Abstract
BACKGROUND AND PURPOSE: Reports of MR imaging in West Nile virus (WNV) meningoencephalomyelitis are few and the described findings limited. The purpose of this study was to review the spectrum of MR imaging findings for WNV meningoencephalomyelitis and investigate whether any of the findings correlates with clinical presentation of flaccid paralysis.
METHODS: We reviewed the MR imaging findings of 17 patients with confirmed WNV encephalitis and/or myelitis. MR imaging brain studies were evaluated for location of signal intensity abnormalities, edema, hydrocephalus, or abnormal enhancement. MR imaging spine studies were evaluated for signal intensity abnormalities in cord and/or enhancement.
RESULTS: Retrospective review of the MR imaging studies of 17 patients was performed by 2 neuroradiologists. Eleven of 16 brain MR images demonstrated abnormalities. Eight (50%) patients had abnormal studies related to meningoencephalitis. All 8 patients had abnormal findings in the deep gray matter and/or brain stem; 2 had additional white matter abnormalities. Three patients with abnormal MR studies of the spine had extremity weakness on examination. The imaging findings included abnormal signal intensity more pronounced in the ventral horns and/or enhancement around the conus medullaris and cauda equina. One patient had additional abnormalities in the pons.
CONCLUSION: Abnormal MR imaging findings in patients with WNV meningoencephalomyelitis are nonspecific but not uncommon. Anatomic areas commonly affected are basal ganglia, thalami, mesial temporal structures, brain stem, and cerebellum. Extremity weakness or flaccid paralysis corresponds to spinal cord/cauda equina abnormalities.
Among patients infected by the West Nile virus (WNV), only a small number will present with clinical symptoms and an even a smaller number with meningoencephalitis. Nonetheless, WNV has become an emerging infection with both rapid increases in incidence, as well as geographic range. Published articles following the 1999 outbreak referred to flaccid paralysis, though they only briefly mentioned MR imaging findings (1, 2). Case reports in the radiology literature described only a few imaging findings in the brain or spine (3, 4). The purpose of this paper was to review the spectrum of MR imaging findings in the brain and spine with documented WNV meningoencephalitis and investigate any possible correlation between certain imaging findings and the clinical presentation of flaccid paralysis.
Methods
The study was approved by our institutional review board (IRB case 6564). We retrospectively retrieved from the electronic data base the MR imaging studies of 17 patients imaged between August 2002 and December 2002 who had been diagnosed with WNV meningoencephalitis and/or myelitis. Diagnosis was established by using Centers for Disease Control and Prevention criteria for WNV infection, which included IgM positivity in CSF by using the capture enzyme-linked immunoassay or immunofluorescence assay technique and/or histopathologic examination of tissue at autopsy (a single case) or biopsy (a single case). Among the 23 patients hospitalized with CNS WNV infections, 16 had at least one MR imaging of the brain. Five of these patients had MR imaging of the entire spine as well. One patient had only MR imaging of the spine. One patient had a follow-up MR imaging of the brain, and one patient had 3 follow-up MR imaging studies of the brain. We reviewed the imaging findings of a total of 20 brain and 6 spine MR imaging studies.
The MR imaging technique varied based on the provided clinical history at the time of imaging. All MR imaging studies were performed on a 1.5T system. Our standard brain protocol included sagittal T1-weighted (TR/TE, 614/12), axial fast spin-echo (FSE) T2-weighted (TR/TE, 3310/93), fluid attenuated inversion recovery (FLAIR; TR/TE, 6490/2000/107) and diffusion weighted (DW) spin-echo echo-planar imaging (TR/TE, 4900/167; 3-direction sensitivity, 3 b values used: 0, 500, 1000), coronal T1-weighted and postcontrast T1-weighted axial and coronal scans. Studies of the spine included sagittal T1-weighted and FSE T2-weighted, axial gradient echo (GRE), and postcontrast sagittal T1-weighted. The imaging studies were as follows: 16 MR imaging studies of the brain included pre- and postcontrast images, 13 of which were supplemented with diffusion-weighted images as well. All 4 unenhanced studies included diffusion-weighted images.
Two neuroradiologists retrospectively reviewed the MR images and analyzed the type of lesions, as well as their distribution and conspicuity, on different MR imaging sequences. Differences in interpretation of subtle findings were resolved by consensus.
