Abstract
BACKGROUND AND PURPOSE: Aneurysm recanalization is an innate problem in endovascular treatment of aneurysms with coils. A coated coil system, covered with a bioabsorbable polymeric material (polyglycolic acid/lactide copolymer, PGLA), was developed to accelerate intra-aneurysmal clot organization and fibrosis. The purpose of this study was to evaluate the efficacy and safety of the PGLA-coated coils in patients with intracranial aneurysms and to compare the outcome with that of bare platinum coils.
PATIENTS AND TECHNIQUES: Fifty-one patients harboring 56 intracranial aneurysms underwent endovascular embolization with the PGLA-coated coils. In the control group were 78 consecutive patients, harboring 87 aneurysms, who underwent coil embolization with bare platinum coils. The authors compared coil volume, packing attenuation, degree of occlusion of aneurysms, procedure-related complications, and follow-up results between the 2 groups.
RESULTS: The PGLA-coil group showed comparable data regarding rate of total or near-total occlusion of the aneurysm, incidence procedure–related thromboembolism, and management outcome. Mean coil volume deployed and packing attenuation of the PGLA-coil group were significantly higher than those of the bare-coil group (P = .0026 and P < .0001, respectively). Radiologic follow-up evaluation revealed recanalization in 14 of 39 aneurysms (major recanalization in 5 [13%] and minor recanalization in 9 [23%]) among the PGLA-coil group and in 29 of 64 aneurysms (major recanalization in 9 [14%] and minor recanalization in 20 [31%]) among the bare-coil group.
CONCLUSION: In this study, the incidence of recanalization was not different in aneurysms treated with PGLA-coated coils compared with historical controls treated with bare platinum coils.
Coil embolization for intracranial aneurysms has been widely used since the introduction of Guglielmi detachable coils (GDCs; Boston Scientific, Fremont, CA). This treatment technique proved to be effective at preventing rebleeding after aneurysmal rupture (1, 2) and showed an even better clinical outcome in terms of disability-free survival as compared with surgical clipping (3). Coil embolization, however, was found to have shortcomings in that it required a further procedure more frequently than surgical clipping (3). Specifically, as many as one-third of patients who had undergone coil embolization experienced aneurysm recanalization (4, 5). Some cases with a recanalized aneurysm are at risk of delayed aneurysm rupture and thus may require further treatment.
To overcome this serious shortcoming, various modifications in coils have been made and a few of them have become available in clinical practice (6–9). Among these, a coated coil system (Matrix; Boston Scientific), covered with a bioabsorbable polymeric material (polyglycolic acid/lactide copolymer [PGLA]) was launched first. The developers showed acceleration of aneurysm fibrosis and intensified neck neointimal formation in an experimental model (7, 10, 11), but efficacy regarding prevention of recurrence was not assessed by appropriate models. Furthermore, its efficacy and safety in clinical practice and the follow-up data for aneurysms embolized by using this PGLA-coated coil system, especially in comparison with preexisting bare platinum coils, are not widely available. In this respect, results of an initial clinical experience in a single center, with historical comparison to consecutive patients treated with standard platinum coils, could provide some estimate of the clinical value of this new material, which is not yet available in the literature.
The purpose of this study was to evaluate the efficacy and safety of the PGLA-coated coils in patients with intracranial aneurysms and to present the short-term outcome of aneurysms treated with the coils in comparison with bare platinum coils in a single institution.
Patients and Techniques
Group Characteristics
To eliminate influence from the confounding variables, we included only cases of saccular aneurysms. We excluded cases of dissecting aneurysms or pseudoaneurysms. Patients who underwent parent arterial occlusions (for giant aneurysms) or prior coil embolizations were excluded. In addition, patients (n = 2; a single case in each group) who underwent coil embolization by using other coated coils (Hydrocoils; MicroVention, Aliso Viejo, CA) were also excluded.
