Abstract
BACKGROUND AND PURPOSE: The purpose of this study was to demonstrate endovascular treatment of wide-necked aneurysms of the internal carotid artery with the liquid embolic agent Onyx HD 500.
METHODS: Twenty-two wide-necked, large or giant aneurysms of the internal carotid artery (ICA) were treated in 22 patients with Onyx HD 500 (15 ophthalmic, 1 clinoid, and 6 cavernous aneurysms). Sixteen patients were asymptomatic, and mass effect of the aneuryms caused cranial nerve palsy in 6. Seven aneurysms showed regrowth after prior endovascular coiling.
RESULTS: Postinterventional angiography demonstrated total occlusion in 18 aneurysms, and a small remnant was left in 4. Clinical and angiographic follow-up data are available in 19 patients (average follow-up, 13 months; range, 5–36 months). Total occlusion was demonstrated in 17 aneurysms (91%), and a partial recanalization was seen in 2. There were 2 ICA occlusions and 1 ICA stenosis. Clinical follow-up demonstrated a transient ischemic attack in 1 patient; 2 with cranial nerve palsy were unchanged, and 2 showed improved symptoms compared with the findings before treatment. The remaining 14 patients were clinically asymptomatic. There was no permanent severe morbidity and no mortality at follow-up.
CONCLUSION: The endovascular treatment of wide-necked, large or giant ICA aneurysms with Onyx HD 500 is a treatment option in these selected cases. The benefit is a primary high and stable occlusion rate and good clinical outcome. ICA occlusion caused by Onyx migration in the parent artery is a typical problem, with a benign clinical course in this series.
The endovascular treatment of intracranial aneurysms with detachable platinum microcoils is an established method. The long-term results in large and wide-necked aneurysms, however, have been unsatisfactory so far. Repeat treatment is often required (1).
Different methodologic approaches have been developed for the treatment of large, wide-necked and other complicated dysplastic aneurysms: balloon-remodeling-techniques, (2–6) stent-assisted coil occlusion, (7–10), or parent vessel occlusion with or without extracranial/intracranial (EC/IC) bypass (11–14). Surgical clipping is not an accepted treatment option in large and giant aneurysms (15, 16). The combination of endovascular and surgical techniques in the treatment of wide-necked aneurysms of the internal carotid artery (ICA) called balloon-assisted microvascular clipping has recently been reported (16).
A new endovascular method is to fill the aneurysms with the liquid embolic agent Onyx HD 500 (Micro Therapeutics, Irvine, CA). Experimental application of this technique has been described elsewhere (17). Aneurysm treatment with Onyx in humans was introduced by Mawad et al (18). A prospective study (CAMEO trial) was conducted in 20 European centers, including 3 aneurysms treated at our centers (19). The present report describes our experience with the liquid embolic agent Onyx in the treatment of the 3 previously reported aneurysms of the CAMEO trial and 19 additional wide-necked aneurysms electively treated at the Alfried Krupp Hospital, Essen, Germany.
Materials and Methods
Between June 2001 and October 2003, a total of 22 patients were treated with the Onyx liquid embolic system HD 500 and HD 500+ (Micro Therapeutics). Table 1 summarizes the clinical data for the aneurysms and follow-up data. Sixteen aneurysms were located near the origin of the ophthalmic artery (paraophthalmic and clinoid), and 6 aneurysms had a cavernous location. On average, the aneurysms were 19 mm high (range, 5–40) and 16 mm wide (range, 4–36). The dimension of the neck of the aneurysms was 9 mm (range, 3–18) on average. In 7 patients, a recurrent inflow into the lumen of the aneurysm was treated after pretreatment with detachable platinum microcoils years ago. Sixteen patients were clinically asymptomatic with respect to the treated aneurysm, whereas 6 aneurysms caused mass effect with cranial nerve paresis. Following the criteria of the CAMEO trial (19), all treated aneuryms were difficult to treat or presented high risk for conventional coil embolization or neurosurgical clipping or recurred following previous coil embolization.