Results
A total of 17 patients hospitalized with WNV meningoencephalomyelitis and imaged at our institution were included in this study. There were 12 male and 5 female patients (age range, 40–85 years; mean age, 68.9 years). Five of 16 patients had negative MR imaging of the brain. The brain and spinal cord abnormalities seen on the MR studies are summarized on Table 1. Three of the 11 positive studies demonstrated acute ischemic changes. The MR imaging studies of the remaining 8 patients demonstrated lesions in the basal ganglia, mesial temporal lobe, thalami, midbrain, pons, cerebellum, and hemispheric white matter. Two of these patients (2 and 17) had follow-up studies. Although follow-up MR imaging of patient 2 revealed persistent posterior fossa abnormalities and new lesion in the spine (Fig 1A–E), 3 consecutive studies on one patient (17) disclosed waxing and waning lesions involving gray and white matter in the supratentorial and infratentorial brain (Fig 2A–Q). A second patient (11) had also cerebral white matter lesions in addition to deep brain abnormalities (Fig 3A–D). Autopsy of this patient performed 25 days after the MR imaging revealed multiple subacute infarcts (Fig 3G) throughout the hemispheric white matter, subacute to remote infarct right internal capsule and lipohyalinosis of cerebral blood vessels. Additional findings such as microglial nodules in the mesial temporal lobe, brain stem, and cerebellar molecular layer, as well as generalized microglial activation throughout the neuroaxis and perivascular lymphocytic infiltration (Fig 3F), supported the diagnosis of encephalitis.
MR findings in WNV meningoencephalomyelitis
| Patient No./Age (y)/Sex | History | MRI Brain | DWI Avail. | Brain MRI Findings | Follow-Up MRI/Other | MRI Spine | Spine MRI Findings | Figures |
|---|---|---|---|---|---|---|---|---|
| 1/75/M | R/o Stroke | W/o | Yes | Right frontal lobe infarct | 5A, -B | |||
| 2/40/M | Encephalitis, extremity weakness | W/ and w/o | Yes | Pons, cerebellar peduncle | MRI brain | W/ and w/o | Cord lesion, nerve root enhancement | 1A–G |
| 3/75/F | Headache | W/o | Yes | Negative | ||||
| 4/79/F | After open heart surgery, r/o stroke | W/o | Yes | Thalamus | W/o | Degenerative changes | ||
| 5/54/M | Meningitis | W/ and w/o | Mesial temporal lobe b/l | |||||
| 6/82/F | Meningitis | W/ and w/o | Yes | Acute lacunar infarct left internal capsule | ||||
| 7/49/M | R/o Stroke | W/ and w/o | Yes | Negative | ||||
| 8/83/M | R/o Stroke | W/ and w/o | Yes | Left PCA infarct | ||||
| 9/62/M | Encephalitis | W and w/o | Yes | Negative | ||||
| 10/74/M | R/o Stroke | W/o | Yes | Negative | W/ and w/o | Negative | ||
| 11/51/M | After renal and pancreatic transplant, r/o stroke | W/ and w/o | Yes | Cerebral white matter, midbrain, temporal lobe | Autopsy | 3A–G | ||
| 12/76/M | Encephalitis | W/ and w/o | Yes | Mesial temporal lobe | ||||
| 13/85/F | Meningoencephalitis | W/ and w/o | Yes | Mesial temporal lobe | ||||
| 14/76/M | Encephalitis | W/ and w/o | Yes | Negative | W/ and w/o | Degenerative changes | ||
| 15/82/M | Extremity weakness | W/ and w/o | Dural enhancement | W/ and w/o | Enhancement of conus and nerve roots | |||
| 16/80/M | Meningitis, extremity weakness | W/ and w/o | Cervical cord lesion, enhancement conus and cauda equina | 4A, -B | ||||
| 17/49/F | Encephalitis, extremity weakness | W/ and w/o | Yes | Cerebral white matter, mesial temporal lobe thalamus, red nucleus substantia nigra dentate nucleus, cerebellum | 3 MRI brain/biopsies | 2A–Q |
Note.—W/ indicates with contrast; w/o, without contrast.
Fig 1.
