Abstract
BACKGROUND AND PURPOSE: We evaluated the differences between percutaneous ethanol injection with and without aspiration of ethanol-mixed fluid for treatment of benign cystic thyroid nodules.
METHODS: We examined 60 patients with benign cystic thyroid nodules confirmed by fine-needle aspiration biopsy and divided them into 2 groups according to nonaspiration (group A, n = 30) or aspiration (group B, n = 30) of ethanol-mixed fluid after intracystic ethanol injection. We evaluated in both groups the complete disappearance of the cystic portion of the thyroid nodule on follow-up ultrasonography (first follow-up ultrasonography; mean, 4.6 months in group A; mean, 4.4 months in group B) (chi-square test), side effects or complications during and after the procedure (chi-square test), and the total procedure time (Student t test).
RESULTS: Most patients showed complete disappearance of the cystic portion of the thyroid nodule (group A, n = 29; group B, n = 28), and they revealed no recurrence on follow-up ultrasonography. There was no statistical difference in the success rates between group A and group B (P > .05). Pain, the most common side effect, and other mild side effects or complications occurred in small numbers of patients in each group, but there was no significant difference in side effects or complications between the 2 groups (P > .05), except for intracystic hemorrhage (P < .05) and the complaint of all group B patients due to a double puncture (P < .001). The total procedure time was nearly double in group B than in group A because of the additional procedures, such as complete evacuation of the ethanol-mixed fluid and the 10-minute compression.
CONCLUSION: Percutaneous ethanol injection without aspiration of ethanol-mixed fluid seems to be the preferable method of treatment of benign cystic thyroid nodules from the perspective of both the physician and the patient.
Sonographically guided percutaneous ethanol injection, an alternative procedure to surgical or medical therapy, is a safe and effective therapeutic tool for the treatment of benign cystic thyroid nodules. Many published studies have reported the efficacy of percutaneous ethanol injection for treating benign thyroid nodules (1–8). In general, 2 sclerotic procedures have been described in those reports: the first, using simple ethanol instillation after aspiration of fluid from a cystic thyroid nodule through the same needle (1–5); and the second, performing the complete evacuation of the ethanol-mixed fluid from the cystic thyroid nodule several minutes after the first method (6–8). To the best of our knowledge, the difference between these 2 methods in ethanol sclerotherapy of benign cystic thyroid nodules has not been reported. The purpose of our study was to compare the sclerotic results, side effects or complications, and the total procedure time of these 2 methods.
Methods
Sixty patients with confirmed benign cystic thyroid nodules by aspiration biopsy underwent ethanol sclerotherapy and follow-up ultrasonography. These patients included 48 women and 12 men (age range, 16–75 years; mean age, 44.7 years).
For ultrasonography, a 128 XP/10 scanner (Acouson, Mountain View, CA) was used with 7-MHz linear probes. In group A (n = 30), using sonographic guidance, a 23-gauge needle was inserted into the cystic portion of the thyroid nodule with the patient in the supine position without local anesthetic after a simple alcohol skin scrub in all patients. In cases with poor aspiration using a 23-gauge needle, we changed the needle size to 21- or 18-gauge. After nearly complete evacuation of fluid from the cystic portion of the thyroid nodule, absolute ethanol (99.9%), in the same or a lesser amount than that of the aspirates, was instilled through the first puncture needle after exchange of the syringe; in cases in which the amount of aspirate was 20 mL. In cases in which the amount of the aspirates was 20 mL, we infused 20 mL of absolute. A positional change with gentle compression to the puncture site was then performed for 10 minutes. In group B (n = 30), after the same procedure as in group A, the ethanol-mixed fluid was completely evacuated through the second puncture needle, and there was then gentle compression to the puncture site for 10 minutes. The amount of total ethanol infusion did not exceed 20 mL in any of the patients in either group, except for one patient. In this one patient from group A, 135 mL of dark, bloody fluid was evacuated and 30 mL of ethanol was then instilled.
