Abstract
BACKGROUND AND PURPOSE: Cases with spinal perimedullary arteriovenous fistulas (SPAVFs) or spinal dural arteriovenous fistulas (SDAVFs) at the cervicomedullary junction are rare. We performed a retrospective, angiographic study of 6 such patients to assess whether available angiographic data were predictive of the risk for hemorrhage.
METHODS: We report 6 patients with arteriovenous fistulas at the cervicomedullary junction. All presented with subarachnoid hemorrhage (SAH). Angiography demonstrated that 4 of the 6 fistulas were SDAVFs fed by the meningeal branch of the vertebral artery; the other 2 were SPAVFs fed by the anterior spinal artery. Drainage was via the perimedullary vein of the cervicomedullary junction.
RESULTS: An ascending venous route into the intracranial sinus was recognized in all 6 cases; in 3 the draining system contained varices. In 2 cases, the venous route was on the ventral side of the brain stem with drainage into the cavernous sinus. In 4 cases, the venous route was lateral at the brain stem with drainage into the inferior petrosal sinus.
CONCLUSION: SPAVFs and SDAVFs at the cervicomedullary junction that manifest an ascending venous route into the intracranial sinus present an increased risk for SAH.
Arteriovenous fistulas in spinal regions are recognized as spinal perimedullary arteriovenous fistulas (SPAVFs) and spinal dural arteriovenous fistulas (SDAVFs). SPAVF shunts are located on the surface of the spinal cord; they are found most frequently in the conus medullaris region. SDAVFs are located in the dura matter of the nerve sleeve; they are often found in the thoracolumbar region. Patients with SPAVFs or SDAVFs usually present with gradual worsening of symptoms such as pain and weakness of the legs (1, 2). Cases with SPAVFs or SDAVFs at the cervicomedullary junction are rare; several of them presented with subarachnoid hemorrhage (SAH). Because there is little documentation regarding the drainage patterns of these fistulas, we performed a retrospective, angiographic study of six such patients to assess whether available angiographic data were predictive of the risk for hemorrhage or ischemic attack.
Patients and Methods
Since 1990, we have treated 113 patients with symptomatic arteriovenous fistulas (AVFs) or dural arteriovenous fistulas (DAVFs). In 6 patients, the fistulas were located at the cervicomedullary junction; 4 were SDAVFs and the other 2 were SPAVFs. All 6 of these patients—5 men and 1 woman, ranging in age from 54 to 75 years—suffered associated SAHs.
Clinical data on the 6 subjects of this study are presented in Table 1. The treatment outcome was assessed at a mean of 6 months after surgery. All 6 patients underwent selective angiograms of the internal, external, vertebral, and thyrocervical arteries to document the location of the fistulas. To obtain detailed information on the location of the fistulas, their arterial supply, and venous drainage pattern, we performed continuous-mode angiography (20 frames/s). In efforts to identify factors that may have placed these patients at increased risk for SAH, we retrospectively evaluated their clinical characteristics and angiographic findings.
TABLE 1:
Summary of the six cases with arteriovenous fistulas at the cervicomedullary junction
| Patient No./Age (y)/Sex | Symptom | Feeder | SDAVF/SPAVF | Fistula Point | Drainer | Related Sinus | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1/61/M | SAH | Meningeal artery | SDAVF | Jugular foramen | Anteromedial medullary vein | Superior petrosal sinus–cavernous sinus | Drainer occlusion | GR |
| 2/56/M | SAH | Meningeal artery | SDAVF | Jugular foramen | Anterior spinal vein | Cavernous sinus | Drainer occlusion | GR |
| Petrosal vein | ||||||||
| 3/75/M | SAH | Ascending pharyngeal artery | SDAVF | Jugular foramen | Pontomedullary vein | Inferior petrosal sinus | Drainer occlusion | MD |
| Inferior petrosal vein | ||||||||
| 4/68/M | SAH | Meningeal artery | SDAVF | Jugular foramen | Anterior hemispheric vein | Inferior petrosal sinus | Drainer occlusion | GR |
| Lateral medullary vein | ||||||||
| 5/54/M | SAH | Anterior spinal artery | SPAVF | Jugular foramen–C1 | Lateral medullary vein | Inferior petrosal sinus | Feeder occlusion | GR |
| 6/56/F | SAH | Anterior spinal artery | SPAVF | Jugular foramen–C1 | Lateral hemispheric vein | Inferior petrosal sinus | Feeder occlusion | GR |
| Inferior vermian vein |
SAH indicates subarachnoid hemorrhage; GR, good recovery; MD, moderately disabled; C, cervical; SDAVF, spinal dural arteriovenous fistula; SPAVF, spinal perimedullary arteriovenous fistula.
Table 2 provides a summary of 41 previously reported patients with SPAVFs or SDAVFs in the cervicomedullary junction. We paid special attention to the reported venous drainage routes in these cases.
