Abstract
Summary: This clinical report is the first to describe angioscopy during carotid angioplasty with stent placement. The average observation time was 3 minutes 43 seconds in 18 cases. The view was clear in 67% of cases. Lesions in the endothelium, rupture of the fibrous cap, clots, debris detaching from plaque, and stent struts were observed. No symptomatic ischemic complications occurred. Diffusion-weighted MR imaging after angioscopy showed asymptomatic ischemic lesions in 47% of cases.
Carotid angioplasty with stent placement became an alternative to carotid endarterectomy in patients at high risk for carotid angioplasty with stent placement (1). One of the distinct differences between carotid angioplasty with stent placement and carotid endarterectomy is that in carotid angioplasty with stent placement, direct visual observation of the carotid lesion is rarely performed.
Because of technological progress, several studies concerning angioscopy in peripheral and coronary arterial lesions (2, 3) and some experimental studies in relation to the carotid artery (4) have been reported in the literature, but to our knowledge, there has been no report on clinical angioscopy in carotid angioplasty with stent placement, probably due to the technical problems associated with carotid observation. One problem is that the continuous irrigation necessary to obtain a clear view may evoke distal embolism or possible hyperperfusion to the brain. Also, temporal interruption of the carotid blood flow may increase the unnecessary risk of cerebral ischemia.
Recently carotid angioplasty with stent placement has been performed with protection devices such as temporal balloon occlusion, filter devices, or the Parodi Antiemboli System (ArteriA Medical Science Inc., San Francisco, CA) to reduce the embolic complications of carotid angioplasty with stent placement (5–7). In our carotid angioplasty with stent placement procedure, as distal protection, the carotid blood flow was temporally occluded by a balloon, which enabled us to perform angioscopy of the carotid artery during carotid angioplasty with stent placement. From June 2003 to July 2004, angioscopy of the carotid artery was performed during carotid angioplasty with stent placement in 18 cases. To detect possible distal embolism, we performed diffusion-weighted MR imaging before and after carotid angioplasty with stent placement. The technique, present benefits, and limitations of angioscopy during carotid angioplasty with stent placement are discussed.
Technique
All 18 male patients with an average age of 70 years had more than 60% carotid stenosis according to the measurements in the North American Symptomatic Carotid Endarterectomy Trial (8) and Asymptomatic Carotid Atherosclerosis Study (9). In Japan, angioscopy is the officially approved diagnostic method in angioplasty, and its expenses are refundable under the Japanese medical insurance system.
Generally, acetylsalicylic acid (100 mg) and ticlopidine (200 mg) were administered for at least 2 weeks before the procedure, and the procedures were performed via the percutaneous transfemoral route with general anesthesia.
In Japan, none of the filter devices have been approved, so protection against distal embolism during carotid angioplasty with stent placement was performed by PercuSurge GuardWire (Medtronic Inc., Minneapolis, MN) or the Parodi Antiemboli System. Distal protection of the internal carotid artery with the PercuSurge GuardWire was used in 13 cases and protection by reversing the carotid blood flow by simultaneous occlusion of the common carotid and the external carotid arteries with the Parodi Antiemboli System, in 6 cases (Table 1). In one case, both protections were used to temporally occlude the common, external, and internal carotid arteries.