Follow-up ultrasonography using the 128 XP/10 scanner or an HDI 5000 scanner (Advanced Technology Laboratories, Bothell, WA) was performed between 1 and 24 months (first follow-up ultrasonography; mean, 4.6 months in group A and 4.4 months in group B) after sclerotherapy, and more than 1 follow-up ultrasonogram was obtained from all patients at least 6 months after percutaneous ethanol injection.
The sclerotherapy results were evaluated on follow-up ultrasonography according to the complete or incomplete disappearance of the cystic portion of the benign cystic thyroid nodules. Side effects or complications in the 2 groups were recorded during the procedure and during follow-up periods, and the total procedure time of the 2 groups was calculated.
We evaluated the differences between the 2 groups in the sclerotherapy results (chi-square test), the side effects or complications (chi-square test), and the total procedure time (Student t test).
Results
The volume of the first aspirates, infused ethanol and ethanol-mixed aspirates from the cystic thyroid nodules, first ultrasonographic follow-up periods, total procedure time, therapeutic results, and side effects and complications in the 2 groups are described in Tables 1 and 2 (also see Figs 1 and 2).
TABLE 1:
Clinical data and results of sclerotherapy in group A
| Group A | Age (y)/Sex | First Aspirates (ml) | Infused Ethanol (ml) | Total Procedure (min) | First Follow-up Ultrasonography (mo) | Therapeutic Result | Complication |
|---|---|---|---|---|---|---|---|
| 1 | 39/F | 2 | 2 | 14 | 2 | + | |
| 2 | 51/F | 7 | 7 | 15 | 1 | + | |
| 3 | 49/M | 2 | 2 | 15 | 8 | + | |
| 4 | 44/F | 5 | 5 | 17 | 15 | + | |
| 5 | 37/F | 10 | 7 | 16 | 15 | + | Headache, dizziness |
| 6 | 43/M | 12 | 9 | 16 | 8 | + | |
| 7 | 43/F | 5 | 5 | 16 | 5 | + | |
| 8 | 43/F | 4 | 4 | 17 | 5 | + | Mild pain |
| 9 | 58/F | 8 | 8 | 16 | 3 | + | Moderate pain |
| 10 | 36/F | 6 | 6 | 15 | 15 | + | Mild pain |
| 11 | 44/M | 15 | 10 | 18 | 3 | + | Mild pain |
| 12 | 27/F | 7 | 6 | 15 | 7 | + | |
| 13 | 38/F | 5 | 5 | 17 | 3 | + | Facial flushing |
| 14 | 47/F | 13 | 12 | 15 | 1 | + | Mild pain |
| 15 | 49/F | 4 | 4 | 14 | 2 | + | |
| 16 | 28/F | 3.5 | 3.5 | 14 | 8 | + | |
| 17 | 38/F | 7 | 7 | 15 | 4 | + | |
| 18 | 43/M | 29 | 16 | 20 | 2 | + | |
| 19 | 32/F | 8 | 8 | 16 | 2 | + | Intracystic hemorrhage |
| 20 | 44/F | 2 | 3 | 20 | 2 | + | Mild pain |
| 21 | 64/F | 2 | 2 | 13 | 3 | + | Mild pain |
| 22 | 46/M | 4 | 4 | 15 | 2 | + | |
| 23 | 47/M | 40 | 15 | 20 | 4 | + | |
| 24 | 75/F | 20 | 12 | 17 | 3 | + | |
| 25 | 47/M | 10 | 7 | 16 | 2 | + | |
| 26 | 35/F | 20 | 17 | 19 | 2 | + | Mild pain |
| 27 | 45/F | 5 | 5 | 15 | 1 | + | Mild pain, drunken sense |
| 28 | 16/M | 96 | 20 | 23 | 2 | + | Facial flushing, drunken sense |
| 29 | 70/F | 135 | 30 | 29 | 3 | + | Perithyroidal leakage |
| 30 | 47/F | 8 | 8 | 15 | 2 | − | |
| Mean | 44.2 | 16.5 | 8.3 | 16.8 | 4.6 |
Note.—+ indicates complete disappearance of the cystic portion of the thyroid nodule; −, incomplete disappearance of the cystic portion of the thyroid nodule.