TABLE 2:
Summary of reported patients with arteriovenous fistula of cervicomedullary junction
| Study | Age (y)/Sex | Symptom | SDAVF/SPAVF | Ascending/Descending | Drainer |
|---|---|---|---|---|---|
| Asakawa et al, 2002 (5) | 64/M | Myelopathy | SDAVF | Descending | Spinal medullary veins |
| Do et al, 1999 (6) | 50/M | SAH | SDAVF | Ascending | Anterior medullary vein |
| Ernst et al, 1997 (7) | 71/M | Myelopathy | SDAVF | Descending | Spinal medullary veins |
| 47/M | Myelopathy | SDAVF | Descending | UD | |
| 58/F | Myelopathy | SDAVF | Descending | Spinal medullary veins, superior petrosal sinus | |
| Fox and Allcock, 1978 (8) | 54/M | Tinnitus | SDAVF | Descending | Jugular vein, varix (+) |
| Gaensler et al, 1990 (9) | 50/M | Myelopathy | SDAVF | Descending | Medullary vein |
| 69/F | Myelopathy | SDAVF | Descending | Medullary vein | |
| Guglielmi et al, 1988 (10) | 62/F | Tinnitus | SDAVF | Descending | Jugular vein |
| Hahnel et al, 1998 (11) | 67/M | Myelopathy | SDAVF | Ascending | Anterior pontomesencephalic vein, superior petrous sinus |
| Hashimoto et al, 2000 (12) | 66/F | SAH | SDAVF | Ascending | Perimedullary vein inferior petrosal sinus |
| Inci et al, 2002 (13) | 22/M | Myelopathy | SDAVF | Descending | Epidural vein |
| Kinouchi et al, 1998 (14) | 10 cases | SAH 7 cases | SDAVF | Ascending: 6 | Vatix: 4 cases |
| Ascending and descending: 3 | |||||
| Descending: 1 | |||||
| Mascalchi et al, 1996 (16) | 69/M | Myelopathy | SDAVF | Descending | Retromedullary vein, inferior petrosal sinus |
| 53/M | Myelopathy | SDAVF | Descending | Anteromedullary veins, retromedullary veins | |
| Miyoshi et al, 1999 (17) | 70/F | Myelopathy | SDAVF | Descending | Anterior and posterior spinal veins |
| Niwa et al, 1997 (18) | 61/F | Asymptomatic | SDAVF | Descending | Medullary vein |
| 61/M | Cerebellar ataxia | SDAVF | Descending | Medullary vein | |
| Oda et al, 1989 (19) | 58/M | Myelopathy | SDAVF | Descending | Medullary vein |
| Oishi et al, 1999 (20) | 62/F | Myelopathy | SDAVF | Descending | Medullary vein |
| Partington et al, 1992 (21) | 63/M | Myelopathy | SDAVF | Descending | Medullary vein |
| Pulido et al, 2004 (22) | 62/M | Myelopathy | SDAVF | Descending | Medullary vein |
| Rodesch and Lasjaunias, 2003 (23) | 36/M | Myelopathy | SDAVF | Descending | Medullary vein |
| Slaba et al, 2000 (24) | 36/M | Myelopathy | SDAVF | UD | Perimedullary veins |
| Symon et al, 1984 (25) | 60/M | Myelopathy | SDAVF | Descending | Perimedullary veins |
| Trop et al, 1998 (26) | 74/M | Myelopathy | SDAVF | Descending | Anterior medullary vein |
| Vinuela et al, 1986 (27) | 30/F | Cerebellar dysfunction | SDAVF | UD | UD |
| Willinsky et al, 1990 (28) | 36/F | Myelopathy | SDAVF | Descending | Anterior medullary vein |
| Bayrakci et al, 1999 (30) | 4 mo/M | Torticolis | SPAVF | Descending | Anterior spinal vein |
| Heros et al, 1986 (31) | 31/M | Myelopathy | SPAVF | Ascending | Anterior spinal vein |
| Hida et al, 2002 (32) | 58/M | SAH | SPAVF | Ascending | UD |
| Nagashima et al, 1996 (33) | 16/F | Myelopathy | SPAVF | Descending | Anterior spinal vein |
SAH indicates subarachnoid hemorrhage: SDAVF, spinal dural arteriovenous fistula; SPAVF, spinal perimedullary arteriovenous fistula; UD, unspecfied data.
