Abstract
OBJECTIVES: Although many previous reports have described age-related changes in regional cerebral blood flow (rCBF), none has used 3D stereotactic surface projection (3D-SSP) analysis, which is able to detect subtle and significant changes in rCBF.
METHODS: The subjects were 31 healthy volunteers (16 men and 15 women; 50–79 years of age) without abnormal MR imaging and MR angiographic findings, cognitive impairment, or depression. For each subject, rCBF was evaluated by using technetium Tc 99m-radiolabeled hexamethylpropyleneamine oxime single-photon emission CT. Maps of rCBF were compared among different age groups (50–59, 60–69, and 70–79 years of age) by using 3D-SSP. The mean z score for each gyrus was calculated for each age group by using a recently developed stereotactic extraction estimation method.
RESULTS: Significant age-related reductions in rCBF were seen in the bilateral cingulate gyri, left inferior gyrus, bilateral medial frontal gyri, left subcallosal gyrus, and left superior temporal gyrus. Extensive and constant reduction in rCBF occurred with increasing age in the bilateral anterior cingulate gyri, and the mean z score for this region was the highest among all the regions examined.
CONCLUSION: The 3D-SSP analysis revealed that the greatest reduction in rCBF occurred within the bilateral anterior cingulate gyri in normal middle-aged and older subjects.
Many previous reports have described age-related changes in regional cerebral blood flow (rCBF) (1–9). In the cingulate gyrus, frontal lobe, parietal lobe, and temporal lobe, rCBF has been reported to diminish with age in normal subjects, even in relatively young subjects (20–40 years of age). Nevertheless, there is at least one report that the reduction in rCBF was negligible in subjects >40 years of age (1). Although several reports describe the anterior cingulate as the main site within which rCBF is reduced with normal aging (2–5), other studies failed to demonstrate any reduction in rCBF in this area (6, 7). These discrepancies may be attributable to differences in the criteria used to select subjects and the diversity of the image analysis methods. We strictly selected healthy middle-aged and older subjects who had undergone 1.5T MR imaging and MR angiography (MRA) and who had no brain lesion or major vessel stenosis, both of which are factors that influence rCBF. This is in contrast to many previous studies in which subjects were not assessed by using MR imaging (1, 5–8). Moreover, in no studies of rCBF has MRA been used to evaluate major vessels. We also assessed the mental status of all subjects.
Some authors have evaluated the relationship between rCBF and normal aging by using statistic parametric mapping (SPM) (2, 3, 5), but, to our knowledge, no one has used 3D stereotactic surface projection (3D-SSP) analysis. 3D-SSP analysis is a fully automated, user-independent method for data extraction that allows (1) pixel-by-pixel analysis of cerebral perfusion, (2) anatomic normalization of individual single photon emission CT (SPECT) data to the standard brain, and (3) comparison of regional voxel data between 2 different groups. 3D-SSP analysis provides a reliable and objective evaluation of the severity, extent, and localization of cortical perfusion abnormalities in 2 groups (10–14). The difference between 3D-SSP analysis and SPM is the intrinsic performance of the algorithms used by each method, including image-matching fusion, pixel interpolation, and axial rotation. The 3D-SSP algorithm uses prior knowledge of brain anatomy and deforms the shape of the brain along the direction of cortical projection fibers (15). This process is thought to be particularly advantageous for standardizing atrophied brains (15). Because brain atrophy occurs with aging in healthy subjects, we believe that 3D-SSP is an excellent method for evaluating changes in rCBF owing to normal aging.