TABLE 2:
Clinical data and results of sclerotherapy in group B
| Group B | Age (y)/Sex | First Aspirates (ml) | Infused Ethanol (ml) | Ethanol Mixed Aspirates | Total Procedure (min) | First Follow-up Ultrasonography (mo) | Therapeutic Result | Complication |
|---|---|---|---|---|---|---|---|---|
| 1 | 50/F | 6 | 5 | 5 | 30 | 1 | + | |
| 2 | 46/F | 2 | 2 | 6 | 32 | 24 | + | Mild pain and intracystic hemorrhage |
| 3 | 32/F | 3.5 | 3 | 3.5 | 27 | 24 | + | |
| 4 | 31/F | 2 | 2 | 2 | 28 | 2 | + | |
| 5 | 35/F | 9 | 9 | 9 | 30 | 24 | + | |
| 6 | 60/F | 2 | 2 | 3 | 33 | 1 | + | Mild pain and intracystic hemorrhage |
| 7 | 32/F | 2 | 1.5 | 2 | 26 | 2 | + | |
| 8 | 24/F | 8 | 5 | 7 | 30 | 1 | − | Mild pain |
| 9 | 66/F | 2 | 2 | 2 | 31 | 1 | + | Mild pain |
| 10 | 74/F | 2 | 2 | 2 | 28 | 8 | + | |
| 11 | 41/F | 4 | 4 | 6 | 29 | 2 | + | Intracystic hemorrhage |
| 12 | 45/M | 5 | 5 | 5 | 27 | 3 | + | |
| 13 | 31/F | 8 | 6 | 8 | 32 | 1 | + | Mild pain |
| 14 | 42/F | 5 | 5 | 6 | 30 | 3 | + | Intracystic hemorrhage |
| 15 | 32/F | 4 | 4 | 4 | 28 | 6 | + | |
| 16 | 60/F | 8 | 9 | 7 | 30 | 3 | + | |
| 17 | 43/F | 2 | 2 | 1.2 | 29 | 4 | + | |
| 18 | 66/F | 20 | 12 | 7 | 33 | 1 | + | |
| 19 | 37/F | 25 | 20 | 20 | 31 | 2 | + | Drunken sense |
| 20 | 55/M | 4 | 4 | 4 | 27 | 2 | + | |
| 21 | 46/F | 4 | 4 | 2 | 33 | 1 | + | Mild dizziness |
| 22 | 20/F | 1 | 1.5 | 1 | 32 | 5 | + | Mild pain and facial flushing |
| 23 | 49/F | 4 | 4 | 3.5 | 28 | 1 | + | |
| 24 | 29/F | 1 | 1 | 1.2 | 30 | 1 | + | Mild pain |
| 25 | 41/M | 5 | 5 | 2 | 36 | 1 | + | Moderate pain and perithyroidal leakage |
| 26 | 47/F | 17 | 10 | 12 | 34 | 1 | − | Intracystic hemorrhage |
| 27 | 66/F | 4 | 4 | 4.5 | 31 | 3 | + | Intracystic hemorrhage |
| 28 | 43/F | 1 | 1 | 1 | 30 | 1 | + | |
| 29 | 41/F | 8 | 8 | 6.5 | 34 | 2 | + | Mild pain |
| 30 | 75/M | 50 | 20 | 22 | 38 | 1 | + | Mild pain and intracystic hemorrhage |
| Mean | 45.3 | 7.3 | 5.4 | 5.5 | 30.6 | 4.4 |
Note.—+ indicates complete disappearance of the cystic portion of the thyroid nodule; −, incomplete disappearance of the cystic portion of the thyroid nodule.
Fig 1.