TABLE 1:
Clinical features of the patients
| Patient No./Sex/Age (y) | Stenosis (%) | Symptom | Occlusion Time: Total/CAS/AS | Protection Device | Ischemic Lesion* |
|---|---|---|---|---|---|
| 1/M/68 | 70 | Minor stroke | 19 min 12 s/16 min 12 s/3 min | PS | Yes |
| 2/M/77 | 80 | Asymptomatic | 21 min 34 s/18 min 34 s/3 min | PS | Yes |
| 3/M/59 | 80 | Asymptomatic | 24 min 24 s/20 min 9s/4 min 15 s | PS | No |
| 4/M/67 | 70 | Asymptomatic | 16 min 8 s/10 min 40 s/5 min 28 s | PS | Yes |
| 5/M/83 | 95 | Minor stroke | 25 min/21 min/4 min | PS | Yes |
| 6/M/70 | 75 | Minor stroke | 15 min 47 s/13 min 2 s/2 min 45 s | PS | Yes |
| 7/M/73 | 70 | Asymptomatic | 19 min/14 min 8 s/4 min 52 s | PS | — |
| 8/M/71 | 70 | Asymptomatic | 8 min 56 s/6 min 58 s/1 min 58 s | PS, PAES | No |
| 9/M/73 | 70 | Asymptomatic | 14 min 2 s/9 min 57 s/4 min 5 s | PS | No |
| 10/M/77 | 60 | Minor stroke | 11 min 30 s/11 min/30 s | PS | Yes |
| 11/M/74 | 70 | Minor stroke | 17 min 47 s/14 min 47 s/3 min | PS | No |
| 12/M/54 | 70 | Asymptomatic | 11 min 10 s/9 min 10 s/2 min | PS | No |
| 13/M/69 | 90 | Asymptomatic | 23 min 1 s/16 min 4 s/6 min 57 s | PAES | No |
| 14/M/76 | 80 | Minor stroke | 13 min/10 min/3 min | PAES | No |
| 15/M/61 | 90 | Asymptomatic | 18 min 20 s/13 min 50 s/4 min 30s | PAES | No |
| 16/M/63 | 70 | Major stroke | 15 min 39 s/10 min 24 s/5 min 15 s | PAES | Yes |
| 17/M/65 | 70 | Asymptomatic | 14 min 6 s/8 min 37 s/5 min 29 s | PAES | Yes |
| 18/M/72 | 60 | Asymptomatic | 13 min 47 s/11 min/2 min 47 s | PS | No |
| Avg 70 | 74 | 16 min 48 s/13 min 5 s/3 min 43s |
Note.—CAS indicates carotid angioplasty with stent; AS, angioscopy; PS, PercuSurge; PAES, Parodi antiemboli system.
Refers to asymptomatic ischemic lesions detected by diffusion-weighted MRI after the procedure.
The procedures were performed by an experienced team. After placement of the sheath introducer, patients were administered 100 IU/kg of heparin intravenously, and the activated clotting time was maintained at 2.5–3 times longer than the control activated clotting time. Heparin was not reversed but was discontinued after completion of carotid angioplasty with stent placement and angioscopy. Acetylsalicylic acid and ticlopidine were continued for another 6 months.
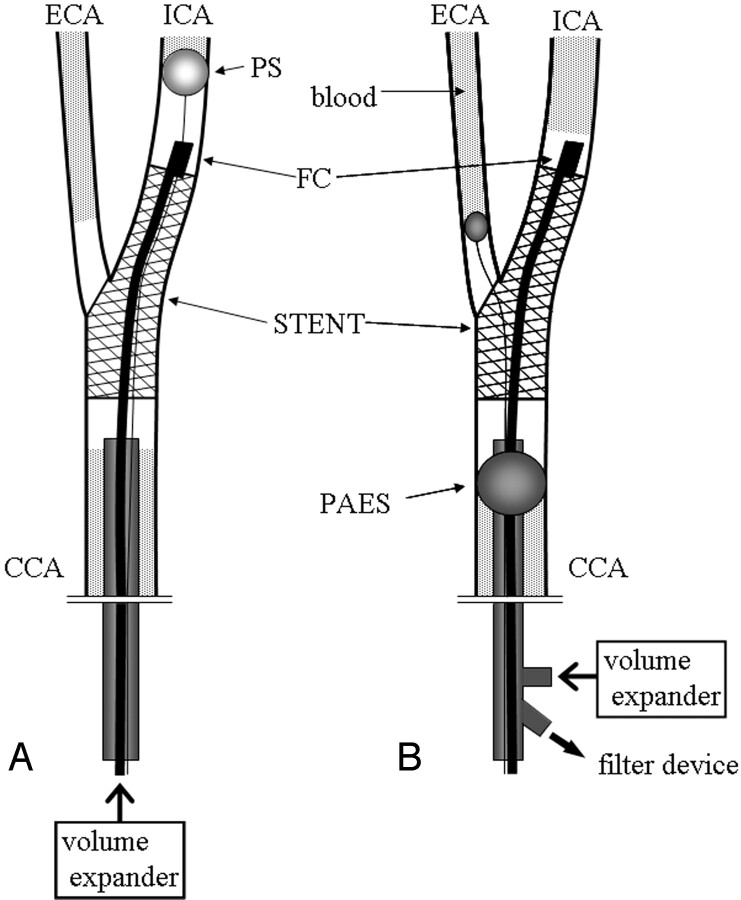
In the 13 patients in whom distal occlusion of the carotid artery was performed, a 7F introducer (Shuttle introducer, Cook Inc., Bloomington, IN) was guided up to the ipsilateral common carotid artery proximal to the carotid stenosis. The PercuSurge GuardWire was gently passed through the stenosis and placed at the distal internal carotid artery. The PercuSurge GuardWire has an occlusion balloon near the tip of the catheter, and this inflatable balloon was used for distal protection. The probe for intravascular sonography was passed through the stenosis and continuously withdrawn to measure the stenosis and observe the nature of the carotid wall. Angioplasty and stent deployment were performed during the temporal inflation of the distal protection balloon (Fig 1A). Either an Easy Wall (Boston Scientific Japan, Tokyo, Japan) or a Smarter (J&J Cordis, Tokyo, Japan) stent was deployed in each case. Angioplasty and stent deployment before angioscopy, with occlusion by the protection balloon, took an average of 13 minutes 35 seconds. Before deflating the protection balloon to