Abstract
BACKGROUND AND PURPOSE: Spinal fractures in ankylosing spondylitis (AS) were difficult to diagnose before CT and MR imaging were available. The purpose of our investigation was to characterize spinal fractures and determine the value of different imaging modalities in AS.
METHODS: Twelve successive cases of spinal fractures were identified in MR imaging files of AS patients. Conventional radiographs were available for 12, CT scans for 7, and 3D-CT scans for 4. We carefully reviewed clinical histories and imaging presentations.
RESULTS: Fractures were found in the cervical spine in 3 patients and in the thoracolumbar spine in 9. The 3 columns of the spine were involved in 11 patients. A routine 4-mm axial CT was not enough to demonstrate all fractures and ligament tears. The sensitivities of 3D-CT scans for demonstration of the following problems were similar to that of MR imaging and were better than that of conventional radiographs: tearing of the posterior longitudinal ligament, the thoracic spinous process fracture, and the facet fracture. MR imaging depicted these following findings that usually were not shown on conventional radiographs or 3D-CT scans: cord deformity, soft tissue disruption, and ligament tears in the posterior column. MR imaging also showed avascular necrosis and occult fractures better than conventional radiographs or CT scans.
CONCLUSIONS: MR imaging shows abnormalities in AS that may not be clear or even detectable by using other imaging methods. With the capability to show lesions in the posterior column, MR imaging can serve to evaluate AS patients with spinal fracture for the possibility of 3-column involvement.
Ankylosing spondylitis (AS), or Marie-Strümpell disease, is an inflammatory disorder of unknown cause that primarily affects the axial skeleton. The human leukocyte antigen HLA-B27 gene is present in approximately 90% of patients, compared with a prevalence of 1–3/1000 in the general population (1, 2).
Radiographically, the earliest manifestation of AS is sacroiliitis, followed by spinal involvement. In the spine, the disease is characterized by ossification of the spinal ligaments, joints, and disks. Syndesmophytes form by means of continuing enchondral ossification until they ultimately bridge the adjacent vertebral bodies with progression to a bamboo spine (3). Patients with AS tend to present with restricted spinal movement and progressive deformity (4). The most serious complication of the disease is spinal fracture, which can occur with even minor trauma because of the rigidity and osteoporotic involvement of the spine (3, 5, 6). Our goal was to characterize spinal fractures in AS, and determine the value of various types of imaging studies.
Methods
Twelve consecutive AS patients with spinal fracture were identified from our records of MR images on the basis of their histories and imaging evidence obtained from May 2001 to November 2003. They included 9 men and 3 women, ranging in age from 41 to 90 years (mean, 58 years). Conventional radiographs were available for all patients, CT scans were available for 7; 4 of them had 3D reconstructed CT scans (3D-CT). MR imaging of the spine had been done by using a 1.5-T unit, including the following pulse sequences: sagittal T1-weighted, fast spin-echo T2-weighted, axial T1-weighted, and axial T2-weighted images. Spatial saturation had been applied anteriorly and posteriorly on T2-weighted images to remove artifacts from heart, great vessel and posterior fat signals. Fat saturation T2-weighted imaging was not performed. Contrast-enhanced T1-weighted images with fat saturation in sagittal and axial views had been obtained in 4 cases. CT scans had been performed with 4-mm contiguous sections. 3D-CT had been performed by using a multidetector-row CT unit based on 0.6–1-mm axial images. Routinely, we perform coronal and sagittal 1-mm reformat and 3D surface reconstruction. Two neuroradiologists (Y.-F.W. and M.M.-H.T.) reviewed these cases separately at first, and then discussed them together. Consultation with the other authors was done when there was any disagreement in diagnosis or structure of involvement. We reviewed patients’ clinical information and radiographic studies. Clinical history and symptoms, afebrile status, and normal white blood cells excluded the possibility of infectious spondylitis in our series. These cases were reviewed by using a combination of clinical records, and radiographic data. Conventional radiographs and CT and MR imaging were reviewed together and compared with each other.
We applied the 3-column concept of the spinal column adapted from Denis and Holdsworth classifications to the whole spine in this study (7). The anterior column contains the anterior two-thirds of the annulus fibrosus, vertebral body and the anterior longitudinal ligament (ALL). The middle column includes the posterior longitudinal ligament (PLL), the posterior third of the vertebral body, and the annulus fibrosus. The posterior column consists of the posterior ligament complex, including the supraspinous and infraspinous ligaments, the capsule of the intervertebral joints, and the ligamentum flavum, as well as the posterior portion of the neural arch. Trauma in the spine may involve different levels in the anterior and posterior columns.
We reviewed MR images carefully and compared them with conventional radiographic and CT scan images to see structures involved in the anterior and posterior columns. We also assessed the appearances in MR images of the fracture line, ligament tears in the posterior column, and adjacent soft tissue disruption. The signal intensities in the fracture, ligament tears in the posterior column, and soft tissue disruption were graded on a 5-point scale: (1) hyperintense, more than bone marrow; (2) isointense to bone marrow; (3) signal intensity between bone marrow and spinal cord; (4) isointense to spinal cord; and (5) signal intensity lower than the spinal cord.
We undertook a careful evaluation to detect the number of true-positives, true-negatives, false-positives, and false-negatives for different imaging modalities for the following lesions: occult fracture, avascular necrosis, pseudoathrosis, tearing of the ALL, tearing of the PLL, ligament tears in the posterior column, cord deformity, spinous process fracture in the cervical region, spinous process fracture in the thoracic region, facet fracture, and any fracture in the posterior column.