Materials and Methods
Subjects
Thirty-one healthy subjects (15 men and 16 women; 10 in their 50s, 12 in their 60s, and 9 in their 70s) were recruited from among persons who received screening for brain health (brain check-up) at Shimane Institute of Health Science for the purpose of making a normal control data base for 3D-SSP analysis by using the following criteria: (1) 50–79 years of age; (2) physically healthy and free of psychiatric disorders; (3) no history of stroke; (4) no findings of brain atrophy, asymptomatic white matter lesion, or stroke lesion by T1, T2, fluid-attenuated inversion recovery, or T2-weighted MR imaging (1.5-Tesla; Siemens Symphony, Munich, Germany); (5) no findings of stenosis of major vessels or aneurysm by head MR angiography; (6) score of >35 points on the Okabe Simplified Intelligence Scale (Okabe Score) (16); and (7) score of <40 points on the Zung Self-Rating Depression Scale (SDS) (17). The Okabe test is a modified and simplified version of the Wechsler Memory Scale and consists of 4 subscales: information, mental control, digit span, and associative learning. The full scores on these 4 subscales total 60 points. The classification criteria are 20–29 points, mild dementia (or notable mental aging); 10–19 points, moderate dementia; <10 points, severe dementia (16). The 20 items of the SDS test are scored on a standard 4-point scale (1–4) for each item, with potential results ranging from 20 to 80. A score of >40 (raw score) is considered to be depressive state. The results of the Okabe test and SDS are shown in Table 1.
TABLE 1:
Mean age, verbal intelligence, and SDS in each age group
| Age group (y) | 50–59 | 60–69 | 70–79 |
| Number (M:F) | 10 (4:6) | 12 (7:5) | 9 (5:4) |
| Mean age (y) | 55.2 ± 3.0 | 63.8 ± 2.9 | 73.2 ± 2.4 |
| Okabe’s Scale, total | 48.9 ± 3.0 | 47.0 ± 5.7 | 45.4 ± 6.9 |
| Information | 18.4 ± 1.6 | 17.5 ± 1.7 | 16.4 ± 1.9 |
| Mental control | 14.5 ± 1.6 | 12.4 ± 3.3 | 12.8 ± 2.9 |
| Digit span | 9.0 ± 1.6 | 8.9 ± 1.5 | 9.2 ± 0.7 |
| Associate learning | 7.0 ± 3.9 | 8.2 ± 3.6 | 7.0 ± 3.6 |
| SDS | 30.8 ± 5.9 | 28.9 ± 4.3 | 29.7 ± 5.7 |
Note.—Okabe Scale indicates Okabe’s Simplified Intelligence Scale (60-point scale); SDS, Zung Self-Rating Depression Scale (80-point scale).
SPECT Imaging
Regional CBF images were obtained by SPECT by using technetium Tc 99m-hexamethylpropyleneamine oxime (HMPAO). All subjects were studied in the supine resting position with closed eyes in a silent room from approximately 16:00 to 17:00 hours. The SPECT images were acquired on a PRISM IRIX (Marconi, Cleveland, OH), 3-headed SPECT camera with ultrahigh-resolution fan-beam collimators. The data acquisition parameters were 128 × 128 matrices (2.33-mm pixel size), 5° per step, 72 views, 30 seconds per view, 2× zoom, and a 140-keV (± 7.5) energy window. The subjects were injected with 370 MBq of technetium Tc 99m-HMPAO in the antecubital vein of the right arm, and the dynamic data acquisition for the Patlak method was performed. Subjects were then injected with 370 MBq technetium Tc 99m-HMPAO again for SPECT acquisition. Reconstruction was performed by filtered back projection by using a Butterworth filter (cut-off frequency, 0.25 cycle/pixel) and ramp filters with attenuation correction by using the Chang 8-order method (μ = 0.09/cm).
3D-SSP Analysis
Because the size and form of each individual’s head is different, it is difficult to compare the SPECT findings between 2 different groups reliably and objectively; however, 3D-SSP analysis enables us to make statistical comparisons easily. Analysis by 3D-SSP anatomically normalizes the individual SPECT data to the standard brain and compares the regional voxel data between 2 different groups (10–14). This procedure was performed by the interface software iSSP (version 3.5, Nihon Medi-Physics Corporation, Nishinomiya, Japan).
First, stereotactic anatomic standardization was performed. Rotational correction and centering in 3 dimensions of the SPECT dataset were conducted, followed by realignment to the anterior commissureposterior commissure line. The anterior commissure–posterior commissure line was estimated by iterative matching between the individual images and the template from the Talairach and Tournoux atlas (18) by using mutual information. Differences in size between the individual brain and standard template were eliminated by linear scaling. Regional anatomic differences between the individual and the standard atlas brain were thus minimized by automated nonlinear warping.