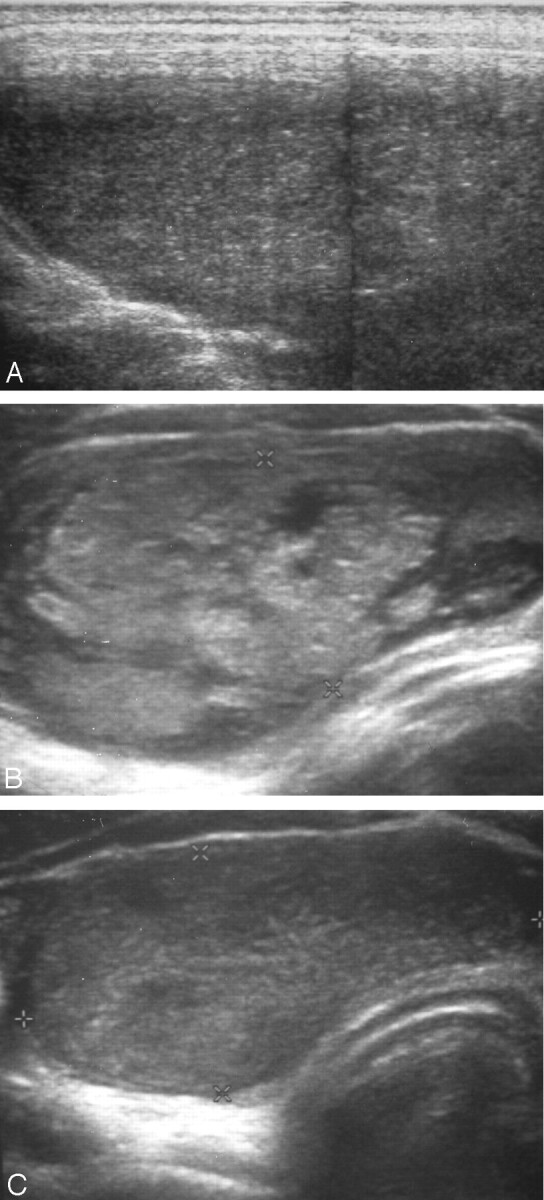
16-year-old boy with a purely cystic thyroid nodule (group A).
A, Ultrasonogram obtained before ethanol sclerotherapy shows a huge hemorrhagic thyroid nodule.
B, Ultrasonogram obtained 2 months after ethanol sclerotherapy reveals the complete disappearance of the cystic portion of the thyroid nodule and replacement by echogenic material.
C, Ultrasonogram obtained 9 months after ethanol sclerotherapy shows a decrease in the size of the postsclerotic thyroid nodule.
Fig 2.
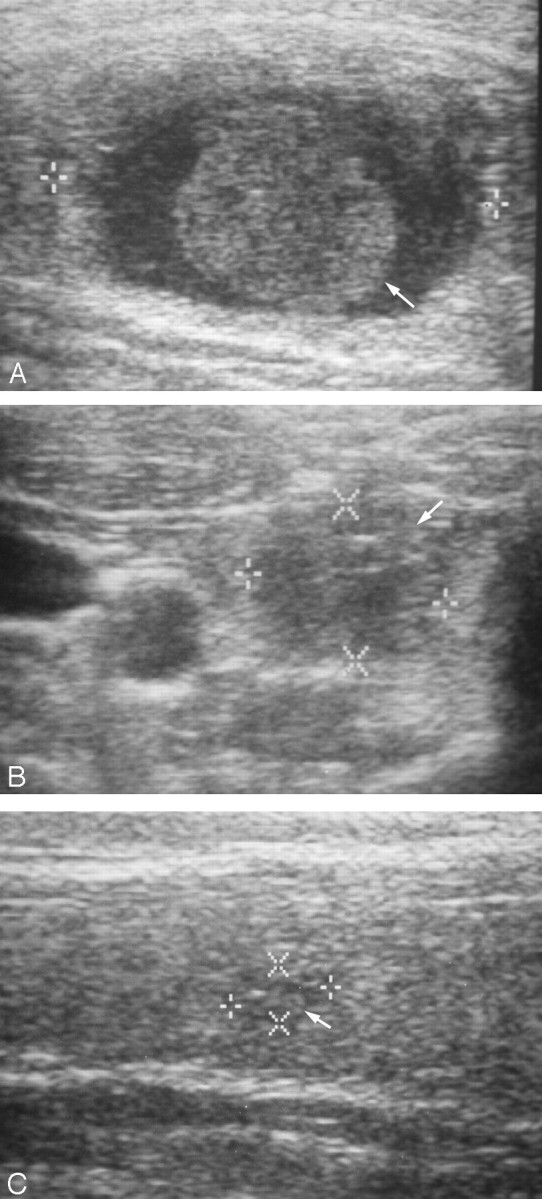
46-year-old woman with a cystic thyroid nodule (group B).