Thus, finally included were 51 patients, harboring 56 intracranial aneurysms, who underwent endovascular embolization with the PGLA-coated coils during the period between September 2003 and June 2004. At least one PGLA-coated coil was used during embolization for these aneurysms. There was no specific selection criteria for patients treated with PGLA coils, but we employed them in technically easier cases in consideration of aneurysm size, neck dimension, and direction of the fundus because the PGLA coils were newer material for aneurysm treatment. There were 16 male and 35 female patients in this series, and age ranged from 31 to 85 years (mean ± SD, 53.8 ± 12.2 years). Nineteen ruptured and 37 unruptured aneurysms were included. Among cases with ruptured aneurysms, Hunt and Hess grades were I in one case, II in 12, III in 2, IV in 3, and V in one.
The control group included 78 consecutive patients, harboring 87 aneurysms (41 ruptured and 46 unruptured), who underwent coil embolization by using bare platinum coils only during the period from November 2002 to September 2003. There were 32 male and 46 female patients in this group, and age ranged from 22 to 83 years (56.4 ± 11.9 years). Among cases with ruptured aneurysms, Hunt and Hess grades were I in 6 cases, II in 12, III in 17, IV in 5, and V in one.
The baseline characteristics showed no significant differences between the 2 groups in terms of sex, age, proportions of ruptured and unruptured aneurysms, aneurysm diameter, and aneurysm volume (Table 1).
TABLE 1:
Group characteristics
| PGLA Coil Group | Bare Coil Group | P | |
|---|---|---|---|
| Total no. of cases | 56 An (51 Pt) | 87 An (78 Pt) | NA |
| Sex (M:F) | 16:35 | 32:46 | .3562 |
| Age (mean ± SD) | 53.8 ± 12.2 | 56.4 ± 11.9 | .2319 |
| Aneurysm | |||
| Ruptured | 19 | 41 | .1653 |
| Unruptured | 37 | 46 | |
| ≥10 mm diam. | 11 | 12 | .4863 |
| <10 mm diam. | 45 | 75 | |
| ≥100 mm3 vol (≥600 mm3) | 23 (3) | 26 (4) | .5326 |
| <100 mm3 vol | 33 | 50 |
Note.—An indicates aneurysms; Pt, patients.
Calculation of Aneurysm Volume, Coil Volume, and Packing Attenuation
Aneurysm volume was calculated on the assumption that aneurysms are ellipsoid and the following formula was applied, that is, aneurysm volume = 4 π (height/2) (length/2) (width/2)/3 (9, 12–14). Each diameter in 3 perpendicular axes was measured from a 3D angiographic image. Angiography was performed by using a commercially available biplanar angiographic unit (Integris Allura; Philips Medical Systems, Best, the Netherlands) in all the cases. Reconstruction of the 3D images by volume rendering was performed with a software package (Integris 3D-RA release 3.2; Philips Medical Systems). The detailed angiographic technique was previously described (15). Coil volume was calculated assuming that a coil is a cylinder with a diameter of the primary coil and was derived from the formula coil volume = π (diameter/2)2 (length). Packing attenuation, also called volume embolization ratio, was calculated as the ratio of the volume of the deployed coils to the aneurysm volume.
For PGLA coils, we employed the outer diameter of the coil, including that of the polymer coat, presented by the company for the attenuation calculation.
In obtaining scatterplots, we adopted a logarithmic scale in aneurysm volumes for better depiction of the data and to enhance interpretability, as previously demonstrated by Sluzewski et al (16).
Endovascular Procedures
Endovascular coil embolization was performed following a standardized protocol in the neuroangiography suite (17, 18). In most cases, general anesthesia was induced. Systemic anticoagulation with heparin was used from the beginning of the procedures in unruptured cases, but only after protective packing with coils in cases with recent hemorrhage. Heparin was administered intravenously just after the introducer sheath was inserted, first as a 3000-U bolus then followed by 1000 U/h. In general, heparin was administered to achieve an activated clotting time approximately twice that of normal. After the procedure, heparin was just discontinued. In the study group, PGLA-coated coils were preferentially deployed until attenuated packing was achieved without compromising the parent arterial lumen. PGLA-coated coils were soaked with saline before deployment within the coil sheath. The operator usually did not change the coil option to bare platinum coils until he felt undue friction and the coils defied deployment into the aneurysm. Bare platinum coils were chosen among GDC, MicroPlex (MicroVention), Trufill-DCS (Cordis, Miami Lakes, FL), and Detach (Cook, Bloomington, IN) at the operator’s discretion.
