord (4). They are concentric, featuring intratumoral cysts more frequently than astrocytomas and ependymomas (9). In most cases (87%), they have a mixed signal intensity on T1-weighted images, representing the different cellular population, with homogeneous hyperintensity on T2-weighted images. After gadolinium administration, more than half (65%) of them show a patchy enhancement, and in 58% of cases, a pial surface enhancement is observed.
In our case, we found an expansive intramedullary mass, with a signal intensity compatible with both astrocytoma and ependymoma on T1-weighted images, more with astrocytoma on T2 image (absence of the cap sign) and more typical of an astrocytoma after gadolinium injection, with irregular and poorly defined enhancement. There were no signs of hemorrhage, cysts, or pial or leptomeningeal involvement. The MR imaging presentation was compatible with a low-grade astrocytoma, with absence of MR imaging clues or signs that could help in making a diagnosis of a higher grade neoplasm, whereas the lesion resulted in a rare case of primitive glioblastoma multiforme of the conus medullaris. Both the kind of tumoral histotype in a young boy and the lack of high-grade malignancy signs at the MR imaging examination are rare in this case.
References
- 1.Baleriaux DLF. Spinal cord tumors. Eur Radiol 1999;9:1252–1258 [DOI] [PubMed] [Google Scholar]
- 2.Costantini S, Hounten J, Miller D, et al. Intramedullary spinal cord tumors in children under the age of 3 years. J Neurosurg 1996;85:1036–1043 [DOI] [PubMed] [Google Scholar]
- 3.Koeller KK, Rosenblum RS, AL Morrison. Neoplasm of the spinal cord and filum terminale: radiologic-pathologic correlation. RadioGraphics 2000;20:1721–1749 [DOI] [PubMed] [Google Scholar]
- 4.Brotchi J, Fischer G. Treatment. In: Fischer G, Brotchi J, eds. Intramedullary spinal cord tumors. Stuttgart, Germany: Thieme;1996;60–84
- 5.Hida K, Iwasaki Y, Cho K, Imamura H, Abe H. Gliomas of the conus medullaris. Paraplegia 1994;32:52–58 [DOI] [PubMed] [Google Scholar]
- 6.Jallo GI, Bong-Soo Kim, Epstein F. The current management of intramedullary neoplasms in children and young adults. Annals of Neurosurgery 2001;1:1–13 [Google Scholar]
- 7.Rondinelli PI, Martinez CA. Spinal cord metastatic glioblastoma multiforme of childhood: case report [in Portuguese]. Arq Neuropsiquiatr 2002;60:643–646 [PubMed] [Google Scholar]
- 8.Aghakhani N, Roux FX, Fallet-Bianco C, Devaux B. Secondary intraspinal localizations of glioblastoma: apropos of a case [in French]. Neurochirurgie 1995;41:363–366 [PubMed] [Google Scholar]
- 9.Patel U, Pinto RS, Miller DC, et al. MR of spinal cord ganglioglioma. AJNR Am J Neuroradiol 1998;19:879–887 [PMC free article] [PubMed] [Google Scholar]