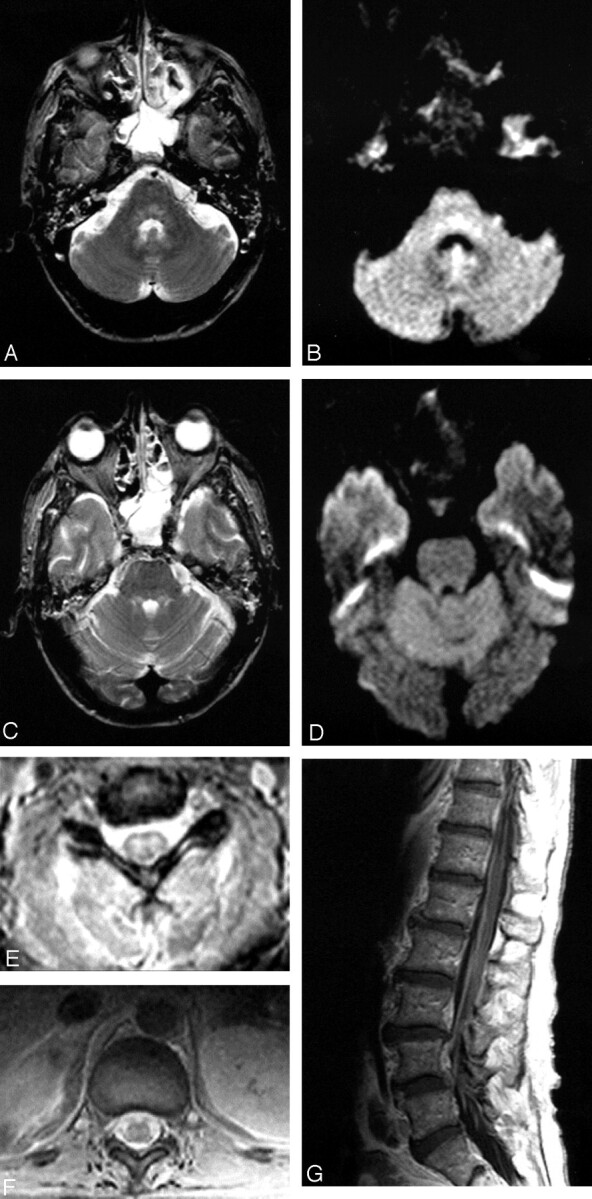
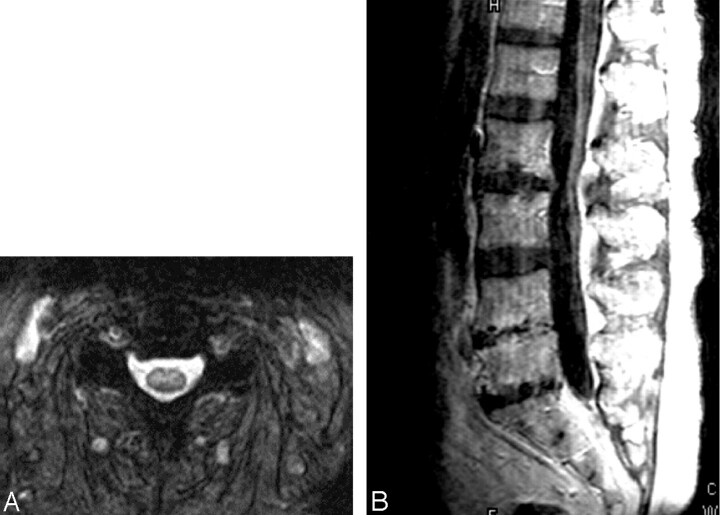
Patient 2, a 40-year-old man admitted with encephalitis and developed flaccid paralysis during hospitalization: axial FSE T2-weighted (A) and trace DW (B) images demonstrate increased signal intensity in the pontine tegmentum. Increased signal intensity was also seen in the superior cerebellar peduncles on FSE T2-weighted image (C), but not on the trace DW image (D). The patient was intubated at the time of imaging, which resulted in opacified sinuses and mastoid air cells. Axial GRE sequences through the cervical (E) and thoracic cord (F) show abnormal signal intensity in the gray matter with more pronounced involvement of the ventral horns. Postcontrast sagittal T1-weighted image (G) demonstrates enhancement of the cauda equina.
Fig 2.
