Abstract
Summary: Solitary fibrous tumors are submesothelial mesenchymal fibroblastic tumors that typically occur in relation to parietal or visceral pleura. However, the tumor also occurs in extrapleural sites, including the peritoneum, mediastinum, orbit, and oral cavity. With the advent of immunohistochemical testing, certain tumors may be more readily identifiable; however, the diagnosis often must be reached by histomorphology and imaging studies alone. We describe a case of a solitary fibrous tumor of the buccal space, including clinical presentation, imaging characteristics, gross pathology, and histopathologic description.
Submesothelial mesenchymal fibroblastic tumors occur in extrapleural sites including the peritoneum, mediastinum, orbit, and oral cavity (1,2). We describe the imaging findings of a solitary fibrous tumor of the buccal space correlated with pathologic findings with emphasis on the importance of imaging when the findings of immunohistochemical testing are negative.
Case Report
An 83-year-old woman presented with a 5-year history of a painless right-sided facial mass. The mass had increased in size during the past month and caused her much difficulty wearing dentures. She denied any pain or numbness. Medical history consisted of only borderline hypertension and a surgical history that included right-ankle surgery and several dental extractions.
On physical examination, right facial swelling was noted, with a firm, palpable mass measuring more than 8.0 cm in diameter. The mass replaced the right malar eminence and zygomatic arch. Oropharyngeal examination demonstrated an exophytic mass with moderate mucosal ulceration near the right anterior alveolar ridge. There were no cranial nerve deficits.
Contrast-enhanced CT disclosed a heterogeneously enhancing 6.0 × 7.0 cm soft-tissue mass in the right buccal and masticator space, completely obliterating the right retroantral fat pad. Significant bone remodeling was noted in the right zygomatic arch, maxillary sinus, and coronoid process of the mandible (Fig 1A). There was indentation of the oral soft tissues with superior extension to the base of the skull. The mass was found to encroach on the foramen ovale; however, there was no evidence of intracranial extension or bony destruction.
Fig 1.
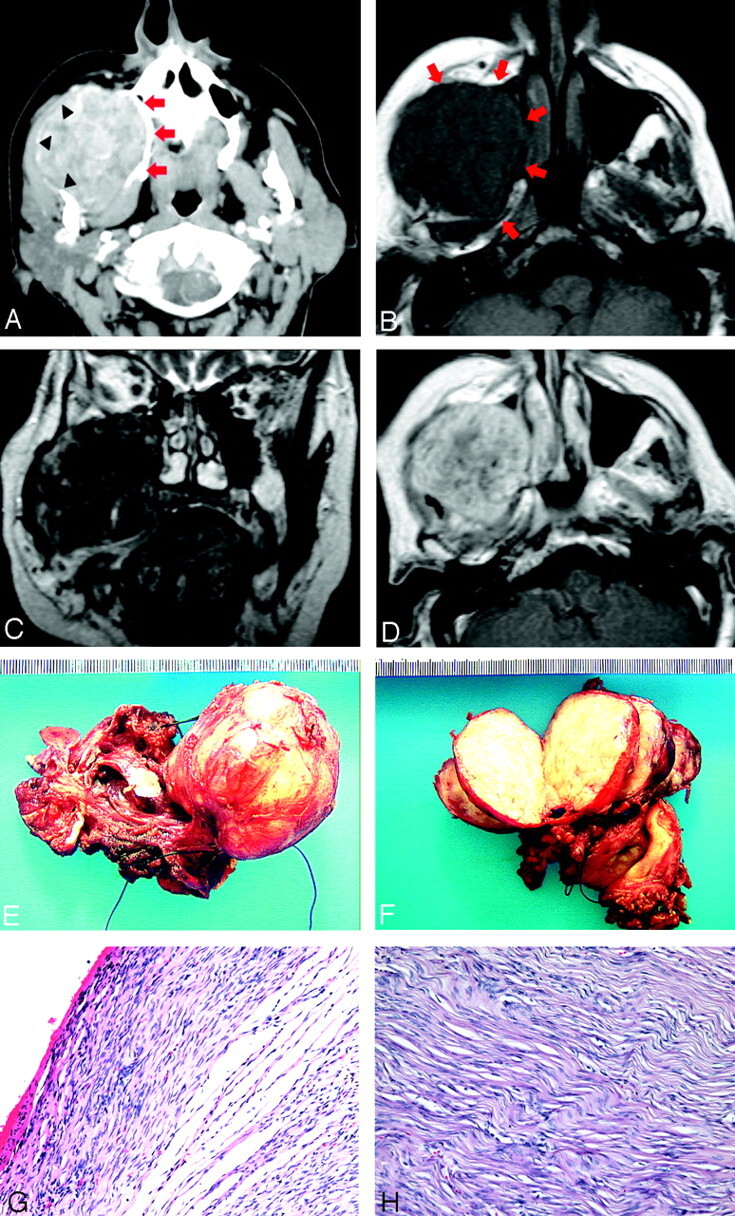
83-year-old woman who presented with right-sided face mass.