Results
Angiography demonstrated that in 4 patients with SDAVFs (cases 1–4), the fistula was fed by the meningeal branch of the vertebral artery or ascending pharyngeal artery. The other 2 patients (cases 5 and 6) had SPAVFs that were fed by the anterior spinal artery. Drainage was via the perimedullary vein of the cervicomedullary junction. All 6 patients manifested an ascending venous drainage route into the intracranial venous system; in 3 cases the draining system contained varices. In cases 1 and 2, the ascending drainage route involved the anteromedial medullary and anterior spinal vein and the superior petrosal sinus, with final drainage into the cavernous sinus. In the other 4 cases, the anterior spinal, anterior hemispheric, lateral hemispheric, lateral medullary, pontomedullary, and inferior petrosal vein were involved in final drainage into the inferior petrosal sinus. In cases 3–6, the ascending route was located on the ventral and lateral side of the brain stem. The point of the fistula in the 4 SDAVF cases was located on the lateral side of the foramen magnum; in the 2 SPAVF cases it was located between the foramen magnum and the C1 portion in front of the anterior surface of the upper cervical cord.
All 6 patients underwent direct surgery via the posterior transcondylar fossa approach because it makes possible the identification of abnormal vessels in front of the medulla oblongata and vertebral artery at the cervicomedullary junction. Before surgical coagulation, intraoperative angiograms were obtained. These proved useful after temporary clipping of the feeding artery (SPAVF cases) or the draining vessel (SDAVF cases). In SPAVF cases with abnormal vessels, the feeding artery was coagulated at a site just proximal to the fistula point. The fistula point was located at the site where the caliber of the vessel changed. In SDAVF cases, the draining vein was coagulated at a site just distal to the exit zone from the dura mater. In all 6 patients, postoperative angiographs revealed the complete disappearance of the SPAVF or SDAVF. The treatment outcome at 6 months after surgery was good in 5 patients; the other patient remained moderately disabled because of initial damage of the medulla oblongata.
Illustrative Cases
Case 1
This 61-year-old man experienced the sudden onset of severe headache. On admission, CT showed SAH involving mainly the posterior fossa. Angiography of the left vertebral artery demonstrated an abnormal vessel at the cervicomedullary junction. The feeding arteries were the dural branches of the left vertebral artery. Drainage was via the anteromedial medullary veins into the superior petrosal sinus and finally the cavernous sinus in the intracranial space. There were no varices in the drainage route (Figs 1 and 2). Direct surgery by using the left transcondylar fossa approach exposed dural penetration by the left vertebral artery and a dilated anterior spinal vein in front of the medulla oblongata. Because the fistula point was located at the site where the left vertebral artery penetrated the dura, a diagnosis of SDAVF was made. After coagulation of the feeding arteries at the dura mater, the color of the draining veins changed from red to dark blue. Finally, the draining veins were coagulated at a site distal to the fistula. Angiography of the left vertebral artery, performed in the operating room immediately following the procedure, showed disappearance of the abnormal vessels.
Fig 1.
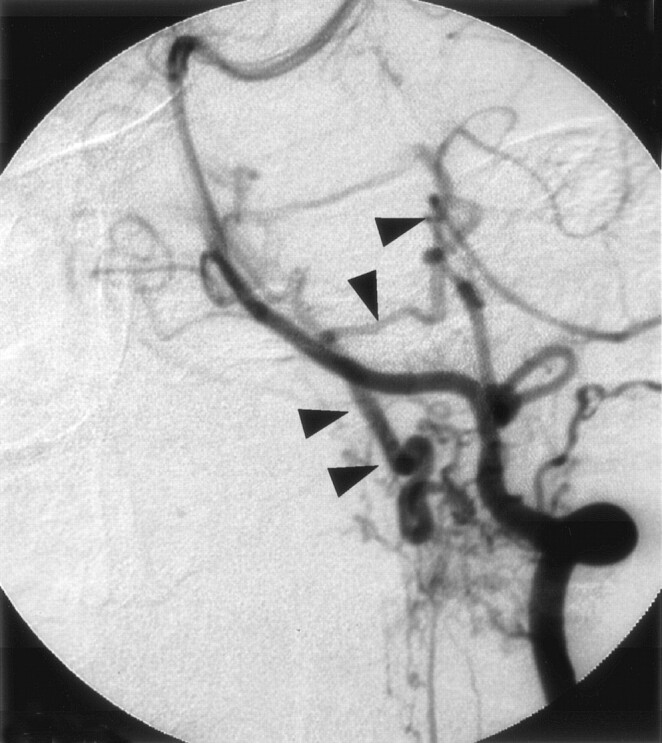
Left vertebral angiogram (early phase), showing abnormal vessels at the cervicomedullary junction. The feeding artery is the meningeal branch of the vertebral artery and ascending drainage route was recognized (arrowhead).
Fig 2.