TABLE 1:
Patient, treatment, and follow-up data
| Patient No./Initials/Age (y)/Sex | Localization | Signs and Symptoms | Aneurysm (mm)* |
Onyx HD 500 (+) ml | Aneurysm Occlusion (%) | FU (mo) | DSA/MRA | Aneurysm Occlusion FU (%) | Signs and Symptoms FU | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Height | Width | Neck | |||||||||
| 1/NN/31/F | Ophth | Asymptomatic | 10 | 15 | 8 | 0.43 | 100 | 22 | DSA | 100 | Asymptomatic |
| 2/GH/41/F | Ophth (GDC) | Asymptomatic | 8 (15) | 10 | 6 | 0.3 | 95 | 12/36 | DSA/MRA | 100 | Asymptomatic |
| 3/KG/41/F | Ophth | Asymptomatic | 15 | 7.5 | 6 | 0.6 | 100 | —† | — | — | — |
| 4/MG/61/M | Clinoidal | Asymptomatic | 20 | 12 | 8 | 1.36 | 100 | 12/25 | DSA/DSA | 100 | Asymptomatic |
| 5/WU/39/F | Ophth | Asymptomatic | 25 | 25 | 12 | 2.5 | 100 | 12 | DSA | 100 | Asymptomatic |
| 6/CM/29/F | Ophth | Asymptomatic | 18 | 18 | 7 | 1.3 | 100 | 6/14 | MRA/MRA | 100 | Asymptomatic |
| 7/KJ/41/F | Ophth | Asymptomatic | 12 | 8 | 6 | 0.27 | 95 | 10 | DSA | 100 | TIA |
| 8/OH/65/F | Cav (GDC) | Cnp III | 20 (30) | 20 (25) | 12 | 4.68 | 100 | 5/18 | DSA/DSA | 100 | Cnp improved |
| 9/THJ/54/M | Ophth | Asymptomatic | 6 | 10 | 7 | 0.16 | 100 | 8 | DSA | 100 | Asymptomatic |
| 10/HB/35/F | Ophth | Asymptomatic | 6 | 12 | 8 | 0.32 (+) | 100 | 2/13 | MRA/DSA | 100 | Asymptomatic |
| 11/LK/52/F | Cav | Cnp III, IV | 25 | 36 | 18 | 2.43 (+) | 90 | 7 | DSA | 90‡ | Cnp improved |
| 12/SMT/47/F | Ophth | Asymptomatic | 10 | 8 | 6 | 0.36 (+) | 95 | 5 | DSA | 100 | Asymptomatic |
| 13/DJ/61/F | Cav | Cnp VI | 25 | 20 | 12 | 2.28 (+) | 100 | 4/16 | MRA/DSA | 85‡ | Unchanged |
| 14/KM/60/F | Ophth | Asymptomatic | 15 | 20 | 8 | 0.51 (+) | 100 | 5 | DSA | 100 | Asymptomatic |
| 15/BE/58/F | Ophth (GDC) | Asymptomatic | 15 (25) | 7 (12) | 6 | 0.42 (+) | 100 | 6 | DSA | 100 | Asymptomatic |
| 16/WA/35/F | Ophth (GDC) | Asymptomatic | 10 (25) | 17 | 8 | 0.73 (+) | 100 | 6 | MRA | 100 | Asymptomatic |
| 17/WG/50/F | Cav | Cnp II | 18 | 20 | 16 | 0.79 (+) | 100 | Pending | Pending | Pending | Pending |
| 18/BI/60/F | Cav | Asymptomatic | 23 | 15 | 8 | 1.90 (+) | 100 | 6 wk | DSA | 100 | Asymptomatic |
| 19/KR/45/F | Ophth (GDC) | Asymptomatic | 25 | 12 | 6 | 0.42 (+) | 100 | 12/30 | DSA/MRA | 100 | Asymptomatic |
| 20/LG/59/F | Cav | Cnp IV | 20 | 15 | 10 | 2.15 (+) | 100 | 7 | DSA | 100 | Asymptomatic |
| 21/WA/61/F | Ophth (GDC) | Cnp II | 18 (30) | 13 (25) | 10 | 1.55 (+) | 100 | 8 | DSA | 100 | Unchanged |
| 22/ML/42/F | Ophth (GDC) | Asymptomatic | 10 (20) | 7 (12) | 8 | 0.41 (+) | 100 | 8 | DSA | 100 | Asymptomatic |
Note.—(+) indicates Onyx HD+ with increased visibility; Ophth = ophthalmic segment of the carotid artery; Cav, cavernous segment of the carotid artery; GDC, recurrent aneurysm after treatment with Guglielmi detachable coils; FU, follow-up; TIA, transient ischemic attack; Cnp, cranial nerve paresis; DSA, digital subtraction angiography; mRA, magnetic resonance angiography.