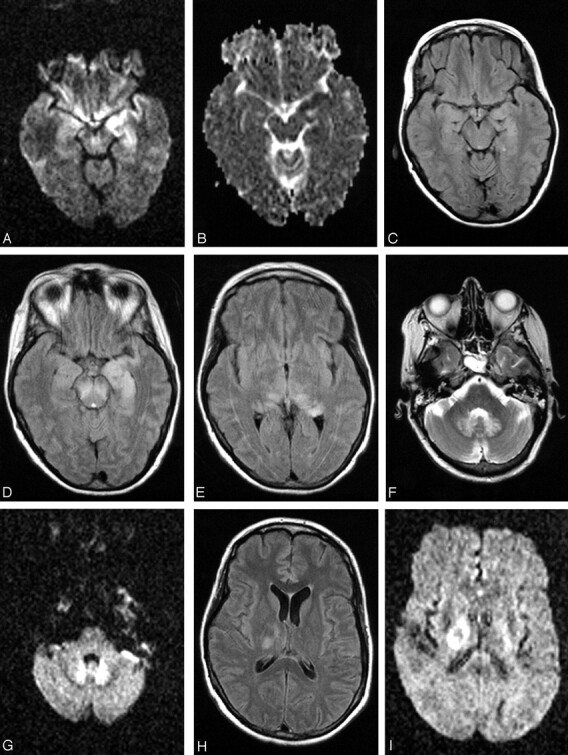
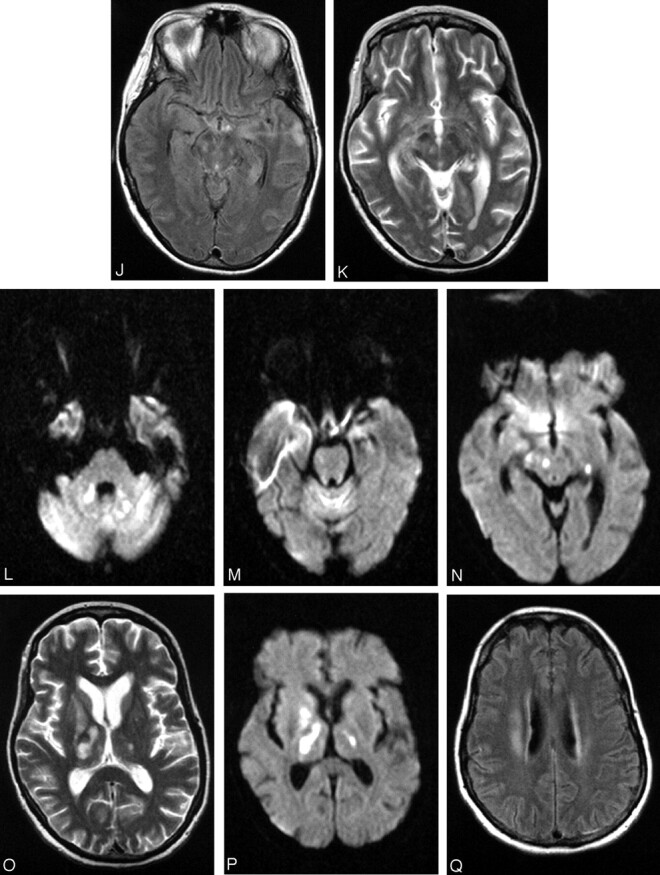
Patient 17, a 49-year-old woman with history of non-Hodgkin lymphoma in remission presented with fever. Work-up was negative for recurrence. The first MR imaging of the brain shows abnormality in the left mesial temporal lobe on trace DW image (A) and ADC (B), though the finding is subtle on the FLAIR sequences (C). At the time of imaging, the findings raised the question of herpes encephalitis, for which the patient was originally treated. Following deterioration of the mental status and development of upper extremity weakness, a second MR imaging was obtained. The abnormality is now apparent on the FLAIR sequences and has progressed to involve not only the contralateral mesial temporal structures, but also the substantia nigra (D), as well as the mesial and dorsal aspect of the thalami (E). Further clinical deterioration with established “polio-like” symptoms prompted a new MR imaging 11 days later, which demonstrated new areas of involvement with resolution of improvement of prior lesions. Images of this MR imaging show increased signal intensity in the dentate nuclei on FLAIR (F) and DW (G) images and right thalamus on FLAIR image (2 hours) and DW image (I) and improvement of the mesial temporal lobe and midbrain abnormalities (J). Focus of T2 hyperintensity is seen in the right red nucleus on FSE T2-weighted image (K). On J, note site of biopsy in the lateral aspect of the left temporal lobe that was negative for herpes encephalitis. The fourth MR imaging was obtained upon patient’s discharge to a nursing facility. DW images show increased signal intensity in the cerebellar hemispheres and right branchium pontis (L), vermis (M), and the right red nucleus (N). Cerebellar hemisphere abnormalities were more conspicuous on DW than FLAIR or FSE T2-weighted images. FSE T2-weighted (O) and DW (P) images through the level of the basal ganglia demonstrate persistent abnormality in the right thalamus and new lesions in the right globus pallidus and left thalamus, whereas the FLAIR image demonstrates involvement of the right corona radiata (Q) as well.
Fig 3.
