Abstract
BACKGROUND AND PURPOSE: Blood oxygen level–dependent functional MR imaging (BOLD fMRI) is a clinically useful technique for preoperative mapping of eloquent cortices in patients with brain tumors. The purpose of this study was to determine the effect on BOLD fMRI accuracy of susceptibility artifacts caused by prior surgery by comparing volumes of activation in the primary motor cortex (PMC) of patients with and without prior brain surgery.
METHODS: The volumes of fMRI activation of the PMC were measured for the tumor and nontumor sides in patients with (n = 13) and without (n = 30) prior neurosurgery. Statistical comparisons of the volumes were performed by using paired t tests and linear regression analysis. The location and degree of susceptibility artifact were subjectively assessed.
RESULTS: No significant difference was found between the mean tumor and nontumor volumes of fMRI activations in patients without prior surgery (P = .51). In patients who had prior surgery, the volume of activation was significantly smaller on the side of the prior operation when compared with the contralateral side (P = .001). The volume of activation on the side of the tumor was also significantly smaller in the patients with prior surgery compared with those without prior surgery (P < .001). Nevertheless, the PMC was identified in all cases, and its location was confirmed intraoperatively.
CONCLUSION: Prior surgery is associated with a decrease in the volume of fMRI activation in patients with prior surgery; however, by examining the T2 images, an astute radiologist can recognize this phenomenon, draw the appropriate conclusions, and correctly identify the PMC.
The primary goal in the neurosurgical treatment of brain tumors is to maximize resection of tumor tissue while minimizing removal of adjacent eloquent cortices. Previous studies have shown that blood oxygen level–dependent functional MR imaging (BOLD fMRI) is a clinically useful tool in the preoperative mapping of eloquent brain areas (1–4). The BOLD fMRI examination can facilitate planning of surgery, shorten the duration of the operation and anesthesia time, and may alleviate the need to awaken the patient during the operation for language and motor mapping. In addition, coregistration of the BOLD fMRI data to the neurosurgical navigational system can guide intraoperative electrocortical stimulation as well as the resection itself (5–9).
BOLD fMRI constructs functional images by exploiting the susceptibility produced by the paramagnetic nature of deoxyhemoglobin (deoxy-Hb) (10–12). Neuronal activation corresponding to specific functional tasks increases local blood flow to the eloquent cortices, which reduces deoxy-Hb concentration and susceptibility and increases signal intensity in a susceptibility-sensitive image such as T2 (13). This signal intensity change is referred to as the BOLD effect. In fact, an echo-planar, gradient-echo sequence is commonly used to emphasize the difference in susceptibility between deoxy-Hb and oxy-Hb.
BOLD fMRI’s reliance on susceptibility is, however, a double-edged sword. The same principles used to identify functional activity through susceptibility differences can also block the detection of that activity. Susceptibility artifacts, for example, are often found at junctions between air and tissue, such as at the temporal lobes (from the tympanomastoid air cells) or orbitofrontal cortex (from the sinuses). The dropout in signal intensity and geometric distortions caused by the susceptibility artifacts may mask the BOLD signal intensity at these sites. If the fMRI data are interpreted on the basis of the measured activation volume, the radiologist may err in determining the location of the eloquent cortex because of the artifact. In extreme cases, such false-negative results may lead the neurosurgeon to resect functioning cortex.
Susceptibility artifacts become a more pressing issue in patients who have undergone prior neurosurgery. The presence of titanium plates to secure skull flaps, metallic staples to close surgical incisions, hemorrhage from surgery or from the tumor, and residual metal from the skull drill (14) can lead to an increase in the susceptibility artifact, which, in turn, can lead to a decrease in the accuracy of BOLD fMRI. In this study, we explore the effect of prior surgery on preoperative BOLD fMRI accuracy by comparing activation volumes in the primary motor cortex (PMC) between patients with and without prior brain surgery.