Results
Table 1 lists the clinical data for the 12 patients, and Table 2 summarizes the structures involved in the fracture and the signal intensity changes in the posterior column shown on MR imaging. These spinal fractures were found in the cervical spine in 3 patients (Fig 1) and in the thoracolumbar spine in 9 (Figs 2–4). One of these patients had an additional old compression fracture probably from an old trauma (case 12).
TABLE 1:
Summary of patients’ clinical and demographic data
| Patient No./Age (y)/Sex | History of Previous Trauma | Time from Trauma | Neurological Defect at Presentation | Surgical Treatment Performed |
|---|---|---|---|---|
| 1/55/M | Traffic accident | ? | Yes | No |
| 2/71/M | Fall | 29 d | No | No |
| 3/45/F | Fall | > 30 mo | No | No |
| 4/47/M | Fall | 38 d | Yes | Yes |
| 5/90/M | Fall | 1 d | Yes | No |
| 6/47/M | Fall | > 15 mo | Yes | Yes |
| 7/61/M | Traffic accident | 1 d | No | No |
| 8/73/F | Fall | 25 d, 5 mo | No | Yes |
| 9/77/M | Fall | 30 d | Yes | Yes |
| 10/42/M | Fall | 2 mo | Yes | Yes |
| 11/49/M | Fall | 4 mo | No | Yes |
| 12/41/F | Fall | > 2 mo, 27 y | Yes | Yes |
TABLE 2:
Lesions detected and signal change in the posterior column
| Patient No. | Lesion in AC and MC | Secondary Change in AC | Lesion in PC* | Lesion Signal on T1WI in PC* | Lesion Signal on T2WI in PC* |
|---|---|---|---|---|---|
| 1 | C4–5 disk | C5 body | C4–5 facet, C4 spinal process | 5 | 5 |
| 2 | C6–7 disk, C7 body | C6 body | C5–6 facet, C5, C4 and C3 spinal processes | 4–5 | 5 |
| 3 | T10–11 disk and bodies | — | T10–11 facet, T10–11 interspinous | 5 | 5 |
| 4 | C7 body | — | C6–7 facet, C5 and C6 spinous processes | 5 | 5 |
| 5 | L1 body | — | T12–L1 facet and interspinous, L1 spinal process | 4 | 5 |
| 6 | T12–L1 disk | T12–L1 bodies | T12–L1 interspinous | 5 | 4–5 |
| 7 | T12–L1 disk and bodies | — | T12 superior articular processes, T11–12 interspinous | 5 | 5 |
| 8 | T11–12 disk and bodies | — | T11–12 facet, T11 spinal process | 5 | 5 |
| 9 | T12–L1 disk and bodies (T12 occult fracture) | — | T12–L1 facet, T12 spinal process | 4–5 | 5 |
| 10 | T12–L1 disk | T12–L1 bodies | Nil | — | — |
| 11 | L1 body, L2 body occult fracture | — | L1 superior articular process, lamina, T12–L1 interspinous space | 4–5 | 4–5 |
| 12 | T11–12 disk | T11–12 bodies | T11–12 facet, T11 spinous process, T10–11 interspinous space | 4–5 | 5 |
Note.—AC indicates anterior column; MC, middle column; PC, posterior column; T1WI, T1-weighted images; T2WI, T2-weighted images.
Signal intensity: 1, >bone marrow; 2, =bone marrow; 3, between bone marrow and spinal cord; 4, =spinal cord; 5, <spinal cord.
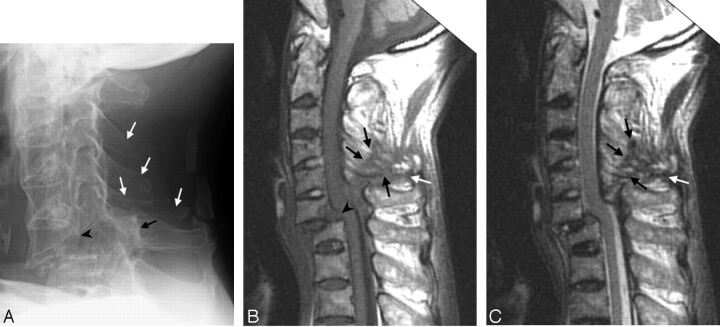
Fig 1.
Case 2. Fracture of the anterior C7 vertebral body and posterior C6 vertebral body with traumatic spondylolisthesis (C6 on C7), tearing of the anterior and posterior longitudinal ligaments. The fracture line extended posteriorly through the C5–C6 facet, to the C3–C5 spinous processes, and involved all 3 spinal columns but at different levels.
A, Conventional radiograph of the cervical spine 16 days after trauma, showing ossification of the ALL due to AS. The distance between the posterior border of the C6 vertebral body (black arrowhead) and the spinolaminar line of C6 (black arrow) is increased, which indicates a fracture of the C6 pedicles bilaterally. There are also fractures in C3–C5 spinous processes (white arrows).
B, T1-weighted image 29 days after trauma, showing the hypointense ligament tears in the posterior column (white arrow) and in C3–C5 spinous process (black arrows). Discontinuity of the normally dark posterior longitudinal ligament at C6–C7 indicates tearing of this ligament (arrowhead).
C, T2-weighted image 29 days after trauma. The ligament tears in the posterior column (white arrow) and the fracture in the spinous process (black arrows) are hypointense. Slight hyperintense dots inside the hypointense area are the displaced spinous processes and fat-containing structures.