A, Ultrasonogram obtained before ethanol sclerotherapy shows a nearly pure thyroid cyst with an intracystic blood clot (arrow).
B, Ultrasonogram obtained after aspiration of infused ethanol shows the disappearance of the cystic portion and incomplete collapse of the cystic nodule due to the blood clot (arrow).
C, Ultrasonogram obtained 24 months after ethanol sclerotherapy shows the markedly decreased size of the thyroid nodule (arrow) without the cystic portion.
The cystic portion of the thyroid nodules disappeared completely in 29 patients from group A (96.7%) and in 28 patients from group B (93.3%). There was no statistical difference in the success rate in group A or B (P > .05, chi-square test) (Table 3). In group A, the previous cystic portion of the thyroid nodule had been replaced by echogenic material without vascularity on the first follow-up ultrasonography and showed progressive decrease in size on long-term follow-up ultrasonography. None of the cases with complete disappearance of the cystic portion of the thyroid nodule showed recurrence on follow-up ultrasonography (0%, 0/57).
TABLE 3:
Comparison of result, complication, and total procedure time
| Group A (%) | Group B (%) | P | |
|---|---|---|---|
| Success result | 29/30 (96.7) | 28/30 (93.3) | >.05 |
| Complication | |||
| Mild pain | 8/30 (26.7) | 9/30 (30) | >.05 |
| Moderate pain | 1/30 (3.3) | 1/30 (3.3) | >.05 |
| Facial flushing | 2/30 (6.6) | 1/30 (3.3) | >.05 |
| Drunken sense | 2/30 (6.6) | 1/30 (3.3) | >.05 |
| Headache | 1/30 (3.3) | 0/30 (0) | >.05 |
| Mild dizziness | 1/30 (3.3) | 1/30 (3.3) | >.05 |
| Perithyroidal ethanol leakage | 1/30 (3.3) | 1/30 (3.3) | >.05 |
| Intracystic hemorrage | 1/30 (3.3) | 7/30 (23.3) | <.05 |
| Complaint: double puncture | − | + | <.001 |
| Total procedure (min) | 16.8 | 30.6 | <.001 |
There was no statistically significant difference in the occurrence of complications between the 2 groups (P > .05, chi-square test) except for intracystic hemorrhage during the procedure (P < .05, chi-square test) and all group B patients’ complaint regarding a double puncture (P < .001, chi-square test) (Table 3). Intracystic hemorrhage during the procedure, more frequent in group B (7/30, 23.3%) than in group A (1/30, 3.3%), had completely disappeared on follow-up ultrasonography.
The total procedure time for sclerotherapy in group B (range, 26–38 minutes; mean, 30.6 minutes) was nearly twice that of group A (range, 13–29 minutes; mean, 16.8 minutes) (P < .001, Student t test) (Table 3).
Discussion
The cystic portion of thyroid nodules is considered to be caused by hemorrhage and subsequent degeneration of preexisting nodules (1). Several methods for treating benign cystic thyroid nodules, such as fine-needle aspiration, thyroid hormone suppression therapy, and sclerotherapy with various sclerosants have been introduced (9–14). Among these methods, simple fine-needle aspiration has been shown to have a high recurrence rate of ≤58% (1,6). Thyroid hormone suppression therapy has been found to have little effect on benign thyroid nodular disease (11). Sclerotherapy with ethanol, tetracycline, or OK-432 is effective to treat cystic thyroid nodules, and the effectiveness of ethanol is similar to that of tetracycline and OK-432 for percutaneous ethanol injection of cystic thyroid nodules, despite its lower cost and ease of repetition (5). Since introduction of percutaneous ethanol injection to treat thyroid cysts in the early 1980s, there have been many studies of the effectiveness of percutaneous ethanol injection in treating cystic thyroid nodules (1–7). Sonographically guided percutaneous ethanol injection for autonomously functioning thyroid nodules was first introduced in 1990 by Livraghi et al (15). The sclerotic mechanism of ethanol is cellular dehydration and protein denaturation in tissue, followed by coagulation necrosis, small vessel thrombosis, hemorrhagic infarct, and reactive fibrosis (15).