restore the anterograde flow, we aspirated debris and blood from a 5F aspiration catheter (Thrombuster, Kaneka Medics, Tokyo, Japan), the tip of which was placed in the internal carotid artery proximal to the occlusion balloon of the PercuSurge GuardWire. In each case, an average of 50 mL of blood was aspirated through this catheter. After aspiration, a mixture of 50 mL of saline (which contained 100 IU/mL of heparin) and 10 mL of contrast medium was slowly injected through the 5F catheter into the lumen to flush out possible remnants of debris to the external carotid artery. Using a TV monitor, we observed the flushed saline flow into the ipsilateral external carotid artery.
Fig 1.
Angioscopic observation with distal protection (A) with PercuSurge GuardWire and (B) with the Parodi Antiemboli System. CCA signifies common carotid artery; ICA, internal carotid artery; ECA, external carotid artery; PS, PercuSurge GuardWire; FC, fiber catheter AS-003 with aspiration catheter; PAES, Parodi Antiemboli System.
In 6 cases, the Parodi Antiemboli System was used. A 10F introducing catheter, which has an inflatable balloon near the tip, was guided via the femoral route and placed in the common carotid artery proximal to the stenosis. In brief, the Parodi Antiemboli System is composed of 2 balloon catheters. One balloon was placed and inflated at the proximal common carotid artery, and the other, at the origin of the external carotid artery. The larger 10F catheter for the common carotid artery has double lumens. One lumen was used for inflation and deflation of the balloon. The other larger and inner lumen is open at the tip of the catheter, so that the blood flow was reversed through this lumen, which was connected to a filter device outside the body. The debris was filtered, and the cleaned blood returned to venous circulation via the sheath placed in the femoral vein. In every case, the reversed flow was confirmed by injection of 3 mL of contrast medium into the distal lumen of the internal carotid artery after inflation of the 2 balloons. After this confirmation, carotid angioplasty with stent placement was performed (Fig 1B). The average time for flow reversal for carotid angioplasty with stent placement was 10 minutes 59 seconds.
After carotid angioplasty with stent placement was completed with one of the previously mentioned procedures, the inner lumen of the artery was observed with an angioscope. In each case, a fiber catheter AS-003 fiber scope (FiberTech Japan Corporation, Tokyo, Japan) with a diameter of 0.75 mm and length of 2800 mm was used. The fiber catheter was so small and fragile that it had to be inserted into the 5F catheter (Thrombuster) beforehand. The tip of the fiber was placed at the tip of the 5F catheter so as not to injure the arterial walls. The catheter harboring the fiber was guided up to the lesion and manipulated to obtain angioscopic observation (Fig 1A). Images were processed with an FT 201(F) imaging device (FiberTech Japan Corporation, Tokyo, Japan) and recorded on a hard disk recorder. When the PercuSurge GuardWire was used, after inflation of the balloon, the volume expander (Low-Molecular Dextran-H [Otsuka Pharmaceutical, Tokyo, Japan] or Hespander [Kyorin Pharmaceutical, Tokyo, Japan]) was manually infused to flush the blood to enable visual observation by the fiberscope. Care was taken not to flush forcefully.