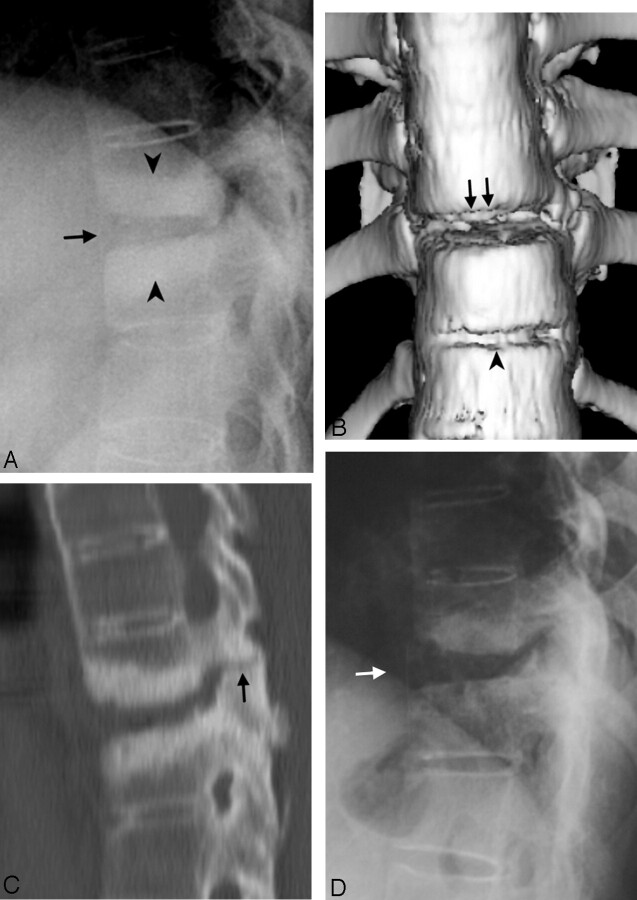
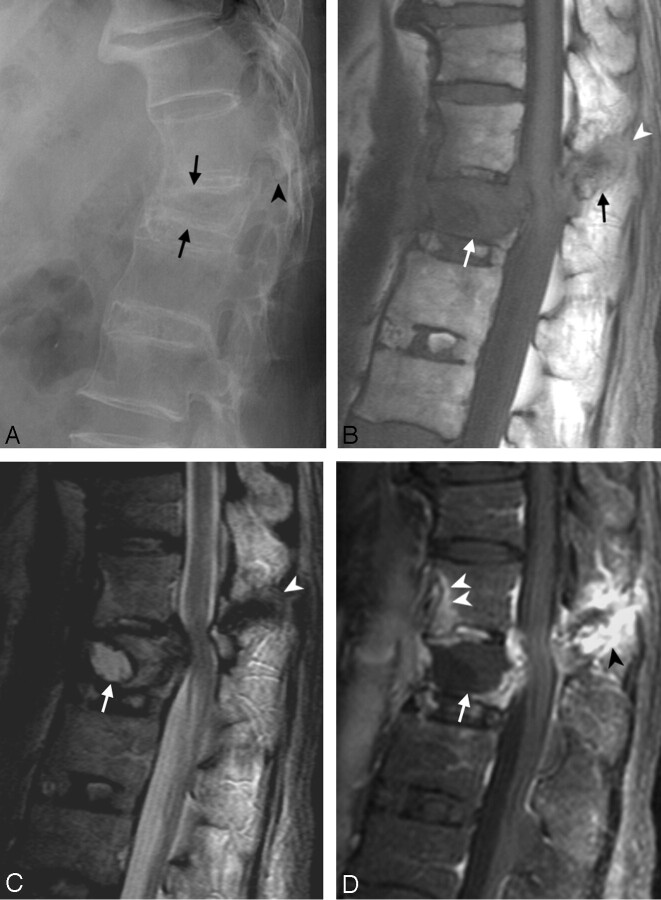
Fig 2.
Case 3. Diskovertebral erosion, pseudarthrosis, with posterior column involvement.
A, Conventional radiograph of the thoracic spine, showing squaring of the vertebral bodies and ossification of the ALL due to AS. Increased disk space of T10–T11 (arrow), endplate erosion, and hyperostotic change in the bone marrow of the adjacent vertebral bodies (arrowheads) were compatible with pseudarthrosis. The hyperostotic change is consistent with a long-term chronic lesion. The facet lesion is not shown well in the conventional radiographs because of overlapping by adjacent structures.
B, Anterior 3D surface-rendered CT reconstruction shows a gap in the ALL and disk space at T10–T11 level (arrows) and another gap in the ALL at the level of the T11–T12 disk (arrowhead), which indicates tearing of the ALL and erosion of the vertebral body at the insertion of this ligament.
C, Sagittal, thin-section, reformatted CT scan image. In addition to the above-mentioned lesions, this reformatted image shows the fracture in the facet (arrow). The diskovertebral lesion is shown better in this image than in conventional radiographs.
D, Conventional radiograph 30 months earlier than A–C, showing squaring of the vertebral bodies and diskovertebral lesion with less extensive sclerotic change (white arrow) than A.
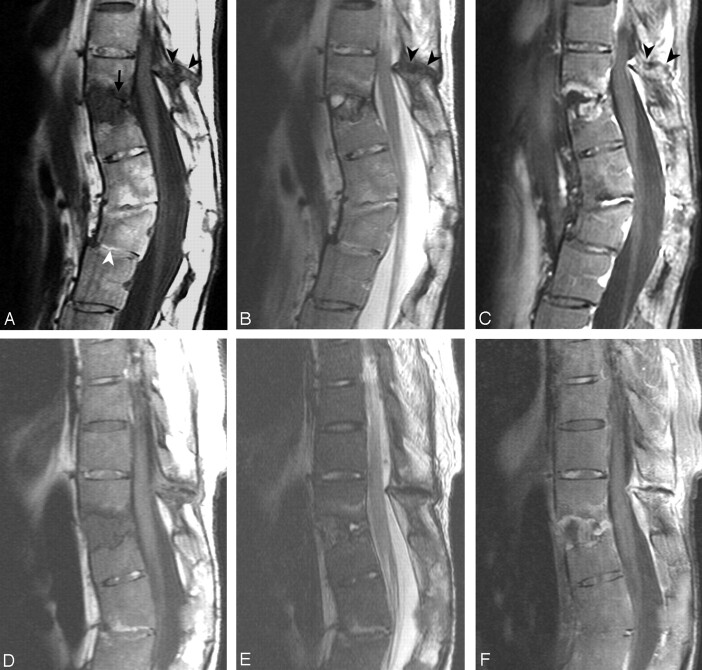
Fig 4.