In the data extraction step, >16,000 surface pixels covering the lateral and medial surfaces of both hemispheres were predetermined with stereotactic coordinates. The peak cortical activity perpendicular to these pixels was projected onto the surface pixels. The pixel values of an individual’s image set were normalized to the mean global CBF before the analysis. Therefore, each brain was stereotactically transformed into a standard surface image format, which enabled us to compare the resultant cortical projections between the 2 groups. To demonstrate the differences in the rCBF patterns, 2-sample t test values were calculated on a pixel-by-pixel basis between 2 groups and then transformed to z values by a probability integral transformation.
Differences in the rCBF Patterns between Age Groups
By using the SPECT data of the subjects, we performed 3D-SSP analysis to compare age groups. To quantify perfusion deficits, pixel-by-pixel z scores were used. z scores ([mean of normalized pixel value of one age group] − [mean normalized pixel value of other age group])/[SD of one age group]) were calculated for each surface pixel. A positive z score represents a reduced rCBF in the one group relative to the other group. We used a z score of 2 as the cutoff value. To assess the rCBF reduction quantitatively, the mean z scores for the each lobe and gyrus level classification was calculated by the recent developed stereotactic extraction estimation (SEE) method (19). Mean z score for each gyrus was automatically measured (average z value of the coordinates with a z value that exceeds 0 of the threshold value).
This study was approved by the local ethics committee of Shimane University Hospital. Written informed consent was obtained from all subjects.
Results
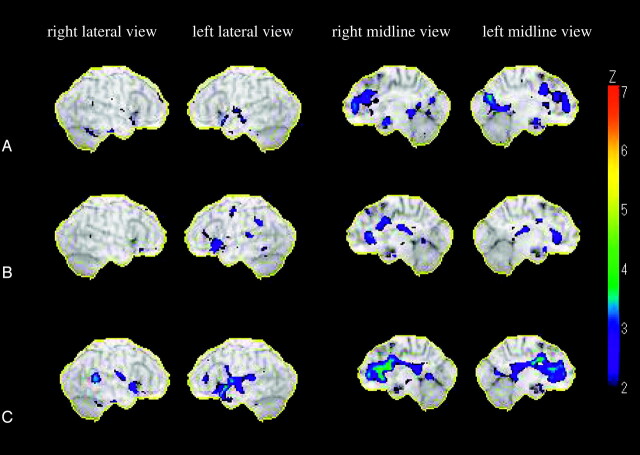
Statistical maps (Fig 1) showed relative reduction of rCBF between each age group (z score ≦2). The extensive and constant reduction of the rCBF was significantly observed in the bilateral anterior cingulate gyri between each age group. The age-related reduction of rCBF in the left superior temporal gyrus and left subcallosal gyrus was also constantly observed between each age group (Fig 1A–C). In addition, there were decreases in the rCBF of the bilateral medial frontal gyri and bilateral posterior cingulate gyri in the 60–69-year-old group and the 70–79-year-old group compared with those in the 50–59-year-old group (Fig 1A, -C). The rCBF of the left inferior frontal gyrus in the 70–79-year-old group was significantly lower than that of the 50–59-year-old group or the 60–69-year-old group (Fig 1B, -C). There were also decreases in the rCBF of right superior and inferior temporal gyrus in the 70–79-year-old group compared with those in the 50–59-year-old group (Fig 1C).
Fig 1.
Statistical maps analyzed by 3D SSP. The color of the outer counter corresponds to a Z score of 7.
A, Relative decreases of rCBF (z score ≦2) in subjects 60–69 years of age compared with rCBF in subjects 50–59 years of age. The most extensive reduction in rCBF was observed in the right anterior cingulate gyrus. There were also decreases in rCBF in the left anterior cingulate gyrus, bilateral posterior cingulate gyri, bilateral medial frontal gyri, bilateral subcallosal gyri, left superior temporal gyrus, and left cuneus.