When the Parodi Antiemboli System was used, the flow reversal was discontinued by a stop-cock during the angioscopic procedure. Even with the occlusion of both the common carotid and external carotid arteries, there was a slight blood flow to the occluded lumen from the distal internal carotid artery and the branches of the external carotid artery such as the superior thyroid artery. Before injection of the volume expander, the flow direction was confirmed by very gentle injection of 3 mL of contrast medium. In all 6 cases, the flow was almost stagnant, or to the branches of the external carotid artery, but not to the distal internal carotid artery. After confirmation of blood flow to the external carotid artery, the volume expander was very gently and continuously injected from the tip of the occlusion balloon catheter placed in the common carotid artery during endoscopic observation (Fig 1B). During the observation time (average, 4 minutes 32 seconds in the Parodi group), 20–60 mL of the volume expander was used.
For both methods, the observation was finished within a few minutes. After the entire procedure was completed, hemostasis was achieved and anticoagulation was discontinued.
Observation Time and Quality of the Images
There were no symptomatic ischemic complications in any of the 18 cases. The average observation time was 3 minutes 43 seconds, with a range from 30 seconds to 6 minutes 57 seconds (Table 1). The short observation times (from 30 seconds to 2 minutes) were due to an unclear view. The view was clear in 12 (67%) of the 18 cases, and in 4 cases, the blood was not well removed because of incomplete occlusion by the balloon or retrograde flow from the external carotid artery. In one case, the catheter tip was caught by the stent struts and the endoscope could not be brought up close enough to the distal lumen. In another case, the fiber tip was covered by a clot, which blocked clear vision.
Although angioscopy usually gives a wide-angle observation, the tip of the angioscope was not parallel to the longitudinal arterial axis. The view was limited to a given direction, and a full 360° observation of the luminal surface could not be obtained for any case.
Angioscopic Findings
The notable and useful findings during angioscopy are listed in Table 2, including the stent attachment to the wall and the protrusion of plaque between the stent struts. Such observations were limited to cases with a clear view.
TABLE 2:
Angioscopic findings in the patients
| Patient No. | Angioscopic Findings |
|---|---|
| 1 | Stent attachment to the wall |
| 2 | Intimal flap |
| 3 | Plaque protrusion |
| 4 | Stent attachment to the wall |
| 5 | Unclear view |
| 6 | Unclear view |
| 7 | Plaque protrusion, debris detaching form the plaque |
| 8 | Stent attachment to the wall |
| 9 | Stent attachment to the wall |
| 10 | Unclear view |
| 11 | Stent attachment to the wall |
| 12 | Unclear view |
| 13 | Stent attachment to the wall |
| 14 | Unclear view |
| 15 | Yellowish surface of carotid artery |
| 16 | Stent attachment to the wall, plaque protrusion |
| 17 | Unclear view |
| 18 | Stent attachment to the wall |
The carotid luminal surface and lesions in the endothelium, rupture of the fibrous cap, clots, debris detaching from plaque, floating debris, and stent struts in relation to the arterial wall were observed by angioscopy (Fig 2). In case 15, yellow streaking was observed on the endoluminal surface, which showed soft plaque on intravascular sonography performed before stent deployment.
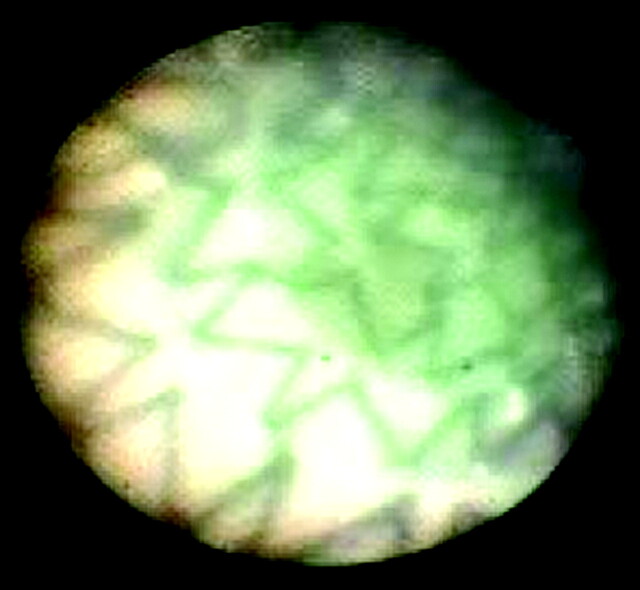
Fig 2.