Dimensions of the contrasted lumen of the aneurysms are followed in parentheses by dimensions of the complete aneurysms before treatment.
Occlusion of the internal carotid artery after vessel rupture due to balloon inflation during the procedure.
Recurrence after Onyx treatment, occluded with coils and self-expandable stents.
Onyx is an ethylene vinyl alcohol copolymer (EVOH), dissolved in dimethyl sulfoxide (DMSO). Tantalum powder gives radiopacity to the material. For the treatment of aneurysms, a solution with high viscosity is used. Onyx HD 500 contains 20% EVOH and approximately 80% DMSO. The material precipitates on contact with aqueous solution and stays cohesive as a “kernel” attached to the tip of the microcatheter (20). Patients 1–12 were treated with Onyx HD 500. All subsequent patients were treated with Onyx HD 500+ (improved visibility due to a higher concentration of tantalum in the embolic material).
Treatment of the patients occurred with general anesthesia. A 6F and a 5F guide system was introduced into the affected internal carotid artery. An Onyx-compatible Hyperglide Balloon (4 mm in diameter and 20 or 30 mm in length; Micro Therapeutics) was introduced by using the 5F guide catheter and placed above the neck of the aneurysm. Thereafter, the 6F guide system was used to introduce an Onyx HD 500 compatible microcatheter (Rebar, Titan; Micro Therapeutics) into the lumen of the aneurysm. The requisite balloon volume needed to seal the aneurysm was then determined (seal test). With the balloon inflated, a slow test injection with contrast material was done. A satisfactory seal test is achieved with stasis of contrast material within the aneurysm.
For the injection techniques of Onyx, we followed the instructions for use from Micro Therapeutics described by Molyneux et al (19). Patients 1–12 were treated with Onyx HD 500 and without the Precision injector with Quick-Stop; the remaining were treated with Onyx HD 500+ and with the Quick-Stop device. For each injection cycle, the internal carotid artery was occluded for a maximum of 5 minutes: 3 minutes for injection of the embolic material and 2 minutes to allow precipitation of the injected embolic material. After an injection cycle, the circulation was reestablished for at least 3 minutes. At the end of the treatment the microcatheter—after aspiration and 10 minutes waiting time to allow for solidification of the embolic material—was withdrawn from the aneurysm under protection of the inflated balloon.
During treatment, the patients were systemically heparinized (partial thromboplastin time [PTT] > 60 seconds), and 500 mg Aspisol intravenously (acetylsalicylic acid) were given after the treatment when the balloon catheter was removed. In addition, a loading dose of 300 mg clopidogrel was administered on the day of the procedure. The PTT-controlled systemic heparinization was continued for a total of at least 72 hours. On day 1 after the procedure, treatment with 75 mg/day clopidogrel and 100 mg/day acetylsalicylic acid for a minimum of 6 weeks was begun.
Results
The quantity of Onyx injected in the aneurysms was 1.12 mL, on average (range, 0.3–4.7 mL). The mean balloon inflation time for the entire treatment of an aneurysm was 44 minutes (range, 21–123 minutes); the mean injection time was 21 minutes (range, 7–47 minutes). Of the 22 treated aneurysms, 18 were completely occluded on postprocedural angiography. Figure 1 demonstrates a successful treatment and a stable result after 12 months. In 4 cases, a small remnant was left after the initial treatment. In 12 cases (44%), a small migration of the embolic material into the parent artery was observed during the last cycles of aneurysm filling (Figs 2 and 3). In 4 of these cases, this also led to a proximal occlusion of the ophthalmic artery, without clinical consequences. We saw no distal migration of Onyx on postprocedural high-resolution CT scans and good collateralization of the retinal supply from the external carotid artery in all cases. Periprocedural complications occurred in 3 cases (14%). In one case, the last inflation of the balloon caused a rupture of the internal carotid artery wall. This problem was managed by occlusion of the internal carotid artery with detachable balloons and coils. The subarachnoid hemorrhage that occurred healed without any consequences for the patient; the occlusion of the internal carotid artery with a detachable balloon was tolerated without neurologic deficit. In 2 cases of giant aneurysms treated previously with platinum microcoils, the microcatheter could not be withdrawn completely from the aneurysm because of rupture in the distal part while it was being removed. This part of the microcatheter was left in the vessel with the tip in the Onyx cast. That did not cause any clinical consequences and required no special treatment. In 21 of the 22 treated patients, a postoperative MR imaging was available. In 6 cases small, circumscribed, ischemic areas could be detected by diffusion-weighted imaging. In 5 of these patients (23%) a transient clinically detectable deficit was present immediately after the procedure but disappeared completely before discharge. Of the 6 patients with space-occupying, cavernous aneurysms, 2 experienced increased ocular symptoms following treatment.