Methods
Subjects
Forty-three consecutive patients with pathologically confirmed brain tumors who were referred to the Functional MR Imaging Laboratory for preoperative localization of the motor cortex were included in this study (Table 1). Thirteen patients had undergone prior neurosurgery, and 30 had not. Of those with prior surgery, 8 were women and 5 were men. The pathologic breakdown was 3 glioblastoma multiforme (GBM), 3 metastases, 2 astrocytomas grade III, 1 oligodendroglioma grade III, 1 astrocytoma grade II, 1 oligodendroglioma grade II, 1 hemangiopericytoma, and 1 meningioma. The average age was 42.6 years, with a range of 19–68 years. None of the lesions demonstrated evidence for hemorrhagic products or calcifications. Of those patients who had not undergone prior surgery, 16 were women and 14 were men. The pathologic breakdown was 12 GBM, 11 metastases, 1 astrocytoma grade III, 1 oligodendroglioma grade III, 2 astrocytomas grade II, 1 oligodendroglioma grade II, 1 oligoastrocytoma, and 1 meningioma. The average age was 56.2 years, with a range of 36–85 years. All patients had tumors within or directly adjacent to the PMC. The study was approved by the institutional review board.
TABLE 1:
Patient characteristics and primary motor cortex volume of activation measurements
| Patient No./Age (y)/Sex | Histologic Diagnosis | Prior Surgery | VoA (NT) | VaO (T) | Ratio (NT/T) |
|---|---|---|---|---|---|
| 1/70/F | GBM | No | 1345 | 3433 | 0.39 |
| 2/79/M | GBM | No | 285 | 459 | 0.62 |
| 3/51/M | GBM | No | 4271 | 4999 | 0.85 |
| 4/61/M | GBM | No | 2246 | 2341 | 0.96 |
| 5/48/F | GBM | No | 4762 | 4414 | 1.08 |
| 6/43/M | GBM | No | 6819 | 5142 | 1.33 |
| 7/69/M | GBM | No | 3449 | 2215 | 1.56 |
| 8/57/M | GBM | No | 4825 | 3038 | 1.59 |
| 9/58/F | GBM | No | 791 | 490 | 1.61 |
| 10/39/F | GBM | No | 2658 | 1503 | 1.77 |
| 11/72/F | GBM | No | 1202 | 380 | 3.17 |
| 12/48/M | GBM | No | 5743 | 1234 | 4.65 |
| 13/65/M | Metastasis | No | 411 | 4888 | 0.08 |
| 14/44/M | Metastasis | No | 949 | 3971 | 0.24 |
| 15/56/F | Metastasis | No | 4113 | 4888 | 0.84 |
| 16/61/F | Metastasis | No | 1819 | 1582 | 1.15 |
| 17/62/F | Metastasis | No | 2452 | 2041 | 1.20 |
| 18/56/F | Metastasis | No | 4588 | 3575 | 1.28 |
| 19/57/F | Metastasis | No | 3939 | 3056 | 1.29 |
| 20/57/M | Metastasis | No | 1440 | 617 | 2.33 |
| 21/53/F | Metastasis | No | 2120 | 870 | 2.44 |
| 22/57/F | Metastasis | No | 2626 | 775 | 3.39 |
| 23/48/M | Metastasis | No | 3212 | 759 | 4.23 |
| 24/52/M | Oligodendroglioma, G-III | No | 633 | 1503 | 0.42 |
| 25/76/M | Astrocytoma, G-III | No | 2167 | 696 | 3.11 |
| 26/43/M | Astrocytoma, G-II | No | 1139 | 4825 | 0.24 |
| 27/36/F | Oligodendroglioma, G-II | No | 4588 | 5585 | 0.82 |
| 28/47/F | Astrocytoma, G-II | No | 5031 | 5031 | 1.00 |
| 29/36/F | Oligoastrocytoma, G-II | No | 4351 | 3196 | 1.36 |
| 30/85/F | Meningioma | No | 1756 | 2136 | 0.82 |
| Avg values of patients without prior surgery (n = 30) | 2858 | 2655 | 1.08 | ||
|---|---|---|---|---|---|
| 31/52/M | GBM | Yes | 6913 | 1883 | 3.67 |
| 32/37/F | GBM | Yes | 6154 | 1471 | 4.18 |
| 33/68/F | GBM | Yes | 4050 | 664 | 6.10 |
| 34/53/M | Metastasis | Yes | 807 | 601 | 1.34 |
| 35/58/F | Metastasis | Yes | 5648 | 2136 | 2.64 |
| 36/46/F | Metastasis | Yes | 4082 | 190 | 21.50 |
| 37/48/M | Astrocytoma, G-III | Yes | 823 | 1867 | 0.44 |
| 38/23/F | Astrocytoma, G-III | Yes | 2832 | 1060 | 2.67 |
| 39/45/F | Oligodendroglioma, G-III | Yes | 2927 | 63 | 46.25 |
| 40/31/M | Astrocytoma, G-II | Yes | 2974 | 823 | 3.62 |
| 41/19/F | Oligodendroglioma, G-II | Yes | 2152 | 253 | 8.50 |
| 42/39/F | Atypical meningioma | Yes | 1218 | 633 | 1.93 |
| 43/35/M | Low-grade hemangiopericytoma | Yes | 4161 | 1946 | 2.14 |
| Avg values of patients with prior surgery (n = 13) | 3442 | 1045 | 3.29 | ||
Note.—VoA indicates volume of activation (mm3); NT, nontumor side; T, tumor side; GBM, glioblastoma multiforme.