Although percutaneous ethanol injection is the most commonly used therapeutic method for treating cystic thyroid nodules in the world, some authors insist on complete evacuation of infused ethanol for prevention of ethanol leakage or other potential complication (6–8), whereas others prefer nonaspiration of infused ethanol for simplicity (1–5). Percutaneous ethanol injection without aspiration of infused ethanol seems to be more popular in recently published articles, whereas Bennedbaek and Hegedus (7) recommended complete aspiration of infused ethanol because of paraglandular fibrosis, caused by ethanol escaping outside the capsule. However, to our knowledge, no studies of the differences between aspiration and nonaspiration of infused ethanol have been found. In our study, a significant difference between the 2 methods was not observed in terms of successful results with complete disappearance of the cystic portion of the thyroid nodule.
Yasuda et al (1) and Cho et al (4) reported that with ethanol sclerotherapy, the cystic volumes decreased by more than half in 72% and 68% of the patients they treated for recurrent thyroid cyst after fine-needle aspiration. In our study, most thyroid nodules had complete disappearance of the cystic portion after percutaneous ethanol injection, as observed on the first follow-up ultrasonography; they showed progressively decreased size or nearly complete obliteration on long-term follow-up ultrasonography for a 1-year period. We obtained excellent results (95%, 3/60) without recurrence in both study groups (0%, 0/57).
Many authors recommend a maximum amount of infused ethanol of ≤10 mL in sclerotherapy of benign thyroid cysts (1–3,6–7). In our study, the total amount of infused ethanol did not exceed 20 mL in any patient, except for one group B patient, and if the first aspirates were <10 mL, nearly the same amount of ethanol was instilled.
Many complications, such as pain, facial flushing, a drunken sensation, headache, mild dizziness, perithyroidal or perinodal ethanol leakage, intracystic hemorrhage, local hematoma, secondary infection, or vocal cord paralysis, can occur during or after percutaneous ethanol injection for cystic thyroid nodule. In this report, no significant statistical differences were observed between 2 groups, except for intracystic hemorrhage during the procedure. Intracystic hemorrhage was more common in group B and indicates that the cystic wall after ethanol instillation is vulnerable to needle puncture or other irritation. On follow-up ultrasonography, all images of patients with intracystic hemorrhage revealed nearly complete disappearance of the cystic portion of the thyroid nodule. However intracystic hemorrhage during percutaneous ethanol injection results in improper reduction of cystic volume and can diminish the patient’s satisfaction with disappearance of the previous palpable thyroid mass in group B. Perithyroidal or pericapsular ethanol leakage during the procedure was observed in 2 patients (group A, n = 1; group B, n = 1). These cases showed progressive disappearance of the perithyroidal abnormality on follow-up ultrasonography and no significant thyroid hormonal alteration on follow-up thyroid function testing. Severe complications, such as vocal cord paralysis or secondary infection, were not observed in our study.
The group B patients’ complaint regarding the double puncture was significant. If a group B patient complained of severe anxiety regarding the needle puncture, local anesthetic was considered. However, percutaneous ethanol injection without aspiration of infused ethanol (group A) demanded one puncture, and local anesthetic was not used, although even one puncture was painful. In addition, percutaneous ethanol injection without aspiration of ethanol-mixed fluid (group A) was much more useful in patients with 2 or more cystic thyroid nodules.