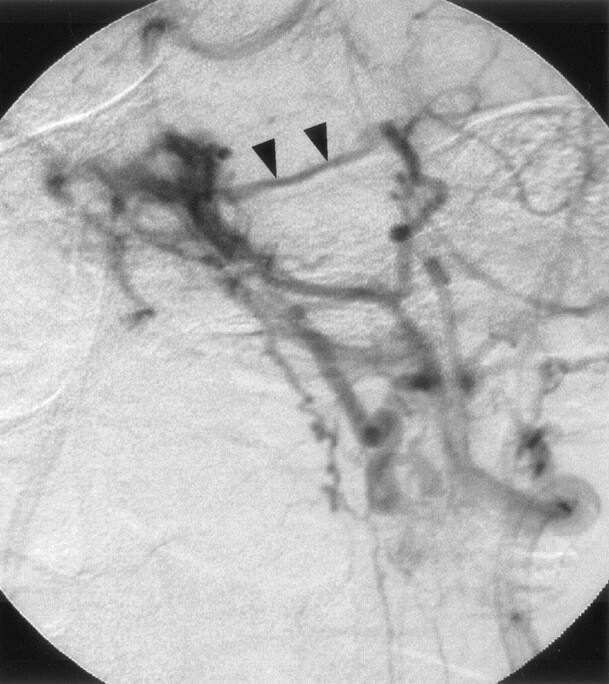
Left vertebral angiogram (late phase), showing shunt flow draining retrogradely into the left superior petrosal sinus (arrowhead).
His postoperative course was uneventful. Angiograms after treatment confirmed the disappearance of the SDAVF (Fig. 3). This patient suffered no neurologic deficits and was able to resume his normal life.
Fig 3.
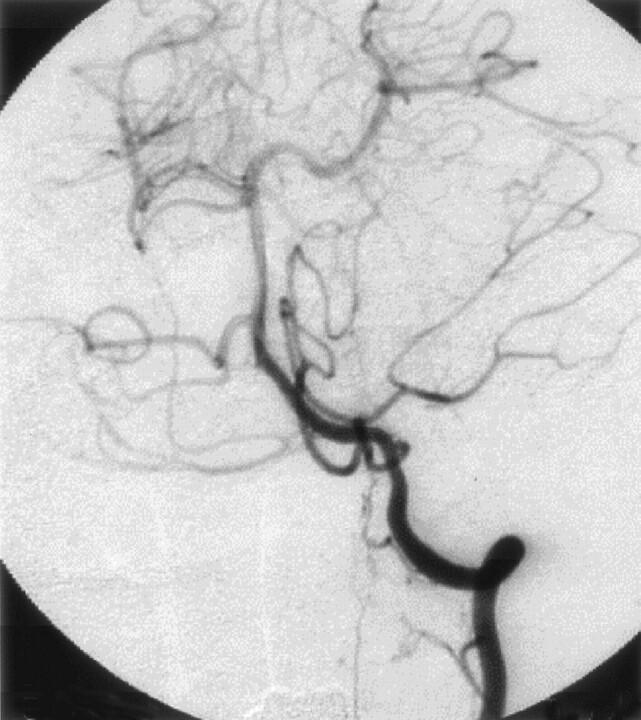
Postoperative left vertebral angiogram, demonstrating complete disappearance of the abnormal vessels.
Case 5
This 54-year-old man experienced the sudden onset of severe headache. On admission, CT showed SAH. Angiography of the left vertebral artery demonstrated an abnormal vessel at the cervicomedullary junction. The feeding artery was the anterior spinal artery. Drainage was via the lateral medullary veins into the inferior petrosal veins in the intracranial space and finally into the inferior petrosal sinus. The caliber of the feeding artery changed at the fistula point. The drainage route contained no varices (Figs 4 and 5). Direct surgery was performed via the left transcondylar fossa approach. The feeding anterior spinal artery was located in front of the medulla oblongata. Because it fed into the anterior spinal vein, a diagnosis of SPAVF was made. Upon temporary clipping of the anterior spinal artery, the color of the anterior spinal vein changed from red to blue. Because intraoperative angiography, performed with the clip in place, showed disappearance of the SPAVF, the anterior spinal artery was clipped at a site just proximal to the fistula.
Fig 4.
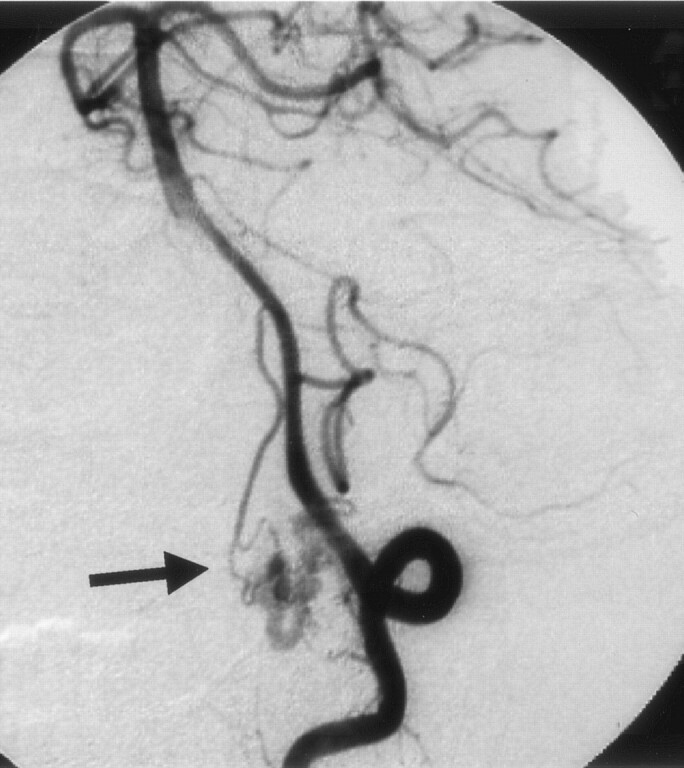
Left vertebral angiogram (early phase), showing abnormal vessels at the cervicomedullary junction. The feeder, the anterior spinal artery, feeds directly into the lateral medullary vein (arrow).