Case 12.
A, T1-weighted image.
B, T2-weighted image.
C, Gadolinium-enhanced T1-weighted image.
There are 2 levels of trauma. The upper one is an anterior opening fracture at T11–T12 with disk-space widening containing a fluid cavity inside (A, arrow) forming a pseudarthrosis. Erosion and enhancement is present in the adjacent endplates and vertebral bodies extending from the anterior border to the posterior border at this level. There are fractures in the facets, spinous process, and the interspinous space of T10–T11, with hypointensity on T1-weighted and T2-weighted images (A–C, black arrowheads). The postcontrast T1-weighted image (C) shows enhancement along the margin of the fracture line.
The lower level is an anterior wedge deformity of the L2 vertebral body (A, white arrowhead). The L2 vertebral body has no edema, enhancement or cavity inside, and is compatible with an old healed fracture. There is suspicious retrolisthesis of L2 on L3, and L3 on L4. The disk space of L1–L2 and L2–L3 are narrowed, with abnormal signal intensity in the disk space and the adjacent bone marrow from ankylosing spondylitis.
D, E and F, MR imaging 14 months later than A–C show little change.
G and H, Sagittal reformatted CT scan 14 months after A–C in the midline (G) and off midline (H) show the fracture line (arrows) and adjacent bony sclerosis.
I, Conventional radiograph 54 months earlier than A–C shows possible avulsion injury with a teardrop fragment in the anterior inferior T11 vertebral body (white arrow) and reactive hyperostosis at anterior superior corner of T12 vertebral body as a result of AS called “shiny corner” configuration (black arrow). There is squaring of the vertebral bodies and ossification of the ALL in other levels due to AS.
The fracture line in the anterior column passed through the disk space (transdiskal) in 4 patients, through the vertebral body (transvertebral) in 3 patients, and through both disk and adjacent vertebral body/bodies in 5 patients. All lesions involved the anterior and middle columns. The anterior, middle, and posterior columns were involved in 11 patients, and the posterior column was spared in one patient.
The following presentations of spinal fracture were found: occult fracture (n = 2; Fig 3), avascular necrosis (n = 2; Fig 3), pseudarthrosis (n = 5; Figs 2 and 4), tearing of the ALL (n = 10), tearing of the PLL (n = 7), ligament tears in the posterior column (n = 11), cord deformity (n = 5), fracture of the spinous process in the cervical spine (n = 3), fracture of the spinous process in the thoracic spine (n = 4), facet fracture (n = 10), any fracture in the posterior column (n = 11). Case numbers for the above findings—including true-positive, true-negative, false-positive, and false-negative—are listed in Table 3. Associated with tearing of the ALL, translation was found in 4 cases and increased disk space in the other 5 cases. An anterior opening wedge–type distraction fracture (n = 1), and a traumatic spondylolisthesis (n = 2; Fig 1) were also found. Encroachment into the spinal canal was found in 11 cases on MR imaging; only 5 of them had spinal cord deformity. Dural and epidural space enhancement was found in all patients who received a postcontrast MR imaging study.
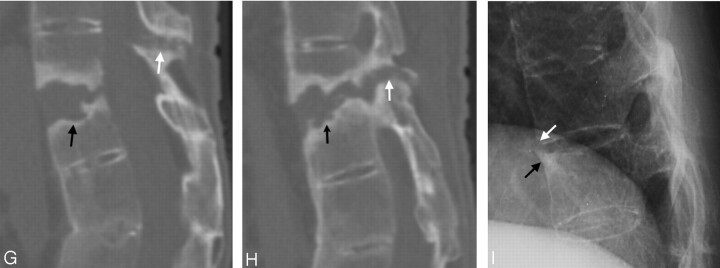
Fig 3.
Case 9. Imaging studies, 1 month after a fall. This patient had fracture in the L1 vertebral body with avascular necrosis. There was also fracture in the anterior inferior T12 vertebral body. The fracture line extended posteriorly with the involvement of the superior articular process of L1, the inferior articular process, and spinous process of T12. All 3 columns were involved.
A, Conventional radiograph, showing ossification of the ALL, and reduced height with wedge deformity of the L1 vertebral body (arrows) and fracture in the facet (arrowhead).
B, T1-weighted image, showing a hypointense signal intensity in the L1 vertebral body (white arrow) due to avascular necrosis and edema, fracture of the T12 spinous process (black arrow), and soft tissue disruption and ligament tears in the posterior column (white arrowhead).
C, T2-weighted image, showing avascular necrosis with fluid inside the L1 vertebral body (white arrow), edema posterior to the fluid cavity, as well as retropulsion with stenosis of the spinal canal and compression of the cord. The bone fracture, ligament tears in the posterior column, and adjacent soft tissue disruption are hypointense (arrowhead).
D, Gadolinium-enhanced T1-weighted image, showing the nonenhancing components of avascular necrosis and edema inside the L1 vertebral body (arrow) with enhancement beyond its border. The fracture in the posterior component is delineated with marginal enhancement (black arrowhead). There is enhancement in the anterior inferior T12 vertebral body (white arrowheads) caused by an occult fracture. The fracture cannot be surely identified in the conventional radiograph (A). It became obvious in the conventional radiograph of the spine taken later (not shown).