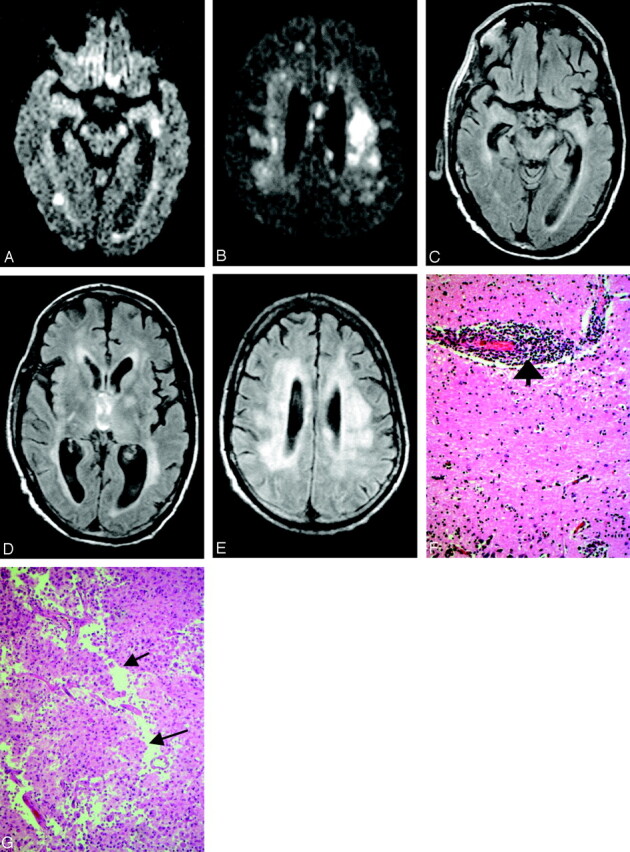
Patient 11, a 51-year-old man post renal and pancreatic transplant with recent mosquito exposure. Axial DW images show patchy areas of increased signal intensity in the periventricular white matter and left cerebral peduncle (A) as well as corona radiata and corpus callosum (B). Axial FLAIR sequences demonstrate subtle abnormalities in the left mesial temporal lobe, both cerebral peduncles, more pronounced in the left one (C), and both thalami and left globus pallidus (D). Diffusely increased signal intensity is also present in the deep hemispheric white matter (D and E). Histopathology (F) shows the hippocampus with marked perivascular (bold arrow) lymphocytic inflammation (hematoxylin and eosin; original magnification 200×). Histopathology (G) demonstrates white matter infarct with necrosis, macrophage and early cavitary changes (cavitation delineated by arrows) (hematoxylin and eosin; original magnification 200×).
Mild thalamic abnormalities were noticed as an isolated finding in one patient. Thalami, however were involved in 2 patients as part of multifocal manifestation. Five patients had involvement of the mesial temporal lobe, 3 of them as a sole finding, one along with other lesions (11), and one as initial finding (17). Left temporal lobe biopsy in the latter patient following the initial MR imaging showed inflammatory changes, but no evidence of necrotizing encephalitis, thus excluding the originally suspected herpes encephalitis.
None of the brain parenchyma lesions was associated with enhancement. One MR imaging of the brain showed pachymeningeal enhancement; however, this patient had undergone lumbar puncture, so the enhancement could be reactive. There were 3 negative MR imaging spine studies of 6 reviewed, one of them unenhanced (Table 1). Two studies were positive for T2 hyperintensity in the cord, especially in the ventral horns along with nerve root enhancement (Fig 4A and -B). One study showed only nerve root enhancement.
Fig 4.
Patient 16, an 80-year-old man presented with extremity weakness. Axial GRE image through cervical spine (A) shows increased signal intensity in the gray matter, whereas postcontrast sagittal T1-weighted sequences (B) reveal nerve root enhancement.
Discussion
WNV is a single-strand RNA virus belonging to the flavivirus family. Phylogenetically closely related viruses include the agents of Japanese encephalitis, St. Louis encephalitis, Murray Valley encephalitis, and Kunjin. The WNV strain circulating in North America is molecularly indistinguishable from WNV strain isolated from an Israeli duck in 1998, identifying the likely source of the virus (5). The WNV appeared unexpectedly in the Western Hemisphere in 1999, with an outbreak of encephalitis in the greater New York area (6). Dead exotic birds in the Bronx Zoo, in New York City, tested positive for the virus and were the harbinger of human cases identified in the state of New York a few months later (7). Since then, WNV meningoencephalitis has become an emerging infection with both rapid increase in incidence, as well as geographic range (8). How the virus arrived in the Western Hemisphere remains an enigma. Possibilities include a viremic person, an infected mosquito, or an infected bird arriving by airplane in New York from an endemic area. The geographic range of the virus spread progressively during the next 2 years, albeit with a limited number of severe human infections, attracting considerable attention to WNV in both the medical and lay communities. Surprisingly, the incidence of WNV infection increased exponentially in 2002, culminating in the largest arbovirus meningoencephalitis outbreak ever documented in the Western Hemisphere. The preponderance of cases occurred in areas on the East Coast of the United States, which had seen little or no human disease previously, and spread to the Pacific Coast. In addition, 4 novel (nonmosquito) modes of WNV transmission have been documented: (1) transplacental; (2) breast feeding; (3) transplantation of infectious organs; and (4) transfusion of infectious blood products (9, 10).