Angioscopic image of the internal carotid artery in case 16 clearly shows stent attachment to the wall.
In case 7, debris detaching from plaque was observed, and in case 2, the dissecting intimal flap trembling in the flow was seen. These cases are described subsequently in more detail.
Detection of Ischemic Lesions by MR Imaging
The MR imaging used in this study was Signa Horizon Echospeed CV 1.5 T (GE Yokokawa Medical System, Tokyo, Japan). The MR imaging studies consisted of axial T2- and diffusion-weighted images with the echoplanar method in the following conditions: TR/TE, 10,000/69.2; section thickness, 5 mm; spacing, 1 mm; b value, 1000 s/mm2; and field of view, 30 cm.
All except one patient were examined by diffusion-weighted MR imaging before carotid angioplasty with stent placement, and the second diffusion-weighted MR imaging was performed 1 or 2 days after carotid angioplasty with stent placement. In one patient, the second MR imaging was performed 4 weeks after carotid angioplasty with stent placement due to mechanical trouble. Between the first MR imaging and carotid angioplasty with stent placement, no new ischemic events, such as transient ischemic attack or stroke, occurred. No angiography was performed during this period.
An experienced neuroradiologist who was not involved in the carotid angioplasty with stent placement or the neurologic evaluation assessed the ischemic lesions. The diffusion-weighed MR images obtained before and after carotid angioplasty with stent placement were compared, and the number of newly appearing ischemic lesions was counted. Newly appearing high-intensity spots were found in 8 (47%) of 17 cases (Table 1). As a mere reference, MR imaging data obtained in the patients who underwent carotid angioplasty with stent placement without angioscopy in the same period were noted. In the reference group, ischemic spots were found in 2 (15%) of 13 cases. However, several limitations did not justify the statistical comparison because of the selection bias. Angioscopy was performed in patients who had better collateral blood flow. Also, the protection methods were not randomly used in both groups.
Case Reports
Case A (Case 7)
Case A was a 73-year-old man. During carotid angioplasty with stent placement with the distal protection by PercuSurge GuardWire, debris was cleared by aspiration of 50 mL of blood and flushing with 50 mL of saline. Even after this aspiration and drainage of debris, some debris was still floating and several other small bits of debris came out of the crushed atherosclerotic wall. The floating debris and its detachment from the plaque could be observed by angioscopy (Fig 3A–F). Aspiration and irrigation of the debris were repeated. Anticoagulation with heparin (10,000 IU/day) was given for 2 additional days after carotid angioplasty with stent placement. In this case, diffusion-weighted MR imaging could not be performed 1 day after carotid angioplasty with stent placement because of mechanical problems. There were no ischemic lesions seen in the T1- and T2-weighted MR images 4 weeks after the procedure. There was no noticeable neurologic change, and the patient remained as before.
Fig 3.
Angioscopic image of the internal carotid artery in case 7. First debris (red arrowheads) detaches from the atherosclerotic carotid wall and then floats to the distal side with time (A–E). Next debris (yellow arrowheads) also detaches from the carotid wall (D–F).
Case B (Case 2)
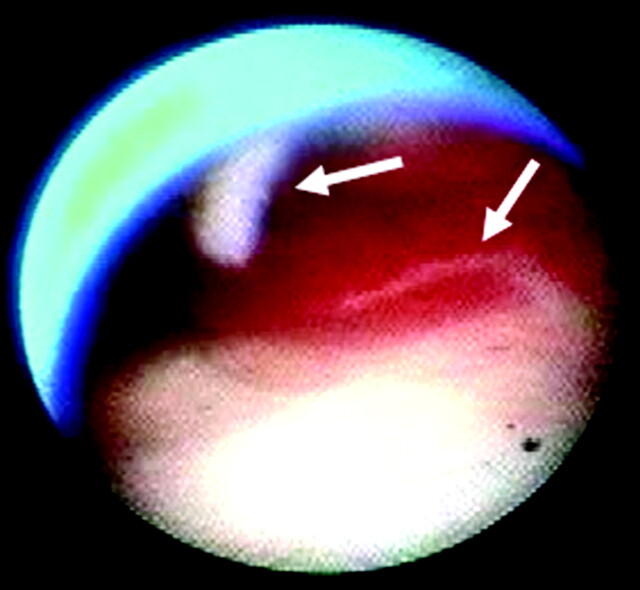
Case B was a 77-year-old man. The carotid angioplasty with stent placement with distal protection by PercuSurge GuardWire was performed, and at 2 locations, angioscopic observation showed the intimal flap fluttering from the arterial wall, which was assumed to be caused by the manipulation of the guidewire or the catheters (Fig 4). After this finding, intravascular sonography was repeated but failed to reveal any particular image of small intimal dissecting flaps. The flaps appeared to be too small to be treated with additional stent deployment, but the patient was more attentively observed with heparin (10,000 IU/day), which was continued for 2 days after carotid angioplasty with stent placement. Except in this case and case A (case 7), in our routine after carotid angioplasty with stent placement, no additional heparin was administered. Diffusion-weighted MR imaging performed 1 day after carotid angioplasty with stent placement showed ischemic lesions, but the patient nevertheless remained completely asymptomatic. There were no apparent adverse symptoms elicited by the flaps. No additional examination was performed to ascertain the fate of the flaps.