TABLE 3:
Case numbers of true-positive (TP), true-negative (TN), false-positive (FP), and false-negative (FN) of different findings on plain film, CT, 3D-CT, and MRI
| Findings | No. of Patients | No. of Positive | Plain film |
CT |
3D-CT |
MRI |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | TN | FP | FN | TP | TN | FP | FN | TP | TN | FP | FN | TP | TN | FP | FN | |||
| Occult fracture | 12 | 2 | 0 | 9 | 0 | 2 | 0 | 6 | 0 | 1 | 0 | 4 | 0 | 0 | 2 | 10 | 0 | 0 |
| Avascular necrosis | 12 | 2 | 1 | 9 | 0 | 1 | 0 | 6 | 0 | 1 | 0 | 4 | 0 | 0 | 2 | 10 | 0 | 0 |
| Pseudarthrosis | 12 | 5 | 5 | 7 | 0 | 0 | 2 | 4 | 0 | 0 | 2 | 1 | 0 | 0 | 5 | 7 | 0 | 0 |
| Tearing of the ALL | 12 | 10 | 8 | 2 | 0 | 2 | 1 | 1 | 0 | 5 | 3 | 1 | 0 | 0 | 8 | 2 | 0 | 2 |
| Tearing of the PLL | 12 | 7 | 0 | 5 | 0 | 7 | 0 | 4 | 0 | 3 | 2 | 1 | 0 | 1 | 5 | 5 | 0 | 2 |
| Ligament tears in the posterior column and adjacent soft tissue disruption | 12 | 11 | 1 | 0 | 0 | 11 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 4 | 11 | 1 | 0 | 0 |
| Cord deformity | 12 | 5 | 0 | 0 | 0 | 12 | 0 | 0 | 0 | 7 | 1 | 0 | 0 | 3 | 5 | 7 | 0 | 0 |
| Spinous process fracture in the cervical region | 3 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Spinous process fracture in the thoracic region | 9 | 4 | 3 | 5 | 0 | 1 | 1 | 5 | 0 | 1 | 1 | 2 | 0 | 0 | 4 | 5 | 0 | 0 |
| Facet fracture | 12 | 10 | 4 | 2 | 0 | 6 | 4 | 2 | 0 | 1 | 4 | 0 | 0 | 0 | 10 | 2 | 0 | 0 |
| Any fractures in the posterior column | 12 | 11 | 6 | 1 | 0 | 5 | 4 | 1 | 0 | 2 | 4 | 0 | 0 | 0 | 11 | 1 | 0 | 0 |
* Sensitivity = TP/(TP + FN).
In 2 cases, the MR imaging detected occult fractures that were not initially shown on conventional radiographs. The occult fracture showed evidence of edema (low signal intensity on T1-weighted images and high signal intensity on T2-weighted images) with enhancement in 1 case (Fig 3). The other case of occult fracture was an old one, showing no edema in adjacent bone marrow on MR imaging.
Pseudarthrosis was found in 5 patients (cases 1, 3, 6, 8, and 12; Figs 2 and 4). MR imaging showed low signals on both T1-weighted and T2-weighted images from fibrosis and bony sclerosis. Two of them had a compartment of low signals on T1-weighted images and hyperintense signals on T2-weighted images inside the pseudarthrosis due to fluid collection. Three patients had postcontrast T1-weighted images; all of these MR imaging images showed enhancement at the margins of the pseudarthrosis. Two patients had old conventional radiographs, and both had initial early signs of abnormality on previous conventional radiographs. Case 6 had a conventional radiographs showing absence of syndesmophytes at the lesion level 60 months before MR demonstration of pseudarthrosis, while adjacent levels had ossification of the ALL. Case 12 had a teardrop fragment in the anterior inferior T11 vertebral body (Fig 4I), and the pseudarthrosis was found 54 months later at the level of the T11–T12 disk space.
For fractures involving vertebral bodies, excluding occult fractures, 3 cases had low signals (lower intensity than the signal intensity of spinal cord) on T1-weighted and T2-weighted images; 1 had low signals on T1-weighted images and signals that were isointense to the spinal cord on T2-weighted images from acute hematoma inside; and 4 had low signals on T1-weighted images (lower than the signal intensity of the spinal cord) and hyperintense on T2-weighted images (signal intensity higher than bone marrow because of acute hematoma in 1, bone marrow edema in 1, avascular necrosis with fluid inside in 2). The fracture tracts in the posterior column appeared as a hypointense band, ranging from isointense to the spinal cord or lower than the signal intensity of the spinal cord on both T1-weighted and T2-weighted images and were most likely fibrosis. No case of hyperintense change on T2-weighted images was found in the fractures, ligament tears in the posterior column, or adjacent soft tissue disruption. Four patients received an intravenous gadolinium-based contrast agent; all of them showed enhancement in the soft tissue next to disruption in the posterior column.
In our 12 patients, surgery was performed on 1 patient with a lesion in the cervical spine and on 6 patients with lesions in the thoracolumbar spine due to the following clinical problems: severe back pain (n = 4), lower leg weakness (n = 4), lower leg numbness (n = 2). The case of C-spine injury received anterior disektomy, bone fusion, and implantation at C6–C7. This patient was intact neurologically initially after a falling accident. Then, gradually, weakness of the lower extremities developed. He became paraplegic before the operation and had not recovered from the paraplegia at follow-up 35 months after the operation. Six patients with lesions in the thoracolumbar region received posterior decompression with laminectomy (n = 6), instrumentation (n = 6), posterolateral bone fusion (n = 3), or posterior fusion (n = 3), anterior fusion (n = 3), or corrective osteotomy of the vertebral body (n = 1). Clinical symptoms improved satisfactorily after the operation in all patients (n = 6) with thoracolumbar lesions. One patient with no neurologic signs at presentation received only conservative treatment and no operation because of his old age. This patient became paraplegic later and expired 30 days after the trauma (case 5). The other 4 patients received no operation, because of relatively mild symptoms and remained stable clinically (cases 1–3 and 7).