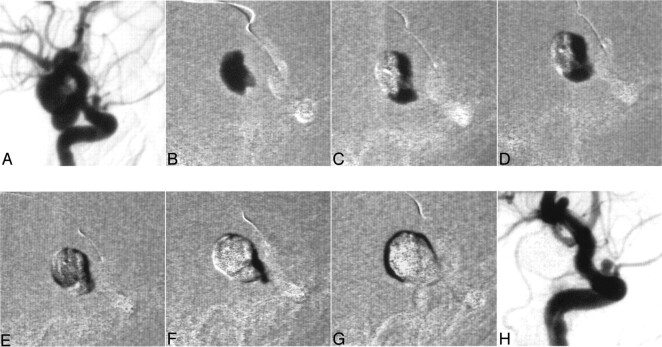
Fig 1.
A 61-year-old male patient presented with an asymptomatic bilobulated aneurysm with a common neck of both sacks (A). Complete occlusion in 7 injection cycles to demonstrate the filling of an aneurysm with Onyx (B–G). Angiography demonstrated a stable result 12 months after the treatment (H).
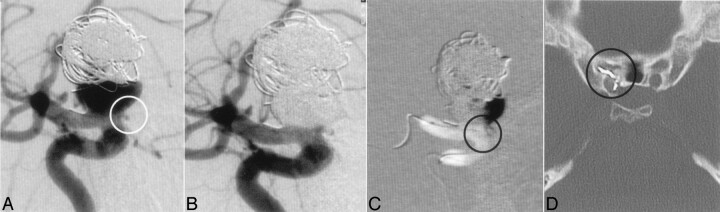
Fig 2.
A 35-year-old female patient with GDC treatment in 1994; regrowth of the aneurysm; the patient was blind on the ipsilateral eye since GDC treatment, so there was no need to protect the ophthalmic artery; origin of the ophthalmic artery at the neck of the aneurysm (A, ring). Complete occlusion of the aneurysm after treatment with Onyx (B). Road map and CT demonstrated migration of Onyx in the proximal part of the ophthalmic artery (C and D, ring).
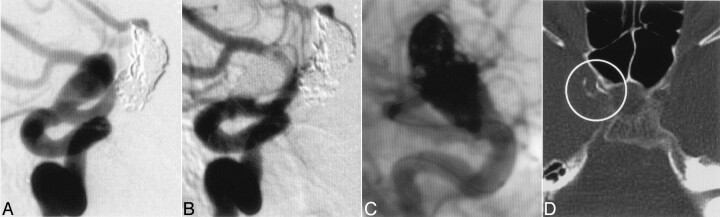
Fig 3.
A 58-year-old female patient years after GDC treatment and regrowth of the partially thrombosed aneurysm (A). Biplanar angiography demonstrated complete occlusion of the aneurysm after treatment with Onyx (B and C). CT after the treatment demonstrated the migration of Onyx in the parent artery forming a thin layer on the inner surface of the right internal carotid artery (D, ring)
For 19 of the 22 patients treated, angiographic follow-up examinations are available (Table 1). Of the remaining 3, 1 was lost, follow-up was not done in 1 patient because the ICA was occluded in conjunction with the aneurysms with a detachable balloon and coils during the initial treatment, and 1 ICA was found occluded on early follow-up angiography after 6 weeks. The mean follow-up time period was 12.5 months (range, 5–36). Seventeen aneurysms were completely occluded, and recanalization was demonstrated in 2. In 16 cases (84%) the parent artery was normally depicted, and in 3 cases (16%) exhibited pathologic changes—namely, a single high-grade stenosis and 2 occlusions.
Nineteen of the 21 (91%) monitored patients—we recently conducted additional telephone interviews in all patients with affected parent arteries—were clinically asymptomatic, unchanged, or improved. Two patients reported clinical symptoms during follow-up. The patient with the high-grade stenosis of the internal carotid artery presented a transient ischemic attack (TIA) episode, and one patient with an aneurysm causing mass effect reported a transient episode of mild diplopia. Therefore, the combined long-term permanent morbidity and mortality rate for the electively treated patients is 0. We have clinical and angiographic follow-up for more than 2 years in the patients treated during the CAMEO trial. All patients are clinically asymptomatic, all aneurysms are occluded, and there is no occlusion or stenosis of the parent artery.