B, Relative decreases of rCBF (z score ≦2) in subjects 70–79 years of age compared with rCBF in subjects 60–69 years of age. The most extensive reduction in rCBF was observed in the bilateral anterior cingulate gyri. There were also decreases in rCBF in the left inferior frontal gyrus, left subcallosal gyrus, left supramarginal gyrus, and left superior temporal gyrus.
C, Relative decreases of rCBFs (z score ≦2) in subjects 70–79 years of age compared with rCBF in subjects 50–59 years of age. The most extensive reduction in rCBF was observed in the bilateral anterior cingulate gyri. There were also decreases in rCBF in the left inferior frontal gyrus, left subcallosal gyrus, left supramarginal gyrus, and left superior temporal gyrus.
Table 2 shows mean z scores for the each lobe and gyrus level classification between each age group. Mean z score of bilateral anterior cingulate gyri was relatively higher than other lobe and gyrus. The z scores for all the significant reductions of rCBF shown above were within 4.5.
TABLE 2:
Mean z score for each region between each age generation
| Relative Decrease of rCBF, Age (y) |
|||
|---|---|---|---|
| 60–69 vs 50–59 | 70–79 vs 60–69 | 70–79 vs 50–59 | |
| Superior frontal gyrus | |||
| L | 0.80 ± 0.51 | 1.59 ± 0.45 | 0.64 ± 0.46 |
| R | 0.73 ± 0.59 | 1.49 ± 0.35 | 0.67 ± 0.41 |
| Middle frontal gyrus | |||
| L | 0.60 ± 0.43 | 1.37 ± 0.32 | 0.99 ± 0.58 |
| R | 0.66 ± 0.53 | 1.49 ± 0.44 | 0.66 ± 0.48 |
| Inferior frontal gyrus | |||
| L | 0.71 ± 0.53 | 1.97 ± 0.57 | 1.53 ± 0.72 |
| R | 0.53 ± 0.37 | 1.39 ± 0.38 | 1.14 ± 0.64 |
| Medial frontal gyrus | |||
| L | 1.38 ± 0.84 | 1.62 ± 0.58 | 1.33 ± 0.99 |
| R | 1.37 ± 0.94 | 1.30 ± 0.16 | 1.37 ± 0.97 |
| Subcallosal gyrus | |||
| L | 1.30 ± 1.04 | 2.08 ± 0.54 | 1.73 ± 0.59 |
| R | 1.47 ± 1.11 | 1.22 ± 0.13 | 0.83 ± 0.37 |
| Superior parietal lobule | |||
| L | 0.24 ± 0.17 | 1.27 ± 0.17 | 0 |
| R | 0 | 0 | 0 |
| Inferior parietal lobule | |||
| L | 0.12 ± 0.12 | 1.50 ± 0.35 | 0.63 ± 0.48 |
| R | 0.63 ± 0.55 | 1.12 ± 0.06 | 0.56 ± 0.40 |
| Superior temporal gyrus | |||
| L | 1.19 ± 0.72 | 1.61 ± 0.43 | 1.97 ± 0.82 |
| R | 1.03 ± 0.69 | 1.37 ± 0.24 | 1.33 ± 0.96 |
| Middle temporal gyrus | |||
| L | 0.95 ± 0.74 | 1.44 ± 0.34 | 1.13 ± 0.72 |
| R | 0.42 ± 0.28 | 1.47 ± 0.27 | 0.82 ± 0.69 |
| Inferior temporal gyrus | |||
| L | 0.76 ± 0.42 | 1.56 ± 0.47 | 0.82 ± 0.45 |
| R | 0.92 ± 0.66 | 1.27 ± 0.19 | 1.20 ± 0.65 |
| Cuneus | |||
| L | 1.29 ± 0.92 | 0 | 0.71 ± 0.63 |
| R | 0.93 ± 0.74 | 1.08 ± 0.04 | 0.36 ± 0.28 |
| Lingual gyrus | |||
| L | 0.70 ± 0.58 | 0 | 0.84 ± 0.55 |