Fig 4.
Angioscopic image of the internal carotid artery proximal to the stent in case 2 shows the intimal flap (arrows) fluttering from the arterial wall.
Discussion
The application of angioscopy for interventional radiologic procedures has been reported mainly in the fields of coronary and peripheral intervention. Ours is the first report of angioscopic observation in clinical cases of carotid angioplasty with stent placement.
In 67% of our cases, the arterial luminal surface was observed by angioscopy, and lesions in the endothelium, rupture of the fibrous cap, clots, debris detaching from plaque, floating debris, and stent struts in relation to the arterial wall were observed. Intravascular sonography also showed sectional images of the inner surface of the vessel, but the images were not as precise as those of angioscopy and did not show the small dissection and debris found in the angioscopic images of cases A and B. This small dissection and the debris were not observed in angiography performed just after carotid angioplasty with stent placement, either. However, intravascular sonography does provide other important information, such as the nature of the intramural structure, which angioscopy cannot. Thus, using both procedures will provide more accurate observation of the lesions.
In coronary intervention, the color of the arterial wall is reported to be closely related to the extent of the atherosclerosis. Uchida et al (10) reported that atheromatous plaque can be observed as yellow or yellow glistening accumulations through the thin fibrous cap. In case 15, a yellow endothelium was observed, and intravascular sonography showed soft plaque with partial calcification.
The angioscope used in this study was 0.75 mm in diameter and could be guided up to the internal carotid artery along a 5F catheter after stent deployment. Uchida et al (11) reported that there are coronary angioscopes ranging from 0.3 to 3.2 mm in diameter. The smaller the angioscope, the weaker the power of the beam conveyed through the fibers, and the bluer the hue of the angioscopic image becomes. They recommend the appropriate diameter to be greater than 0.8 mm. In our experience, the angioscope was not manipulated directly because the tip could not be monitored by x-ray TV. The angioscope was moved indirectly by manipulating the coaxial 5F catheter.
The average ischemic time was 13 minutes 5 seconds for carotid angioplasty with stent placement and 3 minutes 43 seconds for angioscopy (Table 1). Although no neurologic worsening occurred in this series, a shorter occlusion time is better for brain circulation. In each case, the collateral blood flow was evaluated by preoperative diagnostic angiography. The longest observation was 6 minutes 57 seconds, and those cases with longer observation times had a good collateral blood flow, which was confirmed by the diagnostic angiography before carotid angioplasty with stent placement. In coronary angioscopy, the occlusion time is monitored by electrocardiogram and the occlusion is stopped when the QT interval starts to be prolonged (11). In this study, electroencephalography was not used. In the future, angioscopy during carotid angioplasty with stent placement, measurement of carotid back pressure, and electroencephalography are helpful monitors that should be frequently used during carotid endarterectomy.
The volume of the fluid flushed for clearing the view was as small as 100 mL and did not cause any complications in terms of overhydration or embolism.
Mizuno et al (3) reported that in coronary angioscopy, images in 84 (84%) of 100 patients showed a clear view. There was no explanation regarding the 16 cases that did not show a clear view. In our study, a clear view was obtained in 12 (67%) of 18 patients. The major reasons for poor views were that the balloon had not completely occluded the vessels because of incomplete inflation and accidental balloon withdrawal to the proximal larger arterial lumen.