Imaging Sequences
The MR imaging data were acquired with a GE 1.5T LX scanner (GE Medical System, Milwaukee, WI) with a standard birdcage radio-frequency coil. The functional images were acquired by using gradient echo echo-planar imaging (EPI) (TR/TE, 4000/40 ms; 21 axial slices; 128 × 128 matrix; 90° flip angle; 4.5-mm slice thickness with no gap, 240-mm field of view [FOV]). With 4 dumping scans, the total acquisition time for the finger-tapping functional task (described below) was 376 seconds. T1-weighted spin-echo images (TR/TE, 600/8 ms; 21 slices; 256 × 256 matrix; 90° flip angle; 4.5-mm slice thickness with no gap; 240-mm FOV) were obtained for anatomic reference. 3D T1-weighted anatomic images were acquired with a spoiled gradient-recalled sequence (TR/TE, 22/4 ms; 256 × 256 matrix; 30° flip angle; 1.5-mm slice thickness with no gap; 240 mm FOV). The functional EPI and anatomic T1-weighted spin-echo images were acquired with the same number of sections and orientation as with the axial plane for coregistration of fMRI signal intensity on the anatomic images.
Functional Tasks
Patients performed a finger-tapping paradigm, consisting of self-paced bilateral finger-to-thumb tapping, to activate the motor cortex. The patient alternated between execution (6 intervals of 20 seconds each) and rest (6 intervals of 40 seconds each) for a total of 360 seconds. The patient practiced the paradigm before the scan and was observed during the scan to ensure adequate performance. Instructions to start and stop were given over the auditory system.
Image Processing and Analysis
The fMRI data were analyzed by using Analysis of Functional NeuroImaging (AFNI, Milwaukee, WI) software (15). 3D rigid-body registration was used to align the reconstructed fMRI data against a reference image, and spatial smoothing (Gaussian filtering: full width of half maximum = 5 mm) was applied. The volume of activation of the PMC was determined by using a cross-correlation technique (16). A maximal statistical threshold of P < .001 for each patient was determined to localize PMC activation and minimize false-positive pixels.
To obtain the volume of fMRI activation in the PMC, regions of interest were drawn around the precentral gyrus on both the tumor and nontumor sides. The number of activated pixels was then multiplied by the in-plane resolution and section thickness to give total volume of activation.
The location and prominence of the susceptibility artifact, as well as its proximity to the PMC, were assessed subjectively by a fellowship-trained neuroradiologist on the basis of the T2-weighted images. The T2-weighted images were compared to the routine MR imaging, to determine the probable cause the artifact, including prior surgery.
Statistical Evaluation
Paired t tests were used to test for differences between the mean volume of activation in the PMC on the tumor side and the mean volume of activation in the PMC on the nontumor side. A linear regression model was used to assess whether the expected difference in the mean activation volume in the PMC on the tumor side between those patients who had prior surgery and those who did not have prior surgery was significantly different, with adjustments made for the activation volume in the PMC on the nontumor side. All statistical tests were 2-sided, with a P value ≤.05 considered significant. Analyses were performed in S-PLUS (version 6.2 for Windows; Insightful Corp., Seattle WA).
Results
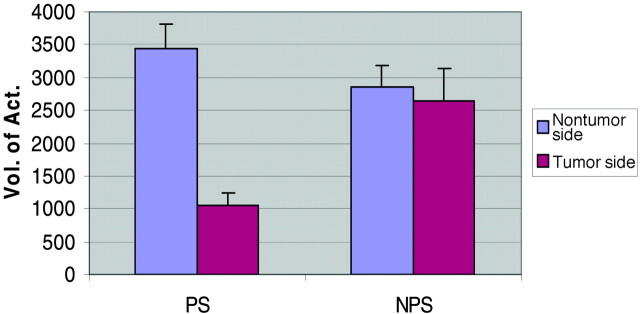
Table 2 provides a statistical summary of the activation volume measurements in the prior surgery and no prior surgery groups. No significant difference was found between the mean tumor and nontumor volumes of activations in patients who did not have prior surgery (P = .51). In contrast, a significant difference was noted between the mean tumor side and nontumor side volumes of activations in patients who had prior surgery (P = .001). Notably, this difference is accounted for primarily by a decrease in the tumor side activation volume, not an increase in the nontumor side activation volume (Fig 1).