| R | 0.49 ± 0.47 | 1.39 ± 0.25 | 0.70 ± 0.38 |
| Anterior cingulate gyrus | |||
| L | 1.55 ± 0.61 | 1.87 ± 0.59 | 2.51 ± 0.81 |
| R | 1.79 ± 0.73 | 2.02 ± 0.60 | 2.89 ± 0.99 |
| Posterior cingulate gyrus | |||
| L | 1.31 ± 1.11 | 1.40 ± 0.27 | 1.42 ± 0.70 |
| R | 1.20 ± 0.81 | 1.56 ± 0.26 | 1.51 ± 0.48 |
| Parahippocampal gyrus | |||
| L | 0.44 ± 0.24 | 1.43 ± 0.29 | 0.97 ± 0.52 |
| R | 1.26 ± 0.65 | 1.73 ± 0.59 | 0.78 ± 0.45 |
| Superior occipital gyrus | |||
| L | 0.06 ± 0 | 0 | 0 |
| R | 0.76 ± 0.28 | 0 | 0.29 ± 0.15 |
| Middle occipital gyrus | |||
| L | 0.47 ± 0.44 | 0 | 0.45 ± 0.19 |
| R | 0.68 ± 0.39 | 0 | 0.56 ± 0.45 |
| Inferior occipital gyrus | |||
| L | 0 | 0 | 0 |
| R | 0.05 ± 0 | 0 | 0 |
Discussion
Although this study revealed age-related reductions in rCBF at several sites, the most marked reduction in rCBF was in the anterior cingulate gyrus, particularly between subjects in their 50s and those in their 70s. Several authors have identified an association between aging and reduced rCBF in the anterior cingulate gyrus. Waldermar et al (8) evaluated rCBF in 53 healthy subjects (21–83 years of age) by using technetium Tc 99m-radiolabeled-HMPAO SPECT and found that rCBF in the upper frontal cortex, superior frontal gyrus, cingulate gyrus, and upper parietal cortex decreased with increasing age. Some studies of age-related changes of rCBF used ethyl cysteinate dimer SPECT. Nakano et al (2) performed rCBF measurements by using the Patlak Plot method in 53 normal volunteers (18–87 years of age). Their results, analyzed by SPM, revealed significant age-related decreases in rCBF in the limbic area and in the associative cortices, such as the prefrontal cortices, anterior cingulate gyri, and insular cortices or bilateral temporal poles. Van Laere et al (3) measured rCBF in 89 healthy volunteers (20–81 years of age) and reported that rCBF declined with age in the anterior cingulate gyrus, the bilateral basal ganglia, and the left prefrontal, left lateral frontal, left superior temporal, and insular cortices. Tanaka et al (4) also reported significant age-related decreases in rCBF in the anterior and posterior cingulate cortex, superior prefrontal and parietal cortex, striatum, and hippocampus in 48 normal subjects (22–95 years of age). Martin et al (5) used positron-emission tomography to demonstrate age-related decreases in rCBF in the cingulate, parahippocampal, superior temporal, medial frontal, and posterior cortices bilaterally and in the left insular and left posterior prefrontal cortices in 30 normal subjects (30–85 years of age).