A, Axial contrast enhance CT demonstrates a soft-tissue mass in the right buccal and masticator space with heterogeneous enhancement. Note the mass effect on the surrounding muscles of mastication and bony remodeling of the right maxilla (arrows) and mandible (arrowhead).
B, Axial T1-weighted MR image (TR/TE, 550/11) demonstrates an isointense mass in the same region (arrows).
C, Coronal T2-weighted MR imaging (TR/TE, 3950/110) reveals a diffusely hypointense mass containing areas of hyperintensity. No infiltrative changes are seen, though there is significant mass effect to the adjacent structures.
D, Axial T1-weighted MR image (TR/TE, 606/16) with gadolinium demonstrates heterogeneous enhancement of the mass.
E, Resected gross specimen shows a well-circumscribed encapsulated soft tissue mass.
F, Cut sections through the tumor reveal homogeneous tan surfaces with a whorled and slightly nodular appearance and focal areas of hemorrhage.
G, Photomicrograph reveals alternating hypocellular and hypercellular areas of spindle-shaped tumor cells with scarce cytoplasm and vesicular nuclei (hematoxylin and eosin, original magnification ×10).
H, High power field demonstrates the classic “patternless pattern” of random wirelike collagen deposition with interlacing bands of fibroblast-like cells (hematoxylin and eosin, original magnification ×20).
On MR imaging, the mass exhibited a low T1 signal intensity, isointense to muscles (Fig 1B), and a very hypointense T2 signal intensity with separate foci of hyperintensity (Fig 1C). Again noted was significant mass effect to the muscles of mastication and bony remodeling in the right maxilla, mandible, and zygomatic arch. The mass showed significant enhancement after contrast administration (Fig 1D). Although the mass deeply extended between the medial and lateral pterygoid muscles, there was no invasion or infiltration of the muscles or evidence of intracranial extension. The mass was thought to arise from the buccal space because of the erosion of the coronoid process and its epicenter being also in the buccal space.
Fine-needle aspiration biopsy demonstrated squamous mucosa and submucosa with ulceration, acute and chronic inflammation, and granulation tissue adjacent to a focus of spindle cell fibroblastic proliferation. At this time, the patient agreed to surgical resection.
In the operating room, a smooth soft-tissue mass was discovered, exhibiting mass effect on the right maxillary sinus. There was erosion of the hard palate on the right side. Dissecting with a 1-cm margin, we peeled the mass off the sinus side of the orbital floor and from around the mandible without difficulty. The right maxilla and pterygoid plate were both subsequently removed. Finally, a palatal prosthesis and right-thigh skin graft were placed.
On gross pathologic review, the tumor was polypoid, well circumscribed, and encapsulated, demonstrating no gross bone or soft palate invasion (Fig 1E). Cut sections through the tumor revealed a homogeneous tan surface with a whorled and slightly nodular appearance and focal areas of hemorrhage (Fig 1F). Histopathologic examination revealed alternating hypocellular and hypercellular areas of spindle-shaped tumor cells with scant cytoplasm and vesicular nuclei. The cells were arranged in short storiform bundles, separated by thick bands of eosinophilic collagen-like material. Surgical margins were free of tumor and rare mitosis was seen (1/10 per high power field) (Fig 1G, -H). Immunohistochemical studies performed on paraffin-embedded tissue sections revealed tumor cells to be negative for CD34, muscle-specific actin, epithelial membrane antigen, and a variety of cytokeratins (AE 1:3). Despite negative staining for CD34, the tumor was histomorphologically consistent with a solitary fibrous tumor.
Discussion
Solitary fibrous tumors are thought to originate from submesothelial mesenchymal fibroblast-like cells, rather than from epithelial or mesothelial tissue, once classified by the World Health Organization (1,2). Although most often occurring in relation to parietal or visceral pleura or peritoneum, the tumor has been reported, as in this case, in extrapleural sites such as the orbit, thyroid, epidural and intradural space, mediastinum, and upper respiratory tract. The absence of mesothelium in these extrapleural locations further supports a mesenchymal origin (3, 4).