TABLE 2:
Comparison of volume of activation measurements (mm3) between tumor side and nontumor side in patients with or without prior surgery
| Prior Surgery (n = 13) |
No Prior Surgery (n = 30) |
|||
|---|---|---|---|---|
| Tumor Side | Nontumor Side | Tumor Side | Nontumor Side | |
| Mean | 1045 | 3442 | 2655 | 2858 |
| Median | 823 | 2974 | 2278 | 2539 |
| SD | 733 | 1976 | 1734 | 1753 |
| Range | 63–2136 | 807–6913 | 380–5585 | 285–6819 |
Fig 1.
Average volume of activation measurements in prior-surgery (PS) and no-prior-surgery (NPS) groups. There is a marked decrease in the tumor side activation volumes of patients with prior surgery.
Patients who had prior surgery showed a significant decrease in tumor side PMC activation volume compared with patients who did not have prior surgery (P ≤ .001; (Table 3). The expected difference in tumor side activation volume between patients with and without prior surgery was 1815 mm3.
TABLE 3:
Multivariate linear regression analysis of expected difference in volume of fMRI activation in motor strip on tumor side in patients with prior surgery
| Expected Difference | 95% CI | P | |
|---|---|---|---|
| Difference in tumor side volume (mm3) associated with a 1-mm3 increase in nontumor side volume | 0.35 | (0.12, 0.59) | .005 |
| Difference in tumor side volume (mm3) associated with having surgery | −1815 | (−2745, −885) | <.001 |
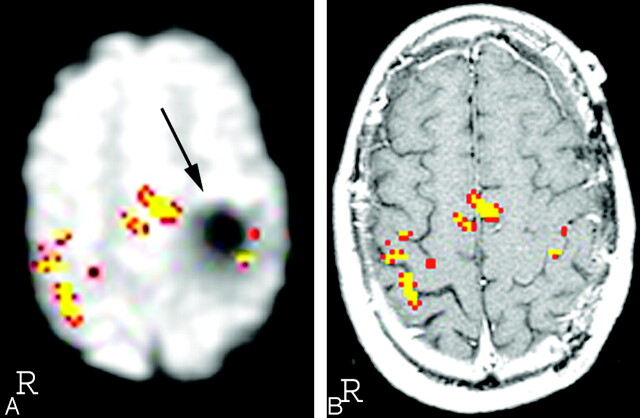
Ten of the 13 patients in the prior surgery group showed the presence of prominent signal intensity dropouts, all of which were on the side of the brain where surgery had been performed. The degree of overlap between the signal intensity dropout and the PMC varied greatly between patients, depending on the location of the susceptibility artifact. If the artifact was directly adjacent to the PMC, fMRI activation was reduced by a prominent degree (Fig 2). In other cases, fMRI activation was either partially reduced (Fig 3) or not affected by the susceptibility artifact.
Fig 2.
In patient 40, there is robust activation in the right motor cortex but little detectable activity in the left motor cortex. In the T2-weighted image (A), the area containing the signal intensity void (arrow) corresponds to the physical location of a titanium plate installed during a prior operation. The susceptibility artifact is not visible in the T1-weighted image (B).
Fig 3.