The total procedure time was twice as long in group B as in group A because of the additional procedures, such as complete evacuation of the ethanol-mixed fluid and the 10-minute compression. This result is important to the physician as well as the patient because of its potential advantages, such as shortening of the total procedure time and decreasing the patient’s anxiety regarding the hospital stay.
A limitation of our study was the insufficient long-term follow-up more than 24 months after percutaneous ethanol injection to evaluate the cyst recurrence.
Conclusion
Percutaneous ethanol injection without aspiration of ethanol-mixed fluid seems to be the preferable method of treatment of benign cystic thyroid nodules from the perspective of both the physician and the patient.
References
- 1.Yasuda K, Ozaki O, Sugino K, et al. Treatment of cystic lesions of the thyroid by ethanol instillation. World J Surg 1992;16:958–961 [DOI] [PubMed] [Google Scholar]
- 2.Verde G, Papini E, Pacella C, et al. Ultrasound guided percutaneous ethanol injection in the treatment of cystic thyroid nodules. Clin Endocinol (Oxf) 1994;41:719–724 [DOI] [PubMed] [Google Scholar]
- 3.Zingrillo M, Torlontano M, Chiarella R, et al. Percutaneous ethanol injection may be a definitive treatment for symptomatic thyroid cystic nodules not treatable by surgery: five-year follow-up study. Thyroid 1999;9:763–767 [DOI] [PubMed] [Google Scholar]
- 4.Cho YS, Lee HK, Ahn IM, et al. Sonographically guided ethanol sclerotherapy for benign thyroid cysts: results in 22 patients. AJR Am J Roentgenol 2000;174:213–216 [DOI] [PubMed] [Google Scholar]
- 5.Kim JH, Lee HK, Lee JH, Ahn IM, Choi CG. Efficacy of sonographically guided percutaneous ethanol injection for treatment of thyroid cysts versus solid thyroid nodules. AJR Am J Roentgenol 2003;180:1723–1726 [DOI] [PubMed] [Google Scholar]
- 6.Monzani F, Lippi F, Goletti O, et al. Percutaneous aspiration and ethanol sclerotherapy for thyroid cysts. J Clin Endocrinol Metab 1994;78:800–802 [DOI] [PubMed] [Google Scholar]
- 7.Bennedbaek FN, Hegedus L. Treatment of recurrent thyroid cysts with ethanol: a randomized double-blind controlled trial. J Clin Endocrinol Metab 2003;88:5773–5777 [DOI] [PubMed] [Google Scholar]
- 8.Bennedbaek FN, Karstrup S, Hegedus L. Percutaneous ethanol injection therapy in the treatment of thyroid and parathyroid diseases. Eur J Endocrinol 1997;136:240–250 [DOI] [PubMed] [Google Scholar]
- 9.Crile G Jr. Treatment of thyroid cysts by aspiration. Surgery 1966;59:210–212 [PubMed] [Google Scholar]
- 10.Miller JM, Zafar SU, Karo JJ. The cystic thyroid nodule. Radiology 1974;110:257–261 [DOI] [PubMed] [Google Scholar]
- 11.Cooper DS. Clinical review 66: thyroxine suppression therapy for benign nodular disease. J Clin Endocrinol Metabol 1995;8:331–334 [DOI] [PubMed] [Google Scholar]
- 12.Edmond CJ, Tellez M. Treatment of thyroid cysts by aspiration and injection of sclerosant. BMJ 1987;295:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hegedus L, Hansen JM, Karstrup S, et al. Tetracycline for sclerosis of thyroid cysts. Arch Intern Med 1988;148:1116–1118 [DOI] [PubMed] [Google Scholar]
- 14.Chang HS, Yoon JH, Chung WY, Park CS. Sclerotherapy with OK-432 for recurrent cystic thyroid nodule. Yonsei Med J 1998;39:367–371 [DOI] [PubMed] [Google Scholar]
- 15.Livraghi T, Paracchi A, Ferrari C, Bergenzi M. Treatment of autonomous thyroid nodules with percutaneous ethanol injection: preliminary results. Radiology 1990;175:827–829 [DOI] [PubMed] [Google Scholar]