Fig 5.
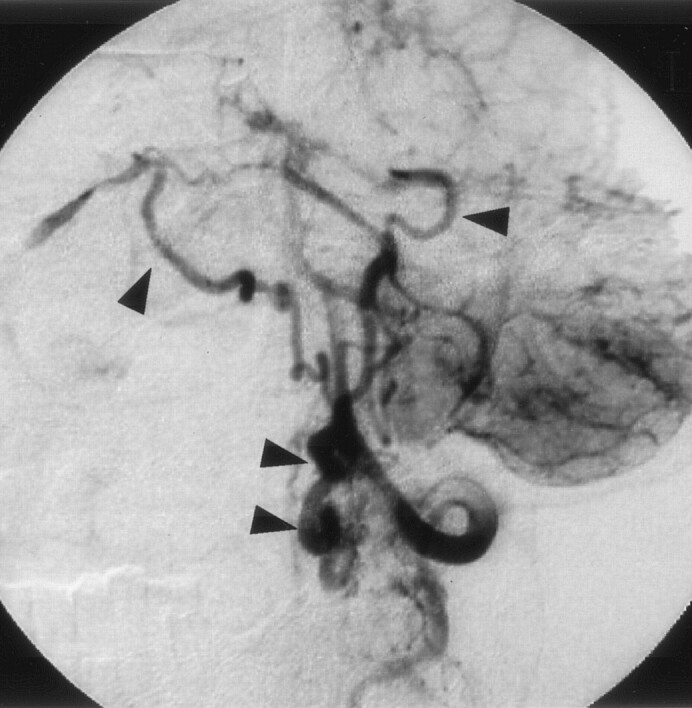
Left vertebral angiogram (late phase), showing shunt flow draining retrogradely into the bilateral inferior petrosal vein (arrowhead).
His postoperative course was uneventful, and angiography after his surgical treatment revealed disappearance of the SPAVF (Fig 6). He suffered no neurologic deficits and was able to resume his normal life.
Fig 6.
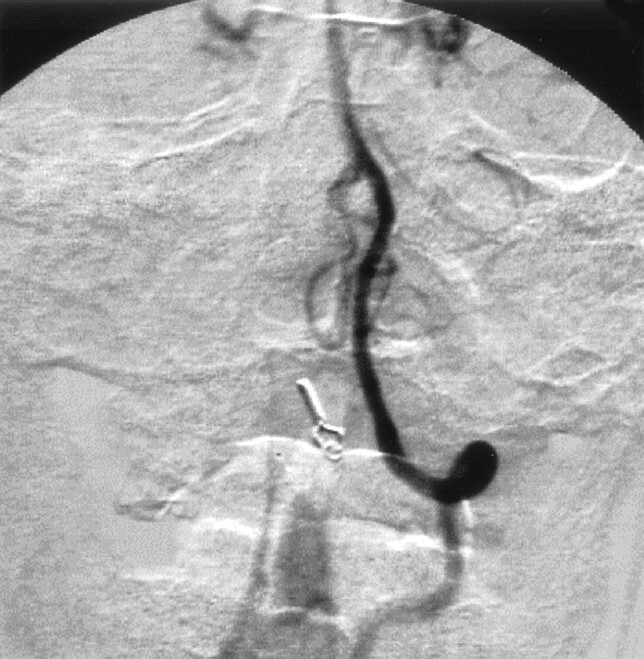
Postoperative left vertebral angiogram, demonstrating complete disappearance of the abnormal vessels.
Discussion
Arteriovenous fistulas in spinal regions are recognized as SPAVFs and as SDAVFs. In cases with SPAVFs, the shunt is located on the surface of the spinal cord; these fistulas are located in the conus medullaris region. SDAVFs are located in the dura mater of the nerve sleeve; they occur in the thoracolumbar region. Patients with SPAVFs and SDAVFs usually experience the gradual worsening of symptoms, such as pain and weakness of the legs; hemorrhagic onset is rare (1–3). Berenstein and Lasjaunias (4) reviewed 172 patients with SDAVF located in the thoracic-, lumbar-, or sacral area; none of these patients suffered SAH. Myelopathy remains the predominant presenting symptom. On the other hand, 10 of 41 previously reported patients with SDAVFs (5–29) or SPAVFs (30–33) at the cervicomedullary junction suffered SAHs. If we include our 6 patients, the incidence of SAH in these patients is 34% (Table 2), which suggests that the location of these fistulas at the cervicomedullary junction plays a role.