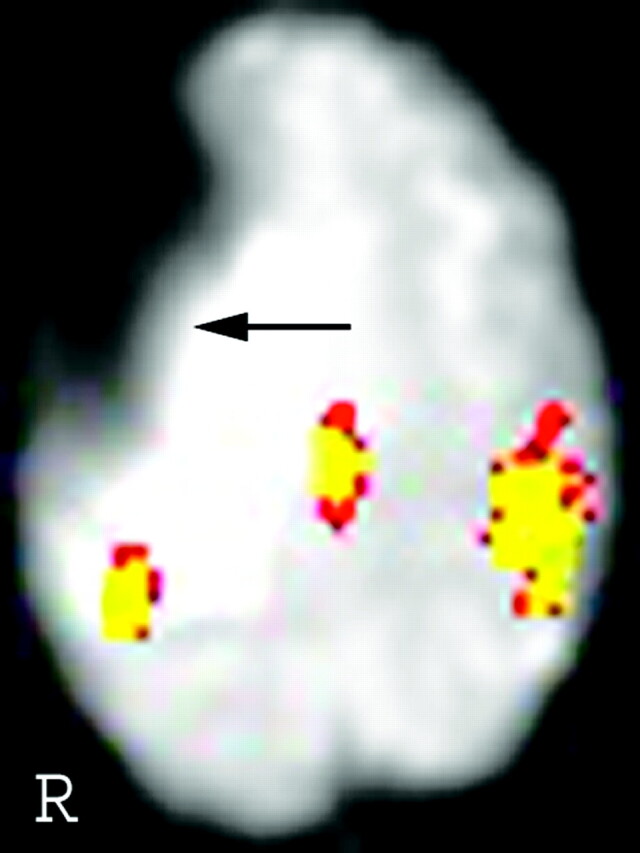
In patient 33, the location of the susceptibility artifact is further away from the motor cortex than in patient 40 (Fig 2). Correspondingly, the signal intensity dropout is less severe.
Notwithstanding the presence of artifacts, we were able to identify the PMC in all patients and to convey this information to the operating neurosurgeon. Intraoperative cortical stimulation confirmed the fMRI results in every case.
Discussion
BOLD fMRI is a noninvasive method increasingly used for the determination of eloquent brain areas in presurgical planning. Despite its growing prevalence, susceptibility artifacts can interfere with BOLD signal intensity acquisition, which may lead to the misinterpretation of images. To enhance the utility of BOLD fMRI in presurgical planning, the conditions in which the procedure is accurate and, more important, inaccurate must be defined.
Our data suggest that BOLD fMRI detects significantly less activation volumes in the tumor side PMC of patients who have undergone prior neurosurgery versus patients without prior neurosurgery. Subjective review of the T2 source images showed that the difference in the volume of activation was most likely due to the presence of susceptibility artifacts from the prior surgery. Studies have shown that the presence of susceptibility artifacts can affect BOLD signal intensity acquisition (17).
A number of other possible explanations exist for the results. First, it has been shown that there is a decrease in the volume of fMRI activation adjacent to GBMs (4, 18, 19). It has been suggested that this may be due to the presence of abnormal tumor neovasculature and the resultant decoupling of neuronal activity and blood flow (4, 18). This explanation, however, does not appear likely, because there were 12 GBMs in 30 cases (40%) in the nonsurgery group and 3 GBMs in 13 cases (23%) in the surgery group. Therefore, had this mechanism played a major role, one would have expected the opposite results (ie, less fMRI activation on the tumor side in the nonsurgery group) of what was observed. We would like to stress that we do not think the present results negate the aforementioned studies; rather, it appears that prior surgery has a greater influence on the volume of fMRI activation than do the effects of abnormal tumor neovasculature.
Another possible explanation is that the decrease in fMRI activation accurately reflects a true decrease in neuronal activity. In such a scenario, the fMRI data may be the result of cortical reorganization due to the growth of an adjacent tumor or due to the actual (rather than artifactual) effects of the surgery itself. Cortical reorganization has been shown to occur due to the growth of brain tumors (20, 21). fMRI studies that showed cortical reorganization have influenced surgical planning and resection of brain tumors (22).
It does not appear that cortical reorganization or the effect of surgery itself played a major role in our results. There was only a slight (statistically insignificant) increase in the volume of activation on the side ipsilateral to the tumor in the surgery group, which may lead one to suspect the presence of cortical reorganization; however, the decrease in fMRI activation was much greater (and statistically significant) on the tumor side in the patients with prior surgery. In addition, it is not immediately clear why cortical reorganization should affect patients with prior surgery more than those without. The timing of cortical reorganization and the relationship to tumor pathology remain to be determined.