Roughly 1/150 individuals infected with WNV will develop significant central nervous system disease, defined as encephalitis, meningitis, or a combination of them (meningoencephalitis) (7). Weakness, though a concomitant clinical feature in all recent outbreaks of WNV disease, has been best described in the United States. In the 1999 New York experience, 27% of individuals exhibited objective motor weakness, with 10% developing diffuse flaccid paralysis, in conjunction with an axonal polyneuropathy on electromyogram testing. Further information regarding acute flaccid paralysis has begun to emerge from the 2002 WNV epidemic, with weakness appearing to result from a poliomyelitis-like syndrome involving anterior horn cells and motor axons (2, 8).
The WNV as a member of flaviviridae family not only has close antigenic relationship with Japanese and St. Louis encephalitides; it also shares some of the imaging findings. Reports on St. Louis encephalitis are relatively limited, but MR imaging findings in Japanese encephalitis have been described extensively (11–14). Signal intensity abnormalities on FLAIR, FSE T2-weighted and, in many cases, DW imaging sequences observed in the thalami, basal ganglia, and midbrain were common findings in our patients. A common finding in 5 (32%) of our patients was the involvement of the mesial temporal structures (Table 1). Although it has been reported with Japanese encephalitis (11), increased intensity signal intensity on T2-weighted sequences in the mesial temporal structures in a clinical setting of encephalitis strongly suggests herpes simplex encephalitis and prompts immediate treatment. The pathologic background of WNV encephalitis, however, is quite different from that of herpes encephalitis, in light of the necrotizing nature of the latter. One of the patients (17) with serial MR images presented with mesial temporal lobe abnormalities (Fig 2A–D), which progressed and then resolved only to involve other areas of the brain. The lack of necrotizing process in WNV encephalitis actually explains the absence of any residual signal intensity abnormality on the follow-up studies. (Fig 2J).
Midbrain involvement was noted in only 3 patients (Table 1); signal intensity abnormalities present in the cerebral peduncle and substantia nigra (Fig 2D, -J, and -K), as well as red nucleus (Fig 2N), are in accordance with prior case reports on Japanese and St. Louis encephalitides (13, 15). Involvements of the pontine tegmentum (Fig 1A and -B) and superior cerebellar peduncles (Fig 1C) were the first abnormalities to be noticed in one of the patients (2), who became ventilator dependent and progressed to develop flaccid paralysis. There were 4 patients who presented at some point with extremity weakness. All, with one exception (17), had MR imaging studies of the spine; abnormalities were seen in the gray matter and particularly the ventral horns in 2 of them, whereas enhancement of the conus medullaris and the cauda equina was seen in all 3. Prior reports have associated the flaccid paralysis with enhancement around the conus and along the cauda equina (2, 3). Anterior myelitis apparently contributes to the clinical picture as well (Figs 1E–G and 4A and -B). Abnormalities in the dentate nuclei, cerebellar white matter, and cortex were present in only one patient (Fig 2F, -G, -L, and -M) and are depicted on one of the follow-up MR imaging studies coinciding with newly developed “polio-like” symptoms. Unfortunately the patient’s spine was never imaged, so whether there were any additional findings of anterior myelitis and/or radiculitis remains unknown.
Imaging findings are corroborated by pathologic findings. Reports in the pathology literature describe the presence of microglial modules but no evidence of necrosis in the hippocampus (16, 17). Sampson et al (17) described the presence of microglial nodules in the gray and white matter, distributed in the basal ganglia, thalami, and medulla, as well as the frontal cortex and molecular layer of the cerebellum. In one case, they did comment on more pronounced involvement of the medulla. Perivascular mononuclear inflammation was found more often in the gray matter vessels. They also commented on the most frequent involvement of the brain stem in WNV in comparison with St. Louis encephalitis. We noticed variable involvement of the cortex specifically in the temporal lobe (5 patients) and cerebellum (one patient). Remarkably, MR imaging of the brain of the very first patient (1) to be diagnosed with WNV encephalitis at our institution showed a single patchy area of increased signal intensity seen on FLAIR and DW images, with minimally low signal intensity on the apparent diffusion coefficient (ADC) that had been interpreted as subacute infarct. In retrospect, this finding might indeed represent inflammatory changes as well (Fig 5A and -B).
Fig 5.