We carefully re-examined angiographic findings on 6 patients with SPAVF and SDAVF at the cervicomedullary junction. All 6 experienced SAH, and in all 6 the venous route displayed the angiographic characteristic of an ascending drainage route into the intracranial sinus system. We recognized 2 types of venous routes. In the first, the route involved the ventral side of the brain stem with final feeding into the cavernous sinus. The second type consisted of a lateral venous route with final feeding into the inferior petrosal sinus. Both types of routes involved final drainage into the intracranial sinus via an ascending drainage route.
In the 41 previously reported cases of SDAVF and SPAVF at the cervical or cervicomedullary junction, venous drainage was via an ascending route in 11, both an ascending and descending route in 3, and a descending route in 25 (Table 2). In the 2 remaining cases, the drainage route was undetected. It is worth noting that all patients with SAHs manifested the ascending route of venous drainage. In 7 cases, the ascending route finally drained into the intracranial sinus. If we include ours, there were 16 patients with SDAVF at the cervicomedullary junction who suffered SAHs; in 13 (81.2%) the drainage route was ascending with final drainage into the intracranial sinus.
We have elsewhere provided angiographic evidence of the existence of 2 types of retrograde leptomeningeal venous drainage (RLVDs) in intracranial DAVFs (34, 35). In type 1, RLVD is into more than one venous sinus and in type 2, RLVD is into a single venous sinus. Patients with type 1 RLVD experienced hemorrhagic episodes at a high rate, whereas no patients with type 2 RLVD suffered hemorrhage. Angiographically, type 1 patients manifested one or more varices in the RLVD route; type 2 patients had no varices. We speculate that, in type 1 patients, a precipitous increase in retrograde venous flow immediately brought about an increase in hemodynamic stress, varix formation, and the development of hemorrhage or neurologic deficits. Our earlier histopathologic study (36) showed that, in type 1 patients, the thickness of the vessel wall was extremely irregular, which suggests that these vessels are at increased risk for developing varices in the RLVD route and for hemorrhagic episodes. On the other hand, in type 2 RLVDs, the volume of retrograde venous drainage is low because of the absence of an accessory route. Therefore, retrograde venous flow does not increase markedly and venous perfusion becomes impaired slowly over a longer period without bleeding. Pathologically, the entire media was thickened and there was prominent local intimal thickening in these patients (36). This may render them less vulnerable to the development of varices and hemorrhagic episodes.
We suspected that the venous drainage pattern in patients with SDAVF and SPAVF at the cervicomedullary junction played a role in their vulnerability for hemorrhage. Therefore, we examined their retrograde venous drainage (RVD) patterns closely and found that there were 2 types of RVD. In type 1, drainage is via an ascending route into the intracranial region with final drainage into the petrosal or cavernous sinus. This pattern is similar to the type 1 RLVD we reported earlier (36). We postulate that, as in type 1 RLVD, the drainage volume is increased in type 1 RVD, rendering these patients with SDAVF or SPAVF at the cervicomedullary junction vulnerable to bleeding. In patients with SDAVFs or SPAVFs that drained via a descending route, RVD did not drain into the intracranial sinus. This draining pattern resembles type 2 RLVD. These patients experienced no hemorrhagic events, because the draining volume was not high (16, 20). In patients with SDAVFs or SPAVFs at the thoracolumbar region there was no RVD into the intracranial sinus and these patients also did not experience hemorrhagic onset (4).
Cognard et al (37) described the correlation between the symptoms and various angiographic patterns in patients with intracranial DAVF. Type V, featuring descending spinal venous drainage, produced progressive myelopathy in 6 of 12 cases. On the other hand, in 5 of 12 patients with hemorrhage there was no descending spinal venous drainage. Kinouchi et al (14) described the connecting pathway between the pontomesencephalic venous system and both the anterior and posterior veins of the spinal cord in DAVF at the cervicomedullary junction. Angiographic studies on patients with only myelopathy showed that the shunt drained inferiorly into a single enlarged spinal medullary vein and that venous flow was slow. On the other hand, in patients with SAH, venous drainage was mainly superiorly into the intracranial sinus, and the venous flow was fast. In addition, some of their patients with SAH manifested varices on the draining vein. Intracranial drainage is associated with relatively fast venous flow and increased hemodynamic stress may lead to the formation of varices on the draining vessels and result in SAH.
We postulate that the anatomic location of SPAVF and SDAVF and their drainage patterns play an important role in placing patients at risk for hemorrhage. Our study showed that, among patients with SPAVF or SDAVF at the cervicomedullary junction, those with an ascending drainage route are at particularly high risk for SAH.