The aneurysm was considered completely occluded angiographically when the sac and the neck were densely packed; near-completely occluded when the sac was packed, but a small neck remained; and incompletely occluded in case of a persistently opacified sac (Fig 1).
Fig 1.
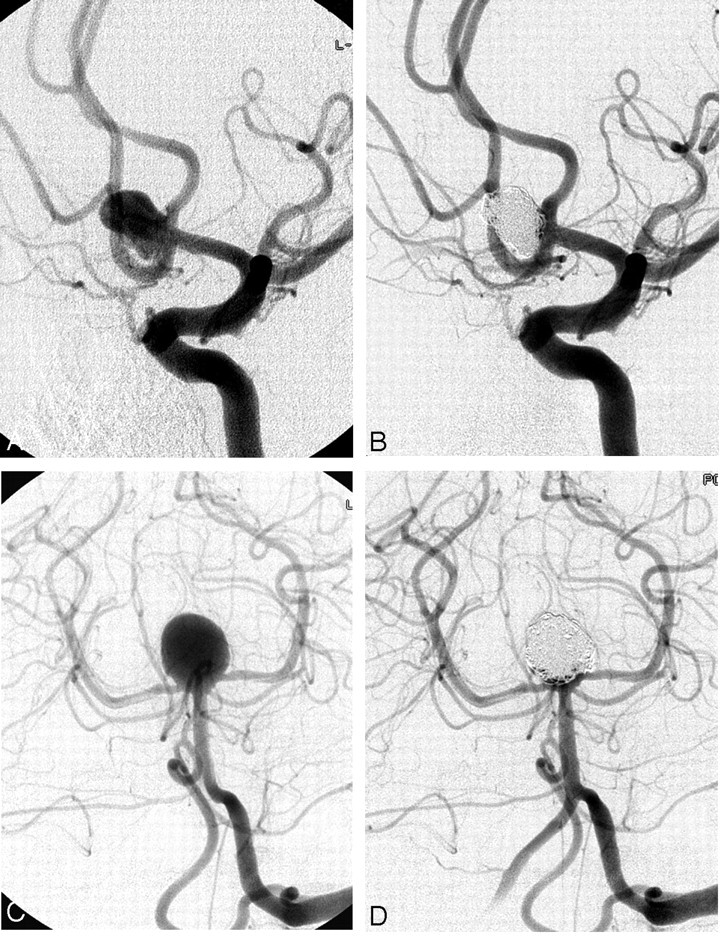
Complete occlusion. Pre-embolization angiogram (A) and postembolization angiogram (B). Complete occlusion was achieved in the anterior communicating aneurysm of a 62-year-old woman. This unruptured aneurysm measured 8.2 mm × 6.0 mm × 6.8 mm. A total of 19 coils (136 cm) were deployed, of which 13 (116 cm) were PGLA-coated. Packing attenuation was calculated to be 68%.
Near-complete occlusion. Pre-embolization angiogram (C) and postembolization angiogram (D). Near-complete occlusion was achieved in the basilar top aneurysm of a 38-year-old man, presenting with subarachnoid hemorrhage. This aneurysm measured 12.8 mm × 10.2 mm × 10.9 mm. A total of 11 coils (185 cm) were deployed, of which 6 (105 cm) were PGLA-coated. Tiny contrast-filled spaces are seen near neck of the aneurysm. Packing attenuation was calculated to be 19%.
Follow-Up Protocol
When an aneurysm was completely embolized, plain radiography, MR angiography with 3D reconstruction (3D MRA), and conventional cerebral angiography were employed 3, 6, and 12 months postembolization, respectively. During plain radiography the same working projections at time of coil embolization were checked to keep comparability. The protocol for 3D MRA was previously described (19). When coil compaction or aneurysmal recanalization was suspected in noninvasive studies, conventional angiography was performed as immediately as possible to check the current state of the aneurysm exactly and to decide the necessity of further treatment.