The main support for the idea that the drop in the volume of fMRI activation is due to susceptibility artifacts from prior surgery is from visual inspection of the T2 images. In each case, visual inspection demonstrated that the decrease in fMRI signal intensity was due to the presence of prominent signal intensity dropout from the susceptibility artifact. Also, the difference in the volume of activation between the 2 sides was greater if the artifact was closer to the PMC (compare Fig 2 and Fig 3). Therefore, it is imperative to examine the T2 images and not just the analyzed fMRI data coregistered onto the T1-weighted images, because the susceptibility artifact present on the T2*-weighted images is no longer visualized on the coregistered images (Fig 2). Analysis of the “source” T2-weighted images (from which the fMRI data are derived) will allow the radiologist to acknowledge the existence of the artifact and draw the appropriate conclusions.
It should be stressed that the correlation of the accuracy of fMRI with the intraoperative results was on the level of the gyrus. Correlation of fMRI activation to intraoperative cortical stimulation was not performed on a subgyral level. Therefore, it is entirely possible (and, in fact, probable) that an area of the PMC that exhibited an increased neuronal activity and a resultant increase in blood flow secondary to the performance of the fMRI paradigm did not show fMRI activation due to the presence of susceptibility artifact. If one defines accuracy as the resolution on the level of the gyrus and in terms of the ability to identify the PMC, BOLD fMRI was successful in all 13 prior surgery patients. In this definition, the accuracy of fMRI is relatively unaffected by susceptibility artifacts caused by prior surgery; however, depending on the location and size of the signal intensity dropout, BOLD fMRI may not be able to define the entire area of increased neuronal activation in the precentral gyrus in all cases.
fMRI scanning parameters are chosen to optimize the BOLD effect—the difference in signal intensity between oxy-Hb and deoxy-Hb, which is based on susceptibility. This optimization of the BOLD effect also causes an increase in the susceptibility artifact. One potential way of overcoming the deleterious effect of susceptibility artifacts may be to implement a BOLD fMRI sequence with a decreased sensitivity to susceptibility. This will reduce the inherent BOLD effect, but may also serve to identify functionally active areas that otherwise would not have been visualized due to susceptibility artifact.
A 3T scanner offers a clear advantage in BOLD fMRI signal intensity over a 1.5T scanner. The physical properties that lead to an improved BOLD fMRI signal intensity on a 3T scanner, however, also lead to an increase in susceptibility artifact. In the present study, we neither used a 3T scanner nor compared the postoperative susceptibility of a 1.5T scanner to a higher field magnet. Nevertheless, the results of the current study, which attribute a significant susceptibility artifact to neurosurgical intervention that would only be exacerbated by using a higher-field-strength machine, may give pause to use of a 3T scanner in a postoperative fMRI study. It is clear that more research, as well as improved methods, is needed in this area to minimize the deleterious effects of postoperative susceptibility artifacts.
Recently, there has been progress in both neurosurgical techniques and instrumentation to minimize susceptibility artifacts. This is probably most evident in aneurysm clips and spinal hardware; however, a neurosurgeon may be reluctant to change the operation or other therapy (for example, whether to insert Gliadel wafers) on the basis of future BOLD fMRI considerations. In conveying the preoperative BOLD fMRI results to our neurosurgical colleagues, we will always mention the presence of susceptibility artifacts on the T2 images, which may influence the accuracy of the BOLD fMRI results. In such cases, the neurosurgeon may elect to map the motor cortex in greater detail, rather than just confirming the BOLD fMRI results.
Conclusion
Our findings indicate that patients who had prior neurosurgery show decreased volumes of fMRI activation on the tumor side compared with patients who have not had prior surgery. This is most likely caused by the prominent signal intensity dropout on the T2 images (used to acquire the BOLD fMRI data) due to the susceptibility artifacts resulting from the previous operation. The astute radiologist who recognizes this phenomenon, however, will still be able to draw the appropriate conclusions and correctly identify the PMC.