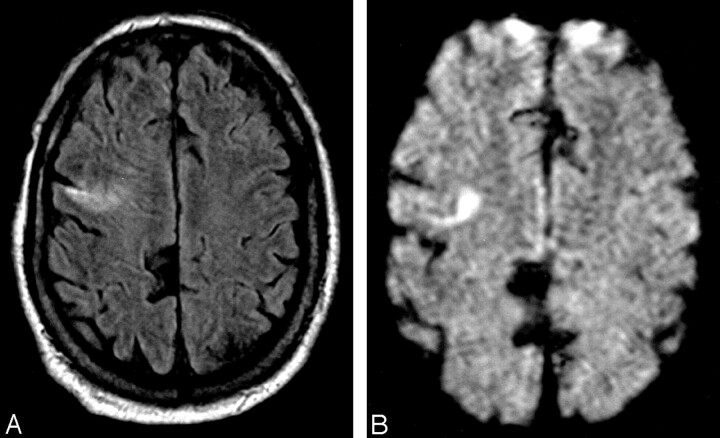
Patient 1, a 75-year-old man showed increased signal intensity in the right frontal lobe (A) with corresponding abnormality on the DW image (B), which was prospectively interpreted as infarct.
The fact that the perivascular inflammation is more often encountered in the gray rather than white matter may reflect on the fact that only 2 of our patients (Table 1) had white matter involvement, both with higher likelihood of impaired immune system and one of them only on late follow-up studies. It is worth commenting on one of these patients (11) who presented with numerous, scattered areas of restricted diffusion throughout the deep hemispheric white matter (Fig 3A and -B). It was this deep and extensive distribution of signal intensity abnormalities that prospectively raised the question of an inflammatory/infectious process. It has been reported that abnormalities on the DW images of variable degree are present in encephalitis (18). Nevertheless, autopsy of the patient revealed frank ischemic changes (subacute infarcts) in the deep hemispheric white matter (Fig 3G). Although pathologists have commented on perivascular infiltration, they had not found any evidence of vasculitis in the 4 cases of WNV on which they reported (17). Abnormalities in the corona radiata in the second patient (17; Fig 2Q) seen on the last follow-up MR imaging could in fact be either inflammatory or ischemic in nature. Both patients had in common significant past medical histories, renal/pancreatic transplant, and non-Hodgkin’s lymphoma, respectively, and both had poor outcomes, with death and severe neurologic impairment, respectively. Regardless of the etiology, ischemic or inflammatory of the diffusion abnormalities in the white matter, whether the white matter involvement represents progression to end-stage disease remains a question. Also under consideration is whether relatively impaired immune status could lead or precipitate such an involvement of the white matter. White matter abnormalities have been reported in Japanese encephalitis also. MR imaging findings in patients with Nipah encephalitis describe almost exclusively white matter abnormalities, at least in early imaging with some involvement of the deep gray matter and cortex on follow-up studies (19, 20). In the patients with WNV we studied, it was the deep gray matter and brain stem involvement that were most often encountered (Table 1).
The follow-up studies in 2 patients disclosed that abnormalities were progressive (2) or transient and migratory (17). The transient nature of the findings may provide at least partial explanation for commonly seen negative studies, because imaging of a patient at different stages of the disease may be elusive. We found the DW images very useful in early detection of the inflammation when the FLAIR abnormalities were absent. The lack, therefore, of DW imaging from the protocol in the very early stages of the disease may very well lead to false-negative study. The abnormality seen on DW images undeniably increased the level of confidence and thus influenced the interpretation of subtle signal intensity changes on FLAIR sequences (Fig 2A–C). The abnormalities we observed either in the brain or spinal cord are nonspecific. Certainly, there are many similarities with Japanese and St. Louis encephalitides. We would expect that there should be similarities with MR imaging findings seen in poliomyelitis, in light of the fact that a number of our patients did present with flaccid paralysis. Many other inflammatory and infectious processes share deep gray matter and cortical involvement. Isolated involvement of the mesial temporal structures should arouse suspicion for herpes encephalitis, which was also seen in patients with WNV encephalitis.
It would be impossible in most instances to suggest WNV encephalomyelitis prospectively and based on MR findings only. On the other hand, certain imaging findings can be suggestive of the disease, if additional factors such geographic area, time of the year, and potential exposure to mosquitoes are taken into consideration. Thus, WNV encephalomyelitis should be included in the differential diagnosis.
The small number of cases and the retrospective approach are the major drawbacks of our report. In light of the retrospective nature of our investigation, the imaging protocols varied because they are routinely designed to address the clinical question. Many of our routine protocols, however, do include DW imaging, which we found helpful, and in most cases postcontrast images were available also. This is a small number of patients for accurate statistical analysis, which may reflect on the relatively higher percentage of positive brain MR imaging sequences in our study (50% instead of the reported 37% reported in the literature). Nevertheless, the reported 37% is much higher than the first impression in the medical community.