Discussion
Ankylosis of the spine results in biomechanical changes that predispose the patient to serious spinal injury (8, 9). The forces necessary to fracture an ankylosed spine are much smaller than those required to fracture a normal spine (6). The tendency of patients with AS to have an injury even after minor trauma has been noted in previous reports (10–12). Sometimes, fractures may occur without any recognizable trauma (5, 11). In our cases, most causes of fractures were minor injuries.
As the joint fuses and the apophyseal joint becomes ankylosed, the ALLs and the interspinous and paraspinous ligaments ossify and the spine becomes osteoporotic. The spine then becomes susceptible to fractures (9, 13). Because of osteopenic changes, the fracture may pass through the vertebral body. Eight of our 12 cases had fractures of the vertebral body. Although ossification of the ligamentous structures occurs in patients with AS, this does not provide extraneous support to the spine. Tearing of the ALL was observed in 10 of our 12 cases. In addition, ligament tears in the posterior column and adjacent soft tissue disruption were noted in 11 of 12 cases. Some AS-related fractures resembled the Chance or seat-belt fractures. Unlike typical Chance fractures, AS injuries often had tearing of the ALL, and translation or displacement at the level of the fracture occurred often. The cervical spine is reportedly the most common site for spinal fracture in patients with AS (5–13). Similar to Shih et al’s report (14), our patients had more cases of thoracolumbar involvement than cervical fractures. A cervical spinal fracture injury in patients with AS can lead to quadriplegia (10, 13). The neurologic deficits due to spinal instability may be acute or delayed (13). Three of our patients had cervical injuries. One had a C6–C7 hyperextension injury-dislocation and cord transection resulting in quadriplegia (case 4).
In 1937, Andersson first described diskovertebral destructive lesions occurring in AS (15). These lesions may become pseudarthrosis later. Two of our patients had early signs of an abnormality on conventional radiograph 2–5 years before the MR demonstration of pseudarthrosis. One had a small avulsion injury. The other one had no ossification of the ALL at the level that later developed pseudarthrosis, whereas ossification of the ALL was found in other adjacent levels. Initially, these patients might not recognize an injury and the fracture may be mild and unnoticeable because of its relatively mild symptoms and presence of a chronic back pain as a result of the AS. Although other levels were ankylosed, movement of the spine mainly took place at the level of the fracture and continued motion or further trauma at the fracture site may eventually lead to formation of a pseudarthrosis and a 3-component fracture. Radiologic demonstration of pseudarthrosis is not difficult. Conventional radiographs may show a widened space caused by bone absorption and adjacent bony sclerosis. The appearance of pseudarthrosis mimics that of an infectious spondylitis (8, 14). The differential diagnosis between pseudarthrosis and infection may be difficult in some cases (16, 17); however, clinical history and symptoms, afebrile status, normal white blood cell count, and the demonstration of a posterior component fracture can help rule out an infection (14).
Two patients had avascular necrosis inside the vertebral body. Whenever MR imaging shows a cavity inside a vertebral body with gas or fluid inside, avascular necrosis must be considered. Sometimes there is abundant marginal enhancement, and an infectious process may be considered as a differential diagnosis (case 9, Fig 3).
Before the use of MR imaging, the diagnosis of a spinal fracture as a complication of longstanding AS was considered difficult without neurologic damage (11). Milicic et al found that 41.67% of the patients were not diagnosed initially and 16.67% had, for this reason, deteriorated neurologically (18). Finkelstein et al proposed reasons why radiographs might not delineate the fractures in AS patients: distortion of the anatomy with surrounding osseous proliferation, ossified spinal ligaments, poor outlining of the disk space, osteoporosis, a history of minor trauma and lack of displacement, and difficulty in visualizing the cervicothoracic junction (1).
Our study indicates that conventional radiographs can show fractures in the anterior column except occult fractures. Some avascular necrosis may be undetected on conventional radiographs (sensitivity 50%). Tearing of the ALL can be demonstrated with conventional radiographs (sensitivity 80%) because of ossification from ankylosing spondylitis. Tearing of the PLL was difficult to demonstrate on conventional radiographs (sensitivity 0%). For the posterior column, conventional radiographs demonstrated the spinous process fracture in the cervical spine with a high sensitivity (100%); yet it was less sensitive in demonstrating fractures in the spinous process in the thoracic spine (75%), any fracture in the posterior column (55%), facet fractures (40%), and ligament tears in the posterior column and adjacent soft tissue disruption (8%; Table 3).
Although standard radiographic techniques may be unsatisfactory (11), MR images are ideal for evaluating soft-tissue injury, because their interpretation is not restricted by osteopenia or distorted anatomy. MR imaging is also a reliable test for detecting occult fractures in AS and obviates reliance on delayed neurologic deficits (1). From our study, MR imaging demonstrated the following findings that usually could also be shown on conventional radiographs, such as anterior column fractures, pseudarthrosis, and tearing of the ALL. MR imaging showed the following findings that usually were not shown by conventional radiographs: occult fractures in the anterior column (sensitivity 100%), cord deformity (100%), tearing of the PLL (71%), ligament tears in the posterior column and soft tissue disruption (100%), fracture in the thoracic spinous process (100%), and facet fracture (100%). On contrast-enhanced T1-weighted images, there was abnormal enhancement at the margin of pseudarthrosis, avascular necrosis, and along the tract of ligament tears in the posterior column and adjacent soft tissue disruption. MR imaging also showed avascular necrosis better than did conventional radiographic and CT scans.