Discussion
The objective of treating intracranial aneurysms is permanent occlusion and anatomic reconstruction of the parent artery. Especially in the case of wide-necked aneurysms, this is not feasible with platinum microcoils, despite several technical improvements, such as 3D coils, Trispan coils, and balloon remodeling (1, 19). The surgical treatment of unruptured aneurysms is associated with a high morbidity and mortality, which is reported to be 0%–4% for mortality and 0%–15.7% for permanent morbidity (21, 22). The mortality rate increases to >20% when giant aneurysms are considered. Endovascular treatment with Onyx should improve the primary occlusion rate and the long-term results. We considered treatment with Onyx to be indicated in wide-necked aneurysms along the intracranial course of the internal carotid artery and in space-occupying, symptomatic aneurysms in the cavernous segment of the internal carotid artery. Most the aneurysms treated were giant or very large and were located in the ophthalmic segment of the internal carotid artery. These aneurysms offer favorable conditions for a balloon-assisted treatment with Onyx because the ophthalmic segment follows a relatively straight course. This is associated with 2 advantages. The first is that the balloon can be reliably positioned in the segment. The second is that the neck of the aneurysm can be completely projected in profile during angiography resulting in easier monitoring during filling as the injected embolic material is not superimposed upon the inflated balloon. In addition, in this segment of the ICA (with the exception of the ophthalmic artery) no important blood vessels (especially no small perforators) that could potentially be compromised by unintended migration of embolic material originate.
It is difficult to set the indication for treatment of aneurysms proximal to the origin of the ophthalmic artery (in the cavernous segment of the ICA) with endovascular occlusion of the aneurysm and reconstruction of the parent artery, because treatment of these aneurysms by occlusion of the parent artery is an established and safe treatment method (23, 24). An important argument for preserving the internal carotid artery and, therefore, for treatment with Onyx is an insufficient collateralization of the hemispheres. An additional argument for preserving the internal carotid artery is evidence that delayed ischemic events also occur after successful test occlusion and/or EC/IC-bypass (14, 18, 25).
The results presented here confirmed our specified indication criteria. All but 2 aneurysms treated are permanently occluded. These results are comparable with those of the CAMEO trial (19) and substantially better than those reported for other endovascular techniques in this type of aneurysm.
At the time of the follow-up examination, all the patients treated in our series were neurologically normal or unchanged compared with the status before treatment. In total there were 4 events (18%) in which Onyx migration interfered with the parent artery. We observed that a gap between the microcatheter and the inflated balloon is the most likely location of the leak at which the embolic material can migrate. Similar to the description of Molyneux et al (19) 3 of these 4 adverse events in our series occurred after treatments with Onyx 500 and without the Quick-Stop device. Onyx HD 500+ has improved radiopacity and the Quick-stop device allows the flow of Onyx to stop immediately. Mass effect after aneurysm filling was not a significant problem in our series.
The further development of this treatment method should prevent leaking of the embolic material into the parent artery and reduce the occlusion time of the parent artery. So far, complete filling of the aneurysm with Onyx has been possible only by accepting the risk of having small quantities of the embolic material pass into the parent artery. At present, it can be stated that complete occlusion is favorable for the long-term results. Thus, the risk of small portions of the embolic material leaking into the parent artery should be accepted. For this reason, antiplatelet therapy is essential.
Within the spectrum of endovascular treatment options, the presented results of treating intracranial aneurysms with Onyx must be measured against the results obtained with other procedures, such as bioactive or coated coils (Matrix; Target/Boston Scientific, Natick, MA; HydroCoil Embolic System, Microvention, Aliso Viejo, CA) in which long-term follow-up data are not available (26–28).
The treatment of cavernous, giant aneurysms of the internal carotid artery through parent artery occlusion is an accepted procedure with low morbidity and mortality (23, 29). Because of these results, the indication for treating a cavernous aneurysm with Onyx must always be weighed against this method.
Concerning the treatment of wide-necked intracranial aneurysms with balloon-expandable stents only limited data are available (10, 21, 30). A large number of patients were presented by Lylyk et al (9), in which 19 cases of 62 aneurysms needed repeat treatment.
Although the results presented concerning the treatment of intracranial aneurysms with balloon-expandable stents are favorable, this method is limited by the inadequate flexibility of the stent and its delivery systems for use in all intracranial locations.