There have been some contradictory results related to the association between aging and a reduction in rCBF in the anterior cingulate gyrus. Pagani et al (6) assessed rCBF in 50 healthy subjects (31–78 years of age) by using HMPAO-SPECT, and found a significant decrease in rCBF with increasing age in the temporocingulate cortex, but not in the anterior cingulate gyrus. Mozley et al (1) evaluated rCBF in 44 healthy subjects (19–73 years of age) by using HMPAO-SPECT and found age-related decreases in rCBF in many parts of the gray matter, including the anterior cingulate gyrus; however, most of the age-related changes were observed in young adults, and the reduction in rCBF was negligible after middle age. Krausz et al (7) compared rCBF in younger healthy subjects (26–47 years of age) and older healthy subjects (47–71 years of age) by using HMPAO-SPECT. Their analysis was performed by applying 3 preformed templates, each of which contained delineated regions of interest, to 3 transaxial brain sections at approximately 4, 6, and 7 cm above the orbitomeatal line. They found no age-related change in rCBF after normalizing the data to rCBF in the cerebellum, whereas significantly increased uptake ratios were observed in the cingulate cortex on the basis of the data normalized to the whole section. Jones et al (9) compared rCBF in healthy subjects 50–72 years of age with those 72–92 years of age and found no significant age-related differences in whole-brain HMPAO uptake, except in the lateral ventricular regions. The results of the study by Krausz et al (7) are exceptional among the studies discussed here: these authors found that rCBF in the anterior cingulate gyrus was reduced with aging in normal subjects, including subjects between 20 and 40 years of age, but that the reduction in rCBF was unremarkable among subjects >50 years of age. In the present study, the age-related reduction in rCBF was extensive in the anterior cingulate in normal subjects 50–79 years of age. All z scores for reductions in rCBF were within 4.5 in the present study, which suggested that the degree of reduction in rCBF was not severe, but significant. To our knowledge, there have been no reports of the relationship between normal aging and rCBF based on the use of 3D-SSP HMPAO SPECT. In light of the fact that 3D-SSP analysis allows pixel-by-pixel analysis of cerebral perfusion and provides a reliable and objective evaluation of the severity and localization of differences in cortical perfusion, this method makes it possible to detect subtle changes in rCBF with aging that might not be detectable by using other methods. Another advantage of 3D-SSP HMPAO SPECT over other methods is the superior mean of standardization of atrophied brains (15).
Some authors reported that patients with pain or psychiatric disorders following anterior cingulotomy exhibited little cognitive impairment (20, 21). The anterior cingulate gyrus is part of the largest formation of the limbic system and is thought to be associated with emotion, attention (22), memory, initiation, motivation, goal-directed behaviors (23), and working memory (24). We believe that reduced rCBF in the anterior cingulate gyrus may not affect general cognition but might cause subtle cognitive impairment with aging.
In conclusion, we observed an age-related reduction in rCBF mainly in the bilateral cingulate gyri, left inferior gyrus, bilateral medial frontal gyri, left subcallosal gyrus, and left superior temporal gyrus in healthy subjects. The greatest reduction in rCBF occurred within the bilateral anterior cingulate gyri.
Acknowledgments
The study was partly supported by a research grant from Nihon Medi-Physics.
References
- 1.Mozley PD, Sadek AM, Alavi A, et al. Effects of aging on the cerebral distribution of technetium-99m hexamethylpropylene amine oxime in healthy humans. Eur J Nucl Med 1997;24:754–761 [DOI] [PubMed] [Google Scholar]
- 2.Nakano S, Asada T, Matsuda H, et al. Effects of healthy aging on the regional cerebral blood flow measurements using 99mTc-ECD SPECT assessed with statistical parametric mapping. Nippon Ronen Igakkai Zasshi 2000;37:49–55 [DOI] [PubMed] [Google Scholar]