Conclusion
WNV is a widespread infectious agent with cases of meningoencephalitis reported throughout the United States. Positive MR imaging studies in patients with WNV meningoencephalitis findings of the brain are not uncommon. Although the findings are in many cases nonspecific, WNV infection should be included in the differential diagnosis of deep gray matter or mesial temporal lobe involvement, especially if exposure to mosquitoes is suspected. Likewise, rhomboencephalitis, another manifestation of the disease, may herald extremity weakness, as has been seen in 2 of our patients. Established extremity weakness with or without progression to flaccid paralysis requires imaging of the entire spine to evaluate for signs of anterior myelitis and/or radiculitis.
References
- 1.Nash D, Mostashari F, Fine A, et al. The outbreak of West Nile virus infection in New York City area in 1999. N Engl J Med 2001;344:1807–1814 [DOI] [PubMed] [Google Scholar]
- 2.Jeha LE, Sila CA, Lederman RJ, et al. West Nile virus infection: a new acute paralytic illness. Neurology 2003;61:55–59 [DOI] [PubMed] [Google Scholar]
- 3.Olsan A, Milburn JM, Baumgarten KL, Durham HL. Leptomeningeal enhancement in a patient with proven West Nile virus infection. AJR Am J Roentgenol 2003;181:591–592 [DOI] [PubMed] [Google Scholar]
- 4.Rosas H, Wippold II FJ. West Nile virus: case report with MR imaging findings. AJNR Am J Neuroradiol 24:1376–1378 [PMC free article] [PubMed] [Google Scholar]
- 5.Lanciotti RS, Roehrig JT, Deubel V, et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 1999;286:2333–2337 [DOI] [PubMed] [Google Scholar]
- 6.Asnis DS, Conetta R, Teixeria AA, et al. The West Nile virus outbreak of 1999 in New York: the Flushing Hospital experience. Clin Infect Dis 2000;30:413–418 [DOI] [PubMed] [Google Scholar]
- 7.Petersen LR, Marfin AA. West Nile virus: a primer for the clinician. Ann Intern Med 2002;137:173–179 [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Acute flaccid paralysis syndrome associated with West Nile virus infection: Mississippi and Louisiana, July–August. MMWR Morb Mortal Wkly Rep 2002;51:825–828 [PubMed] [Google Scholar]
- 9.Iwamoto M, Jernigan DB, Guasch A, et al. Transmission of West Nile virus from an organ donor to four transplant recipients. N Engl J Med 2003;348:2196–2203 [DOI] [PubMed] [Google Scholar]
- 10.Morse DL. West Nile virus: not a passing phenomenon. N Engl J Med 2003;348:2173–2174 [DOI] [PubMed] [Google Scholar]
- 11.Abe T, Kojima K, Shoji H, et al. Japanese encephalitis. J Magn Reson Imaging 1998;8:755–761 [DOI] [PubMed] [Google Scholar]
- 12.Kumar S, Misra UK, Kalita J, et al. MRI in Japanese encephalitis. Neuroradiology 1997;39:180–184 [DOI] [PubMed] [Google Scholar]
- 13.Kalita J, Misra UK. The substantia Nigra is also involved in Japanese encephalitis. AJNR Am J Neuroradiol 2000;21:1978–1979 [PMC free article] [PubMed] [Google Scholar]
- 14.Shoji H, Kida H, Hino H, et al. Magnetic resonance imaging findings in Japanese encephalitis: white matter lesions. J Neuroimaging 1994;4:201–211 [DOI] [PubMed] [Google Scholar]
- 15.Cerna F, Mehrad B, Luby J, et al. St Louis encephalitis and the substantia nigra: MR imaging evaluation. AJNR Am J Neuroradiol 1999;20:1281–1283 [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly TW, Prayson RA, Ruiz AI, et al. The neuropathology of West Nile meningoencephalitis. Am J Clin Pathol 2003;119:749–753 [DOI] [PubMed] [Google Scholar]
- 17.Sampson BA, Ambrosi C, Charlot A, et al. The pathology of human West Nile virus infection. Hum Pathol 2000;32:527–531 [DOI] [PubMed] [Google Scholar]
- 18.Tsuchiya K, Katase S, Yoshino A, Hachiya J. Diffusion-weighted MR imaging of encephalitis. AJR Am J Roentgenol 1999;173:1097–1099 [DOI] [PubMed] [Google Scholar]
- 19.Lim CCT, Sitoh Y, Hui F, et al. Nipah viral encephalitis or Japanese encephalitis? MR findings in a new zoonotic disease. AJNR Am J Neuroradiol 2000;21:455–461 [PMC free article] [PubMed] [Google Scholar]
- 20.Tchoyoson Lim CC, Lee KE, Lee WL, et al. Nipah virus encephalitis: serial MR study of an emerging disease. Radiology 2002;22:219–226 [DOI] [PubMed] [Google Scholar]