Recent results have been presented concerning the treatment of intracranial aneurysms with self-expandable stents (Neuroform, Smart Therapeutics, Inc., Boston Scientific, San Leandro, CA) (7, 31–33). These studies show limitations either in follow-up data or the occlusion rate (32, 33). An improved Neuroform stent and a new self-expandable intracranial stent (Leo; BALT, Montmorency, France) have recently been invented, but there are no clinical data available up to now. Compared with all of the published data concerning the treatment of wide-necked aneurysms, our results and those of the CAMEO study (19) showed more favorable occlusion and complication rates.
Conclusion
We reported the treatment of 22 unruptured wide-necked, large or giant aneurysms of the ICA with Onyx. Of the 21 aneurysms monitored, we found a complete occlusion in 19 (90%) cases on follow-up angiography; the parent artery was occluded in 3 cases and the stenotic in 1 case. The combined rate for permanent morbidity and mortality was 0.
Compared with the published data on the natural history of unruptured large and giant aneurysms, the results of surgical series and the endovascular treatment with platinum coils and self-expandable intracranial stents this is a favorable result for this type of aneurysm.
Our results are compatible with the results of the CAMEO trial. A broadening of the indication spectrum by using the liquid embolic material Onyx in aneurysm therapy could result from the combination of a more viscous embolic material with a self-expandable stent, (34) to reduce balloon occlusion time of the carotid artery and prevent unintended migration of Onyx into the parent artery.
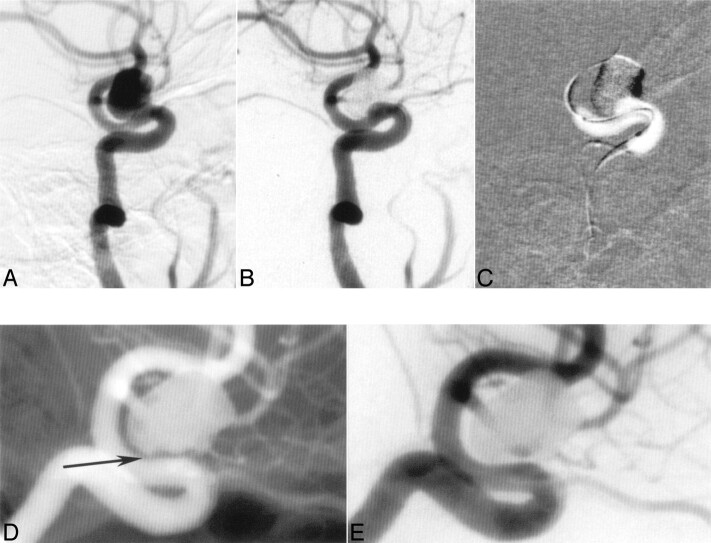
Fig 4.
A 60-year-old female patient with an asymptomatic ophthalmic aneurysm (A). Complete occlusion of the aneurysm post Onyx (B). Bulging of the balloon into the aneurysm neck during the injection of Onyx (C). Follow-up angiography 5 months after the treatment demonstrated complete occlusion of the aneurysm, reconstruction of the parent artery, and “healing reaction” at the neck (D and E, arrow).
TABLE 2:
Complications and aneurysm regrowth
| Patient No. | Cause | Treatment | Clinical Outcome | |
|---|---|---|---|---|
| Periprocedural complications | ||||
| 3 | ICA rupture/SAH | Inflation of the Hyperglide balloon | Parent artery occlusion with detachable balloon and coils | Asymptomatic |
| 10, 23 | Stuck catheter | Catheter rupture during removing | 72 h heparin IV | Asymptomatic |
| 1, 4, 5, 9, 10 | TIA | 72 h heparin IV | Asymptomatic | |
| 11, 20 | Steroids | Improved | ||
| Postprocedural complications | ||||
| 7 | ICA stenosis | Onyx migration in the parent artery | Acetylsalicylic acid 100 mg | TIA |
| 5, 9, 13 | ICA occlusion | Onyx migration in the parent artery | — | Asymptomatic |
| Aneurysm regrowth | ||||
| 11, 13 | Aneurysm regrowth | — | Self-expandable stent and coils | Asymptomatic |
Note.—ICA indicates internal carotid artery; IV, intravenously; TIA, transient ischemic attack.