From Shih et al’s report, about 69% of fractures in the posterior column had high signal intensity on T2-weighted images (14). It is reasonable that the bone and soft tissue near the fracture line could become edematous for some time after trauma and present high signal intensity on the T2-weighted images. The fracture line itself should be low on both T1-weighted and T2-weighted images, unless fluid collection and hematoma were present inside the fracture line. From our study, the fracture, ligament tears in the posterior column, and adjacent soft tissue disruption were all low in signal intensity on both T1-weighted and T2-weighted images. Only one case showed high signal intensity on T2-weighted images at the bone near the fracture line. The causes of this discrepancy between Shih et al’s and our study were probably (1) differences in time until the MR imaging examination after trauma, and our study was done at a stage with no fluid or hematoma collection in the fracture line, ligament tears, or soft tissue disruption and (2) the fact that we did T2-weighted images with spatial saturation instead of fat suppression. T2-weighted images with fat suppression may be more sensitive in detecting edema in the posterior column mixing with fat signal intensity than T2-weighted images with spatial saturation. We did not do short time inversion recovery (STIR) pulse either. STIR is capable of removing fat signal intensity and thus is more sensitive in showing edema. Similar to Shih et al’s report, we found that MR imaging was very helpful for identification of any abnormalities of the dura, ligaments, and soft tissue.
In comparison to MR imaging, CT of the spine requires a shorter scanning time, and it can show bony details, including fractures and fragments (5, 19). From our study, a routine noncontrast 4-mm axial CT was poor in sensitivity in demonstrating the following lesions: tearing of the ALL (sensitivity 17%), tearing of the PLL (0%), ligament tears in the posterior column (0%), and cord deformity (0%). The new-generation CT scanners with multiplanar reconstruction ability can also provide information regarding the extent of the lesion and are helpful in visualization of the details of these fractures (3, 19). Four of our cases that underwent 3D-CT had good demonstration of the spinal fractures (Fig 2). From our study, tearing of the ALL was best demonstrated by 3D-CT (sensitivity 100%), such as anterior view of anterior 3D surface-rendered CT scan (Fig 2B) or sagittal thin-section reformatted CT scan (Fig 2C). The sensitivities of 3D-CT for demonstration of the following problems were similar to that of MR imaging: tearing of PLL (66%), and cervical or thoracic spinous process fracture (100%), and facet fractures (100%).
With modern imaging modalities, the potential difficulties in detecting such spinal fractures come chiefly from the patient’s side. Unless there was an acute trauma with acute new back pain, spinal fracture in these patients may be easily overlooked. These patients tended to have minor trauma with daily back pain. A pseudarthrosis often develops long after an initial minor abnormality at the level of the lesion, similar to our cases 6 and 12. Because of the presence of a chronic problem, the patient and physicians might not become aware when a pseudarthrosis has developed.
Milicic et al found 16.67% of patients had deteriorated neurologically because of late diagnosis (18). From our study, 11 patients had 3-column involvement. Paraplegia occurred in 2 patients before adequate surgical treatments were undertaken for them (cases 4 and 5). Therefore, early diagnosis and proper early management of these 3-column spinal fractures are important. Any patient with AS who seeks treatment for a new complaint of neck or back pain with or without neurologic deficit should be treated as if he or she has an unstable spinal fracture until proved otherwise (5, 20). Usually, spinal instability was determined through dynamic (flexion/extension) views or a standing lateral view of the spine, which was usually done in cases of degenerative spine disorder. Three-column injury is an extremely unstable fracture, and patients should be immobilized and not be moved or manipulated once the fracture is identified. The above-mentioned instability evaluation in these patients with 3-column involvement may result in severe neurologic deficits. All of our patients had anterior and middle component involvement, and most our patients had 3-component involvement. To have 3-component involvement could be from an acute onset lesion as a result of a significant trauma like our case 5, or as a result of a chronic process like our cases 6 and 12. Cardiopulmonary resuscitation, intubation, patient transfer, and improper application of traction devices to reduce a fixed flexion deformity of the cervical spine have all been implicated as causes of acute traumatic fractures of the spine, aggravation of previous spinal lesions, or development of new neurologic deficits in patients with AS (13).
Surgical management of these fractures has traditionally resulted in a high morbidity rate (5). With benefits from improved instrumentation, the morbidity should now be much lower. Surgical treatment of fractures in ankylosing spondylosis depends on its location (cervical, thoracic, thoracolumbar, or lumbar), level of involvement (multiple or single), acute or chronic status (such as pseudarthrosis), structures involved, and stability (3-column involvement), displacement, or spinal cord compression or spinal stenosis (soft tissue, dural thickening or bony compression, hematoma). An imaging study should try to answer the above questions. Surgical operations include anterior surgery, posterior surgery, or posterior surgery with an anterior approach; decompression with laminectomy, instrumentation, bone fusion (anterior, posterior, posterolateral), and various ways of doing osteotomy for deformity correction (3, 20–22). Nondisplaced or minimally displaced acute traumatic lesions in AS patients may be treated successfully by conservative methods, such as plaster cast or brace; but the inherent instability of the fracture is reflected by its high potential of acute displacement, which may cause a catastrophic situation. Therefore, surgical fixation with long segmental instrumentation and fusion is highly recommended for this lesion. Surgical intervention with long segmental posterior fixation and fusion followed by anterior fusion must be done for significant displacement or pseudarthrosis. Decompression may be performed if there is localized stenosis in the spinal canal, especially when the patient has neurologic deficit. Another important part of the management program is education of the patient and his or her caregivers about the risks of injury (6). This education principally involves alerting patients to the fragility of their spine and to the importance of avoiding spinal trauma (3, 6, 11, 19, 20).