Conclusion
SPAVFs and SDAVFs at the cervicomedullary junction tended to present with hemorrhagic onset. On the other hand, patients with SPAVFs or SDAVFs in the thoracolumbar region usually experienced gradual neurologic worsening. We postulate that the anatomic location of SPAVF and SDAVF and their drainage patterns play an important role in placing patients at risk for hemorrhage and that patients with SPAVFs or SDAVFs at the cervicomedullary junction that manifest an ascending drainage route are at particularly high risk for SAHs.
References
- 1.Jellema K, Tijssen CC, van Rooij WJ, et al. Spinal dural arteriovenous fistulas: long-term follow-up of 44 treated patients. Neurology 2004;62:1839–1841 [DOI] [PubMed] [Google Scholar]
- 2.Koch C, Gottschalk S, Giese A. Dural arteriovenous fistula of the lumbar spine presenting with subarachnoid hemorrhage: case report and review of the literature. J Neurosurg Spine 2004;100:385–391 [DOI] [PubMed] [Google Scholar]
- 3.Brunereau L, Gobin YP, Meder JF, et al. Intracranial dural arteriovenous fistulas with spinal venous drainage: relation between clinical presentation and angiographic findings. AJNR Am J Neuroradiol 1996;17:1549–1554 [PMC free article] [PubMed] [Google Scholar]
- 4.Berenstein A, Lasjaunias P. Surgical neuroangiography. 5. Endovascular treatment of spine and spinal cord lesions. Berlin: Springer-Verlag;1992. :5–24
- 5.Asakawa H, Yanaka K, Fujita K, et al. Intracranial dural arteriovenous fistula showing diffuse MR enhancement of the spinal cord: case report and review of the literature. Surg Neurol 2002;58:251–257 [DOI] [PubMed] [Google Scholar]
- 6.Do HM, Jensen ME, Cloft HJ, et al. Dural arteriovenous fistula of the cervical spine presenting with subarachnoid hemorrhage. AJNR Am J Neuroradiol 1999;20:348–350 [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst RJ, Gaskill-Shipley M, Tomsick TA, et al. Cervical myelopathy associated with intracranial dural arteriovenous fistula: MR findings before and after treatment. AJNR Am J Neuroradiol 1997;18:1330–1334 [PMC free article] [PubMed] [Google Scholar]
- 8.Fox AJ, Allcock JM. Successful embolization of a fistula between the ascending pharyngeal artery and internal jugular vein. Neuroradiology 1978;15:149–152 [DOI] [PubMed] [Google Scholar]
- 9.Gaensler EH, Jackson DE Jr, Halbach VV. Arteriovenous fistulas of the cervicomedullary junction as a cause of myelopathy: radiographic findings in two cases. AJNR Am J Neuroradiol 1990;11:518–521 [PMC free article] [PubMed] [Google Scholar]
- 10.Guglielmi G, Guidetti G, Mori S, Silipo P. Therapeutic embolization of an ascending pharyngeal artery-internal jugular vein fistula: case report. J Neurosurg 1988;69:132–133 [DOI] [PubMed] [Google Scholar]
- 11.Hahnel S, Jansen O, Geletneky K. MR appearance of an intracranial dural arteriovenous fistula leading to cervical myelopathy. Neurology 1998;51:1131–1135 [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto H, Iida J, Shin Y, Hironaka Y, Sakaki T. Spinal dural arteriovenous fistula with perimesencephalic subarachnoid haemorrhage. J Clin Neurosci 2000;7:64–66 [DOI] [PubMed] [Google Scholar]
- 13.Inci S, Bertan V, Cila A. Angiographically occult epidural arteriovenous fistula of the craniocervical junction. Surg Neurol 2002;57:167–173 [DOI] [PubMed] [Google Scholar]
- 14.Kinouchi H, Mizoi K, Takahashi A, et al. Dural arteriovenous shunts at the craniocervical junction. J Neurosurg 1998;89:755–761 [DOI] [PubMed] [Google Scholar]
- 15.Koenig E, Thron A, Schrader V, Dichgans J. Spinal arteriovenous malformations and fistulae: clinical, neuroradiological and neurophysiological findings. J Neurol 1989;236:260–266 [DOI] [PubMed] [Google Scholar]
- 16.Mascalchi M, Scazzeri F, Prosetti D, et al. Dural arteriovenous fistula at the craniocervical junction with perimedullary venous drainage. AJNR Am J Neuroradiol 1996;17:1137–1141 [PMC free article] [PubMed] [Google Scholar]
- 17.Miyoshi Y, Taniwaki T, Arakawa K, et al. A case of cervical myelopathy due to dural arteriovenous fistula at the craniocervical junction [in Japanese]. Rinsho Shinkeigaku 1999;39:836–841 [PubMed] [Google Scholar]