References
- 1.Muramaya Y, Nien YL, Duckwiler G, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years’ experience. J Neurosurg 2003;98:959–966 [DOI] [PubMed] [Google Scholar]
- 2.Aletich VA, Debrun GM, Misra M, et al. The remodeling technique of balloon-assisted Guglielmi detachable coil placement in wide-necked aneurysms: experience at the University of Illinois at Chicago. J Neurosurg 2000;93:388–396 [DOI] [PubMed] [Google Scholar]
- 3.Cottier JP, Pasco A, Gallas S, et al. Utility of balloon-assisted Guglielmi detachable coiling in the treatment of 49 cerebral aneurysms: a retrospective, multicenter study. AJNR Am J Neuroradiol 2001;22:345–351 [PMC free article] [PubMed] [Google Scholar]
- 4.Lefkowitz MA, Gobin YP, Akiba Y, et al. Balloon-assisted Guglielmi detachable coiling of wide-necked aneurysm. Part II. Clinical results. Neurosurgery 1999;45:531–537 [DOI] [PubMed] [Google Scholar]
- 5.Malek AM, Halbach VV, Phatouros CC, et al. Balloon-assisted technique for endovascular coil embolization of geometrically difficult intracranial aneurysms. Neurosurgery 2002;46:1397–1406 [DOI] [PubMed] [Google Scholar]
- 6.Moret J, Cognard C, Weill A, et al. Reconstruction technique in the treatment of wide-neck intracranial aneurysms: long-term angiographic and clinical results: apropos of 56 cases. J Neuroradiol 1997;24:30–44 [PubMed] [Google Scholar]
- 7.Henkes H, Bose A, Felber S, et al. Endovascular coil occlusion of intracranial aneurysms assisted by a novel self-expandable nitinol microstent (Neuroform). Intervent Neuroradiol 2002;8:107–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lanzino G, Wakloo AK, Fessler RD, et al. Efficacy and current limitations of intravascular stents for intracranial internal carotid, vertebral, and basilar artery aneurysms. J Neurosurg 1999;91:538–546 [DOI] [PubMed] [Google Scholar]
- 9.Lylyk P, Cohen JE, Ceratto R, et al. Endovascular reconstruction of intracranial arteries by stent placement and combined techniques. J Neurosurg 2002;97:1306–1313 [DOI] [PubMed] [Google Scholar]
- 10.Sekhon LHS, Morgan MK, Sorby W, et al. Combined endovascular stent implantation and endosaccular coil placement for the treatment of a wide-necked vertebral artery aneurysm: technical case report. Neurosurgery 1998;43:380–383 [DOI] [PubMed] [Google Scholar]
- 11.Bavinski G, Killer M, Ferraz-Leite H, et al. Endovascular therapy of idiopathic cavernous aneurysms over 11 years. AJNR Am J Neuroradiol 1998;19:559–565 [PMC free article] [PubMed] [Google Scholar]
- 12.Niiro M, Shimozuru T, Nakamura K, et al. Long-term follow-up study of patients with cavernous sinus aneurysm treated by proximal occlusion. Neurol Med Chir (Tokyo)2000;40:88–96 [DOI] [PubMed] [Google Scholar]
- 13.Serbienko FA, Filatov JM, Spallone A, et al. Management of giant intracranial ICA aneurysms with combined extracranial-intracranial anastomosis and endovascular occlusion. J Neurosurg 1990;73:57–63 [DOI] [PubMed] [Google Scholar]
- 14.Zhou LF, Jiang DJ. Cerebral artery reconstruction in the treatment of large and giant intracranial aneurysms. Chin Med J 1994;107:41–46 [PubMed] [Google Scholar]
- 15.Brennan JW, Schwartz ML. Unruptured intracranial aneurysms: appraisal of the literature and suggested recommendations for surgery, using evidence-based medicine criteria. Neurosurgery 2000;47:1359–1371 [PubMed] [Google Scholar]
- 16.Thorell W, Rasmussen P, Perl J, et al. Balloon-assisted microvascular clipping of paraclinoid aneurysms. J Neurosurg 2004;100:713–716 [DOI] [PubMed] [Google Scholar]
- 17.Muramaya Y, Vinuela F, Tateshima S, et al. Endovascular treatment of experimental aneurysms by use of a combination of liquid embolic agents and protective devices. AJNR Am J Neuroradiol 2000;21:1726–1735 [PMC free article] [PubMed] [Google Scholar]