References
- 1.Lee CC, Ward HA, Sharbrough FW, et al. Assessment of functional MR imaging in neurosurgical planning. AJNR Am J Neuroradiol 1999;20:1511–1519 [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller WM, Yetkin FZ, Hammeke TA, et al. Functional magnetic resonance imaging mapping of the motor cortex in patients with cerebral tumors. Neurosurgery 1996;39:515–521 [DOI] [PubMed] [Google Scholar]
- 3.Jack CR, Thompson RM, Butts RK, et al. Sensory motor cortex: correlation of presurgical mapping with functional MR imaging and invasive cortical mapping. Radiology 1994;190:85–92 [DOI] [PubMed] [Google Scholar]
- 4.Holodny AI, Schulder M, Liu WC, et al. The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: implications for image-guided neurosurgery. AJNR Am J Neuroradiol 2000;21:1415–1422 [PMC free article] [PubMed] [Google Scholar]
- 5.Maldjian JA, Schulder M, Liu WC, et al. Intraoperative functional MRI using a real-time neurosurgical navigation system. J Comput Assist Tomogr 1997;21:910–912 [DOI] [PubMed] [Google Scholar]
- 6.McDonald JD, Chong BW, Lewine JD, et al. Integration of preoperative and intraoperative functional brain mapping in a frameless stereotactic environment for lesions near eloquent cortex. J Neurosurg 1999;90:591–598 [DOI] [PubMed] [Google Scholar]
- 7.Gumprecht H, Ebel GK, Auer DP, Lumenta CB. Neuronavigation and functional MRI for surgery in patients with lesion in eloquent brain areas. Minim Invasive Neurosurg 2002;45:151–153 [DOI] [PubMed] [Google Scholar]
- 8.Nimsky C, Ganslandt O, Kober H, et al. Integration of functional magnetic resonance imaging supported by magnetoencephalography in functional neuronavigation. Neurosurgery 1999;44:1249–1255 [DOI] [PubMed] [Google Scholar]
- 9.Schulder M, Maldjian JA, Liu WC, et al. Functional image-guided surgery of intracranial tumors located in or near the sensorimotor cortex. J Neurosurg 1998;89:412–418 [DOI] [PubMed] [Google Scholar]
- 10.Belliveau JW, Rosen BR, Kantor HL, et al. Functional cerebral imaging by susceptibility-contrast NMR. Magn Reson Med 1990;14:538–546 [DOI] [PubMed] [Google Scholar]
- 11.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 1990;87:9868–9872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeYoe EA, Bandettini P, Neitz J, et al. Functional magnetic resonance imaging of the human brain. J Neurosci Methods 1994;54:171–187 [DOI] [PubMed] [Google Scholar]
- 13.Toronov V, Walker S, Gupta R. The roles of changes in deoxyhemoglobin concentration and regional cerebral blood volume in the fMRI BOLD signal. Neuroimage 2003;19:1521–1531 [DOI] [PubMed] [Google Scholar]
- 14.Vlieger EJ, Majoie CB, Leenstra S, Den Heeten GJ. Functional magnetic resonance imaging for neurosurgical planning in neurooncology. Eur Radiol 2004;14:1143–1153 [DOI] [PubMed] [Google Scholar]
- 15.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29:162–173 [DOI] [PubMed] [Google Scholar]
- 16.Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med 1993;30:161–173 [DOI] [PubMed] [Google Scholar]
- 17.Haberg A, Kvistad KA, Unsgard G, Haraldseth O. Preoperative blood oxygen level-dependent functional magnetic resonance imaging in patients with primary brain tumors: clinical application and outcome. Neurosurgery 2004;54:902–914 [DOI] [PubMed] [Google Scholar]
- 18.Holodny AI, Schulder M, Liu WC, et al. Decreased BOLD functional MR activation of the motor and sensory cortices adjacent to a glioblastoma multiforme: implications for image-guided neurosurgery. AJNR Am J Neuroradiol 1999;20:609–612 [PMC free article] [PubMed] [Google Scholar]
- 19.Schreiber A, Hubbe U, Ziyeh S, Hennig J The influence of gliomas and nonglial space-occupying lesions on blood-oxygen-level-dependent contrast enhancement. AJNR Am J Neuroradiol 2000;21:1055–1063 [PMC free article] [PubMed] [Google Scholar]
- 20.Fandino J, Kollias SS, Wieser HG, et al. Intraoperative validation of functional magnetic resonance imaging and cortical reorganization patterns in patients with brain tumors involving the primary motor cortex. J Neurosurg 1999;91:238–250 [DOI] [PubMed] [Google Scholar]
- 21.Holodny AI, Schulder M, Ybasco A, Liu WC. Translocation of Broca’s area to the contralateral hemisphere as the result of the growth of a left inferior frontal glioma. J Comput Assist Tomogr 2002;26:941–943 [DOI] [PubMed] [Google Scholar]
- 22.Petrovich NM, Holodny AI, Brennan CW, Gutin PH. Isolated translocation of Wernicke’s area to the right hemisphere in a 62-year-man with a temporo-parietal glioma. AJNR Am J Neuroradiol 2004;25:130–133 [PMC free article] [PubMed] [Google Scholar]