Extrapleural solitary fibrous tumors are typically observed in adults between 20 and 80 years of age, occurring equally among men and women. Although their presentation may vary, imaging often provides the first clue in identifying these tumors. In this case, initial imaging demonstrated a well-defined mass occupying the buccal and masticator space. Differential diagnosis for masses in this region includes schwannoma, sarcoma, squamous cell carcinoma, lymphoma, lymphangioma/hemangioma (lymphatic or venous malformation), ameloblastoma, and abscess from dental infection. The primary site of this tumor was difficult to determine. The center of the mass was located in the buccal space, and erosion of the coronoid process supported its buccal space origin. CT imaging of solitary fibrous tumors has been reported to demonstrate a soft-tissue mass with varying heterogeneous or homogeneous enhancement. Occasional internal calcifications or necrosis has been reported in some tumors (1, 4). MR imaging reveals solitary fibrous tumors to be mostly isointense to muscle on T1 and hypointense on T2, reflecting their primary fibrous-tissue component, with heterogeneous or homogeneous enhancement (3,4). In our case, the tumor displayed several areas of T2 hyperintensity, likely because of both internal hemorrhage and relatively fresh fibrosis visualized on histopathologic examination.
Solitary fibrous tumors were first described histologically by Stout and Murray (5) as a classic “patternless pattern” of random wirelike collagen deposition with interlacing bands of fibroblast-like cells. The cells contain inconspicuous nucleoli, supporting the benign nature of the tumor. Additionally, solitary fibrous tumors are typically well marginated with a hemangiopericytoma-like vascular pattern (5). Keloid-like bundles of collagen, calcification, ossification, and psammoma bodies are rarely seen in these neoplasms (6). These histologic features, in conjunction with the tumor phenotype, are particularly helpful in recognizing solitary fibrous tumors occurring in sites away from mesothelial-lined tissues.
Recent advancements in immunohistochemistry have allowed more definitive characterization of solitary fibrous tumors when the histomorphologic diagnosis is uncertain. This allows differentiation from other benign soft-tissue tumors such as fibroblastic or neurogenic tumors. CD34 is a transmembrane glycoprotein expressed by hematopoietic progenitor cells, endothelium, and some mesenchymal stromal cells in the dermis (7). Tumor cells in solitary fibrous tumors are characteristically immunoreactive for CD34 in 90%–95% of cases (8). Although this marker was not expressed on the tumor cells described in our case, the histomorphology and imaging supported the diagnosis, emphasizing the importance of correlating imaging studies with gross and histopathological findings.
Although most reported solitary fibrous tumors pursue a benign course, approximately 10%–15% behave aggressively. Rare cases have shown abrupt transition from a benign solitary fibrous tumor to a high-grade sarcoma (8). The largest reported series proposed criteria for malignancy that included the presence of more than 4 mitotic figures per 10 high power field, cellular pleomorphism, or tumor giant cells (6). On imaging studies, malignant solitary fibrous tumors tended to be larger and more frequently necrotic. Typically, they were more likely to arise from the parietal pleura or mediastinum, compared with benign solitary fibrous tumors. Metastasis is most frequently observed in the lung, bone, and liver.
In conclusion, we reported CT and MR imaging findings in a case of a solitary fibrous tumor of the buccal space, unlike the typically reported solitary fibrous tumors in the parietal and visceral pleura. Bone remodeling and low signal intensity on T2-weighted MR images strongly suggested its slow-growing fibrous nature. With the absence of positive immunohistochemical staining, imaging once again became crucial in establishing the diagnosis.
References
- 1.Iwai S, Nakazawa M, Yoshikawa F, Amekawa S, Sakuda M. Solitary fibrous tumor of the buccal mucosa: report of a case with immunohistochemical studies. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;88:461–465 [DOI] [PubMed] [Google Scholar]
- 2.Sobin LH, ed. Histological typing of soft-tissue tumours. In: Weiss SW, ed. WHO: World Health Organization—international histological classification of tumours. Berlin, Germany; Springer-Verlag;1994
- 3.Shin J, Sung I, Suh J, et al. Solitary fibrous tumor in the buccal space: MR findings with pathologic correlation. AJNR Am J Neuroradiol 2001;22:1890–1892 [PMC free article] [PubMed] [Google Scholar]
- 4.Kim TA, Brunberg JA, Pearson JP, Ross DA. Solitary fibrous tumor of the paranasal sinuses: CT and MR appearance. AJNR Am J Neuroradiol 1996;17:1767–1772 [PMC free article] [PubMed] [Google Scholar]
- 5.Stout AP, Murray MR. Localized pleural mesothelioma. Arch Pathol 1942;34:951–964 [Google Scholar]
- 6.England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura: a clinicopathologic review of 223 cases. Am J Surg Pathol 1989;13:640–658 [DOI] [PubMed] [Google Scholar]
- 7.van de Rijn M, Lombard CM, Rouse RV. Expression of CD34 by solitary fibrous tumors of the pleura, mediastinum, and lung. Am J Surg Pathol 1994;18:814–820 [DOI] [PubMed] [Google Scholar]
- 8.Guillou L, Fletcher JA, Fletcher CDM, Mandaki N. Extrapleural solitary fibrous tumour and haemangiopericytoma. World Health Organization classification of tumours: pathology and genetics of tumours of soft tissue and bone. Albany, NY: WHO Publication Center,2002;86–90