- 18.Mawad ME, Cekirge S, Ciceri E, et al. Endovascular treatment of giant and large intracranial aneurysms by using a combination of stent placement and liquid polymer injection. J Neurosurg 2002;96:474–482 [DOI] [PubMed] [Google Scholar]
- 19.Molyneux AJ, Cekirge S, Saatci I, Gál G. Cerebral Aneurysm Multicenter European Onyx (CAMEO) trial: results of a prospective observational study in 20 European centers. AJNR Am J Neuroradiol 2004;25:39–51 [PMC free article] [PubMed] [Google Scholar]
- 20.Molyneux AJ, Ellison DW, Morris J, et al. Histological findings in giant aneurysms treated with Guglielmi detachable coils: report of two cases with autopsy correlation. J Neurosurg 1995;83:129–132 [DOI] [PubMed] [Google Scholar]
- 21.Wiebers DO, Whisnant JP, Huston J 3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome and risks of surgical and endovascular treatment. Lancet 2003;362:103–110 [DOI] [PubMed] [Google Scholar]
- 22.Gruber A, Killer M, Bavinzski G, Richling B. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: a 7-year, single-center experience. Neurosurgery 1999;45:793–803 [DOI] [PubMed] [Google Scholar]
- 23.Higashida RT, Halbach VV, Dowd C, et al. Endovascular detachable balloon embolization therapy of cavernous carotid artery aneurysms: results in 87 cases. J Neurosurg 1990;72:857–863 [DOI] [PubMed] [Google Scholar]
- 24.Larson JJ, Tew JM Jr, Tomsick TA, et al. Treatment of aneurysms of the internal carotid artery by intravascular balloon occlusion: long-term follow-up of 58 patients. Neurosurgery 1995;36:26–30 [PubMed] [Google Scholar]
- 25.Graves VB, Perl J 2nd, Strother CM, et al. Endovascular occlusion of the carotid or vertebral artery with temporary proximal flow arrest and microcoils: clinical results. AJNR Am J Neuroradiol 1997;18:1201–1206 [PMC free article] [PubMed] [Google Scholar]
- 26.Barrocas AM, Derdeyn CP, Cross DT 3rd, et al. Histologic and hemodynamic effects of endovascular platinum coils for intracranial aneurysms. J Long Term Eff Med Implants 2004;14:225– 242 [DOI] [PubMed] [Google Scholar]
- 27.Muramaya Y, Vinuela F, Tateshima S, et al. Bioabsorable polymeric material coils for embolization of intracranial aneurysms: a preliminary experimental study. J Neurosurg 2001;94:454–463 [DOI] [PubMed] [Google Scholar]
- 28.Cloft HJ, Kallmes DF. Aneurysm packing with HydroCoil embolic system versus platinum coils: initial experience. AJNR Am J Neuroradiol 2004;25:60–62 [PMC free article] [PubMed] [Google Scholar]
- 29.Schaaf van der IC, Brilstra EH, Buskens E, et al. Endovascular treatment of aneurysms in the cavernous sinus: a systematic review on balloon occlusion of the parent vessel and embolization with coils. Stroke 2002;33:313–318 [DOI] [PubMed] [Google Scholar]
- 30.Highashida RT, Smith W, Gress D, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery. J Neurosurg 1997;87:944–949 [DOI] [PubMed] [Google Scholar]
- 31.Wanke I, Doerfler A, Schoch B, et al. Treatment of wide-necked intracranial aneurysms with a self-expanding stent system: initial clinical experience. AJNR Am J Neuroradiol 2002;24:1192–1199 [PMC free article] [PubMed] [Google Scholar]
- 32.Benitez RP, Silva MT, Klem J, et al. Endovascular occlusion of wide-necked aneurysms with anew intracranial microstent (Neuroform) and detachable coils. Neurosurgery 2004;54:1359–1367 [DOI] [PubMed] [Google Scholar]
- 33.Fiorella D, Albuquerque FC, Han P, McDougall CG. Preliminary experience using the Neuroform stent for the treatment of cerebral aneurysms. Neurosurgery 2004;51:16–17 [DOI] [PubMed] [Google Scholar]
- 34.Raymond J, Salazkin I, Stavros G, et al. Endovascular treatment of experimental wide-neck aneurysms: comparison of results using coils or cyanoacrylate with the assistance of an aneurysm neck bridge device. AJNR Am J Neuroradiol 2003;23:1710–1716 [PMC free article] [PubMed] [Google Scholar]