- 3.Van Laere K, Versijpt J, Audenaert K, et al. 99mTc-ECD brain perfusion SPET: variability, asymmetry and effects of age and gender in healthy adults. Eur J Nucl Med 2001;28:873–887 [DOI] [PubMed] [Google Scholar]
- 4.Tanaka F, Vines D, Tsuchida T, et al. Normal patterns on 99mTc-ECD brain SPECT scans in adults. J Nucl Med 2000;41:1456–1464 [PubMed] [Google Scholar]
- 5.Martin AJ, Friston KJ, Colebatch JG, Frackowiak RSJ. Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab 1991;11:684–689 [DOI] [PubMed] [Google Scholar]
- 6.Pagani M, Salmaso D, Jonsson C, et al. Regional cerebral blood flow as assessed by principal component analysis and 99mTc-HMPAO SPET in healthy subjects at rest: normal distribution and effect of age and gender. Eur J Nucl Med 2002;29:67–75 [DOI] [PubMed] [Google Scholar]
- 7.Krausz Y, Bonne O, Gorfine M, et al. Age-related changes in brain perfusion of normal subjects detected by 99mTc-HMPAO SPECT. Neuroradiology 1998;40:428–434 [DOI] [PubMed] [Google Scholar]
- 8.Waldemar G, Hasselbalch SG, Andersen AR, et al. 99mTc-d,l-HMAPO and SPECT of the brain in normal aging. J Cereb Blood Flow Metab 1991;11:508–521 [DOI] [PubMed] [Google Scholar]
- 9.Jones K, Johnson KA, Becker JA, et al. Use of singular value decomposition to characterize age and gender differences in SPECT cerebral perfusion. J Nucl Med 1998;39:965–973 [PubMed] [Google Scholar]
- 10.Minoshima S, Frey KA, Koeppe RA, et al. A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med 1995;36:1238–1248 [PubMed] [Google Scholar]
- 11.Burdette JH, Minoshima S, Vander Borght T, et al. Alzheimer disease: improved visual interpretation of PET images by using three-dimensional stereotaxic surface projections. Radiology 1996;198:837–843 [DOI] [PubMed] [Google Scholar]
- 12.Honda N, Machida K, Matsumoto T, et al. Three-dimensional stereotactic surface projection of brain perfusion SPECT improves diagnosis of Alzheimer’s disease. Ann Nucl Med 2003;17:641–648 [DOI] [PubMed] [Google Scholar]
- 13.Hanyu H, Shimuzu S, Tanaka Y, et al. Cerebral blood flow patterns in Binswanger’s disease: a SPECT study using three-dimensional stereotactic surface projections. J Neurol Sci 2004;220:79–84 [DOI] [PubMed] [Google Scholar]
- 14.Kaneko K, Kuwabara Y, Sasaki M, et al. Posterior cingulate hypoperfusion in Alzheimer’s disease, senile dementia of Alzheimer type, and other dementias evaluated by three-dimensional stereotactic surface projections using Tc-99m HMPAO SPECT. Clin Nucl Med 2004;29:362–366 [DOI] [PubMed] [Google Scholar]
- 15.Ishii K, Willoch F, Minoshima S, et al. Statistical brain mapping of 18F-FDG PET in Alzheimer’s disease: validation of anatomic standardization for atrophied brains. J Nucl Med 2001;42:548–557 [PubMed] [Google Scholar]
- 16.Fukunisi I, Okabe S, Nakagawa T, Hosokawa K. The assessment of intelligence function of aged chronic schizophrenia. Jpn J Psychiatry Neurol. 1990;44:503–509 [DOI] [PubMed] [Google Scholar]
- 17.Zung WW. A self-rating depression scale. Arch Gen Psychiatry 1965;12:63–70 [DOI] [PubMed] [Google Scholar]
- 18.Talairach J, Tournoux P. Co-planar stereotactic atlas of the human brain. New York: Thieme;1988
- 19.Mizumura S, Kumita S, Cho K, et al. Development of quantitative analysis method for stereotactic brain image: assessment of reduced accumulation in extent and severity using anatomical segmentation. Ann Nucl Med 2003;17:289–295 [DOI] [PubMed] [Google Scholar]
- 20.Kim CH, Chang JW, Koo MS, et al. Anterior cingulotomy for refractory obsessive-compulsive disorder. Acta Psychiatr Scand 2003;107:283–290 [DOI] [PubMed] [Google Scholar]
- 21.Dougherty DD, Baer L, Cosgrove GR, et al. Prospective long-term follow-up of 44 patients who received cingulotomy for treatment-refractory obsessive-compulsive disorder. Am J Psychiatry 2002;159:269–275 [DOI] [PubMed] [Google Scholar]
- 22.Cohen RA, Paul R, Zawacki TM, et al. Emotional and personality changes following cingulotomy. Emotion 2001;1:38–50 [DOI] [PubMed] [Google Scholar]
- 23.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain 1995;118:279–306 [DOI] [PubMed] [Google Scholar]
- 24.Osaka N, Osaka M, Kondo H, et al. The neural basis of executive function in working memory: an fMRI study based on individual differences. Neuroimage 2004;21:623–631 [DOI] [PubMed] [Google Scholar]