- 18.Niwa J, Matsumura S, Maeda Y, Ohoyama H. Transcondylar approach for dural arteriovenous fistulas of the cervicomedullary junction. Surg Neurol 1997;48:627–631 [DOI] [PubMed] [Google Scholar]
- 19.Oda Y, Konishi T, Suzui H, et al. Partially thrombosed radiculomeningeal arterio-venous fistula in spinomedullary junction [in Japanese]. No Shinkei Geka 1989;17:63–68 [PubMed] [Google Scholar]
- 20.Oishi H, Okuda O, Arai H, et al. Successful surgical treatment of a dural arteriovenous fistula at the craniocervical junction with reference to pre- and postoperative MRI. Neuroradiology 1999;41:463–467 [DOI] [PubMed] [Google Scholar]
- 21.Partington MD, Rufenacht DA, Marsh WR, Piepgras DG. Cranial and sacral dural arteriovenous fistulas as a cause of myelopathy. J Neurosurg 1992;76:615–622 [DOI] [PubMed] [Google Scholar]
- 22.Pulido Rivas P, Villoria Medina F, Fortea Gil F, Sola RG. Dural fistula in the craniocervical junction: a case report and review of the literature. Rev Neurol 2004;38:438–442 [PubMed] [Google Scholar]
- 23.Rodesch G, Lasjaunias P. Spinal cord arteriovenous shunts: from imaging to management. Eur J Radiol 2003;46:221–232 [DOI] [PubMed] [Google Scholar]
- 24.Slaba S, Smayra T, Hage P, et al. An unusual cause of acute myelopathy: a dural arteriovenous fistula at the craniocervical junction. J Med Liban 2000;48:168–172 [PubMed] [Google Scholar]
- 25.Symon L, Kuyama H, Kendall B. Dural arteriovenous malformations of the spine: clinical features and surgical results in 55 cases. J Neurosurg 1984;60:238–247 [DOI] [PubMed] [Google Scholar]
- 26.Trop I, Roy D, Raymond J, et al. Craniocervical dural fistula associated with cervical myelopathy: angiographic demonstration of normal venous drainage of the thoracolumbar cord does not rule out diagnosis. AJNR Am J Neuroradiol 1998;19:583–586 [PMC free article] [PubMed] [Google Scholar]
- 27.Vinuela F, Fox AJ, Pelz DM, Drake CG. Unusual clinical manifestations of dural arteriovenous malformations. J Neurosurg 1986;64:554–558 [DOI] [PubMed] [Google Scholar]
- 28.Willinsky R, Lasjaunias P, Terbrugge K, Hurth M. Angiography in the investigation of spinal dural arteriovenous fistula: a protocol with application of the venous phase. Neuroradiology 1990;32:114–116 [DOI] [PubMed] [Google Scholar]
- 29.Yoshida S, Oda Y, Kawakami Y, Sato S. Progressive myelopathy caused by dural arteriovenous fistula at the craniocervical junction: case report. Neurol Med Chir (Tokyo) 1999;39:376–379 [DOI] [PubMed] [Google Scholar]
- 30.Bayrakci B, Aysun S, Firat M. Arteriovenous fistula: a cause of torticollis. Pediatr Neurol 1999;20:146–147 [DOI] [PubMed] [Google Scholar]
- 31.Heros RC, Debrun GM, Ojemann RG, et al. Direct spinal arteriovenous fistula: a new type of spinal AVM: case report. J Neurosurg 1986;64:134–139 [DOI] [PubMed] [Google Scholar]
- 32.Hida K, Iwasaki Y, Ushikoshi S, et al. Corpectomy: a direct approach to perimedullary arteriovenous fistulas of the anterior cervical spinal cord. J Neurosurg 2002;96:157–161 [DOI] [PubMed] [Google Scholar]
- 33.Nagashima C, Miyoshi A, Nagashima R, et al. Spinal giant intradural perimedullary arteriovenous fistula: clinical and neuroradiological study in one case with review of literature. Surg Neurol 1996;45:524–531 [DOI] [PubMed] [Google Scholar]
- 34.Kai Y, Hamada J, Morioka M, et al. Correlation between magnetic resonance images and draining patterns in dural arteriovenous fistulas with leptomeningeal venous drainage. Acta Neurochir (Wien) 2000;142:413–418 [DOI] [PubMed] [Google Scholar]
- 35.Kai Y, Hamada J, Morioka M, et al. Pre- and post-treatment MR imaging and single photon emission CT in patients with dural arteriovenous fistulas and retrograde leptomeningeal venous drainage. AJNR Am J Neuroradiol 2003;24:619–625 [PMC free article] [PubMed] [Google Scholar]
- 36.Hamada J, Yano S, Kai Y, et al. Histopathological study of venous aneurysms in patients with dural arteriovenous fistulas. J Neurosurg 2000;92:1023–1027 [DOI] [PubMed] [Google Scholar]
- 37.Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology 1995;194:671–680 [DOI] [PubMed] [Google Scholar]