In coronary and peripheral vasculature, complications with angioscopy, such as arterial damage, spasm, thrombosis, embolism, and fluid overload, have been reported. We did not experience any symptomatic complications in our series, but the diffusion-weighted MR imaging showed more frequent appearance of silent cerebral ischemic lesions in carotid angioplasty with stent placement with angioscopy (47%) than in carotid angioplasty with stent placement without angioscopy (15%). This difference was not statistically significant. Also, this comparison had limitations as we have mentioned. In previous reports, the ischemic lesions in MR imaging appeared in the range of 25%–43% in carotid angioplasty with stent placement itself (12, 13). In this study, diffusion-weighted MR imaging was performed after carotid angioplasty with stent placement, and whether endoscopic observation played a major role in producing ischemic lesions was not clear, but care must be taken not to produce microemboli during endoscopic observation.
Conclusion
Ours is the first clinical report of endoscopic observations of the carotid artery related to carotid angioplasty with stent placement. In 18 cases, the arterial luminal surface and lesions in the endothelium, rupture of the fibrous cap, clots, debris detaching from plaque, floating debris, and stent struts in relation to the arterial wall were observed by angioscope. In 2 cases, as a result of angioscopic findings, additional treatments such as repeated aspiration and additional anticoagulation were required. There were no symptomatic complications. Diffusion-weighted MR imaging after carotid angioplasty with stent placement and angioscopy showed asymptomatic ischemic lesions in 8 (47%) of 17 cases.
References
- 1.Yadav JS. Carotid stenting in high-risk patients: design and rationale of the SAPPHIRE trial. Cleve Clin J Med 2004;71:S45–46 [DOI] [PubMed] [Google Scholar]
- 2.Diethrich EB, Yoffe B, Kiessling JJ, et al. Angioscopy in endovascular surgery: recent technical advances to enhance intervention selection and failure analysis. Angiology 1992;43:1–10 [DOI] [PubMed] [Google Scholar]
- 3.Mizuno K, Miyamoto A, Satomura L, et al. Angioscopic coronary macromorphology in patients with acute coronary disorders. Lancet 1991;337:809–812 [DOI] [PubMed] [Google Scholar]
- 4.Grutenhuis JA, de Varies J, Tacl S. Angioscopy-guided placement of balloon-expandable stents in the treatment of experimental carotid aneurysms. Minim Invasive Neurosurg 1994;37:56–60 [DOI] [PubMed] [Google Scholar]
- 5.Henry M, Polydorou A, Henry I, Hugel M. Carotid angioplasty under cerebral protection with the PercuSurge GuardWire System. Catheter Cardiovasc Interv 2004;61:293–305 [DOI] [PubMed] [Google Scholar]
- 6.Kasirajan K, Schneider PA, Kent KC. Filter devices for cerebral protection during carotid angioplasty and stenting. J Endovasc Ther 2003;10:1039–1045 [DOI] [PubMed] [Google Scholar]
- 7.Adami CA, Scuro A, Spinamano L, et al. Use of the Parodi anti-embolism system in carotid stenting: Italian trial results. J Endovasc Ther 2002;9:147–154 [DOI] [PubMed] [Google Scholar]
- 8.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis: North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1991;325:445–453 [DOI] [PubMed] [Google Scholar]
- 9.Endarterectomy for asymptomatic carotid artery stenosis: Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995;273:1421–1428 [PubMed] [Google Scholar]
- 10.Uchida Y, Nakamura F, Tomaru T, et al. Prediction of acute coronary syndromes by percutaneous coronary angioscopy in patients with stable angina. Am Heart J 1995;130:195–203 [DOI] [PubMed] [Google Scholar]
- 11.Uchida Y. Cardioangioscopy. Tokyo: Medicalview;1995. :21–47
- 12.Schluter M, Tubler T, Steffens JC, Mathey GD, Schofer J. Focal ischemia of the brain after neuroprotected carotid artery stenting. J Am Coll Cardiol 2003;42:1014–1016 [DOI] [PubMed] [Google Scholar]
- 13.Flach HZ, Ouhlous M, Hendriks JM, et al. Cerebral ischemia after carotid intervention. J Endovasc Ther 2004;11:251–257 [DOI] [PubMed] [Google Scholar]