Conclusions
Spinal fractures in AS often involve 3 columns of the spine and are unstable. Early recognition and correct diagnosis of these unstable 3-column lesions are important for proper treatment. The following various spinal lesions may be found in these patients: occult fracture, avascular necrosis, pseudarthrosis, tearing of the anterior longitudinal ligament, tearing of the posterior longitudinal ligament, ligament tears in the posterior column, and adjacent soft tissue disruption, cord deformity, fracture in the posterior column, and traumatic spondylolisthesis. MR imaging shows abnormalities in AS that may not be clear or even detectable by using other imaging methods. With the capability to show lesions in the posterior column, MR imaging can serve to evaluate AS patients with spinal fracture for the possibility of 3-column involvement.
References
- 1.Finkelstein JA, Chapman JR, Mirza S. Occult vertebral fractures in ankylosing spondylitis. Spinal Cord 1999;37:444–447 [DOI] [PubMed] [Google Scholar]
- 2.Lawrence JS. The prevalence of arthritis. Br J Clin Pract 1963;17:699. [PubMed] [Google Scholar]
- 3.Hitchon PW, From AM, Brenton MD, et al. Fractures of the thoracolumbar spine complicating ankylosing spondylitis. J Neurosurg 2002;97(2 suppl):218–222 [DOI] [PubMed] [Google Scholar]
- 4.Graham GP, Evans PD. Spinal fractures in patients with ankylosing spondylitis. Injury 1991;22:426–427 [DOI] [PubMed] [Google Scholar]
- 5.Gartman JJ Jr, Bullitt E, Baker ML. Axis fracture in ankylosing spondylitis: case report. Neurosurgery 1991;29:590–593 [DOI] [PubMed] [Google Scholar]
- 6.Wade W, Saltzstein R, Maiman D. Spinal fractures complicating ankylosing spondylitis. Arch Phys Med Rehabil 1989;70:398–401 [PubMed] [Google Scholar]
- 7.Denis F. The three column spine and its significance in the classification of acute thoracolumbar spine injuries. Spine 1983;8:817–831 [DOI] [PubMed] [Google Scholar]
- 8.Resnick D, Niwayama G. Discovertebral destruction in a man with chronic back problems. Invest Radiol 1981;16:89–94 [DOI] [PubMed] [Google Scholar]
- 9.Graham B, Van Peteghem PK. Fractures of the spine in ankylosing spondylitis: diagnosis, treatment, and complications. Spine 1989;14:803–807 [DOI] [PubMed] [Google Scholar]
- 10.Rowed DW. Management of cervical spinal cord injury in ankylosing spondylitis: the intervertebral disc as a cause of cord deformity. J Neurosurg 1992;77:241–246 [DOI] [PubMed] [Google Scholar]
- 11.Hunter T, Dubo H. Spinal fractures complicating ankylosing spondylitis. Ann Int Med 1978;88:546–549 [DOI] [PubMed] [Google Scholar]
- 12.Hunter T, Dubo HIC. Spinal fractures complicating ankylosing spondylitis: long-term follow-up study. Arthritis Rheum 1983;26:751–759 [DOI] [PubMed] [Google Scholar]
- 13.Fox MW, Onofrio BM, Kilgore JE. Neurological complications of ankylosing spondylitis. J Neurosurg 1993;78:871–878 [DOI] [PubMed] [Google Scholar]
- 14.Shih TTF, Chen PQ, Li YW, Hsu CY. Spinal fractures and pseudarthrosis complicating ankylosing spondylitis: MRI manifestation and clinical significance. J Comp Assist Tomogr 2001;25:164–170 [DOI] [PubMed] [Google Scholar]
- 15.Andersson O. Rontgenbilden vid spondylarthritis ankylopoetica. Nord Med 1937;30:2000–2002 [Google Scholar]
- 16.Pettersson T, Laasonen L, Leirisalo-Repo M, Tervahartiala P. Spinal pseudarthrosis complicating ankylosing spondylitis: a report of two patients. Br J Rheumatol 1996;35:1319–1323 [DOI] [PubMed] [Google Scholar]
- 17.Eschelman DJ, Beers GJ, Naimark A, Yablon I. Pseudarthrosis in ankylosing spondylitis mimicking infectious diskitis: MR appearance. AJNR Am J Neuroradiol 1991;12:1113–1114 [PMC free article] [PubMed] [Google Scholar]
- 18.Milicic A, Jovanovic A, Milankov M, et al. Fractures of the spine in patients with ankylosing spondylitis. Medicinski Pregled 1995;48:429–431 [PubMed] [Google Scholar]
- 19.Fitt G, Hennessy O, Thomas D. Case report 709: transverse fracture with epidural and small paravertebral hematomata, in a patient with ankylosing spondylitis. Skeletal Radiol 1992;21:61–63 [DOI] [PubMed] [Google Scholar]
- 20.Knoringer P. Osteosynthesis of injuries and rheumatic or congenital instabilities of the upper cervical spine using double-threaded screws. Neurosurg Rev 1992;15:275–283 [DOI] [PubMed] [Google Scholar]
- 21.Dihlman W, Delling G. Disco-vertebral destructive lesions (so-called Andersson lesions) associated with ankylosing spondylitis. Skeletal Radiol 1978;3:10 [Google Scholar]
- 22.Taggard DA, Traynelis VC. Management of cervical spinal fractures in ankylosing spondylitis with posterior fixation. Spine 2000;25:2035–2039 [DOI] [PubMed] [Google Scholar]