In cases with incomplete aneurysm occlusion on initial embolization, we recommended conventional angiography follow-up at 3 months postembolization. The subsequent follow-up protocol depended on the stability of the coil mass and configuration of the embolized aneurysm. When a minor aneurysmal recanalization—that is, minimal coil compaction at the aneurysmal neck—occurs during the follow-up, another 6-month MRA and 1-year angiography were recommended (Fig 2). Meanwhile, urgent repeat embolization was recommended when a major recanalization—that is, contrast filling within the aneurysm dome—or significant coil loosening or compaction occurred (Fig 3).
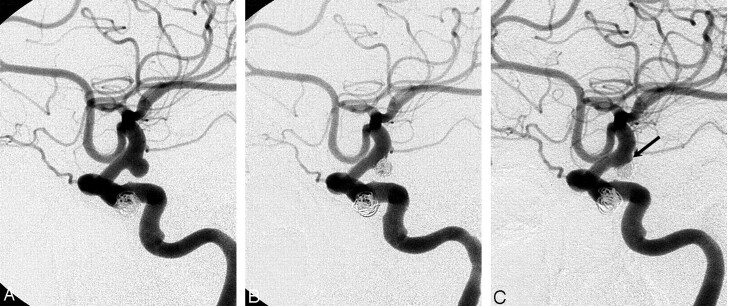
Fig 2.
Minor recanalization. Pre-embolization angiogram (A), postembolization angiogram (B), and control angiogram at 12 months postembolization (C). Near-complete occlusion was achieved in the unruptured internal carotid artery aneurysm of a 61-year-old woman (B). Follow-up angiogram (C) shows minimal recanalization of the aneurysm neck with compaction of the coil mesh (arrow).
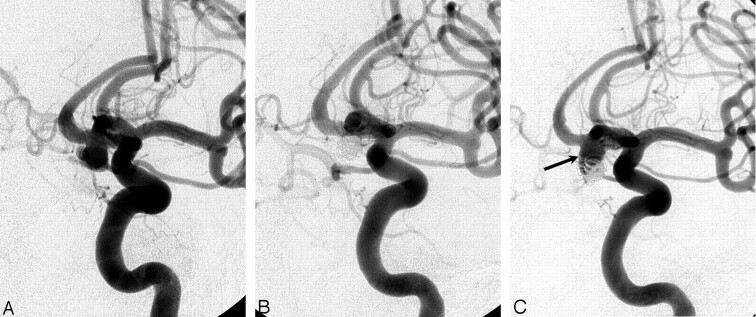
Fig 3.
Major recanalization. Pre-embolization angiogram (A), postembolization angiogram (B), and control angiogram at 7 months postembolization (C). Complete occlusion was achieved in the ruptured anterior communicating artery aneurysm of a 35-year-old man (B). Follow-up angiogram (C) shows significant recanalization of the aneurysm; coil mesh is compacted into the dome of aneurysm and loosened at its upper aspect (arrow).
Statistic Analyses
Mann-Whitney test, unpaired Student t test, linear regression analysis, χ2 test for trend, and Fisher exact test were performed by using GraphPad InStat (version 3.05 for Windows 95/NT; GraphPad Software, San Diego, CA). Open Epidemiological Calculators (http://www.openepi.com/Jan2004/menu/OpenEpiMenu.htm) were used for acquisition of confidence intervals for single-person time rates and for comparison of 2-person time rates. For this person-time incidence-rate analyses, the denominator was the sum of the follow-up period in months and the numerator was the number of events of aneurysmal recanalization in each group. A P value <0.05 was considered significant.
Results
Procedural Events
In the PGLA-coil group, complete or near-complete occlusion of the aneurysms could be achieved in almost all cases (54/56). Procedure-related thromboembolism occurred in 6 cases (11% of all aneurysms); in 4 cases intra-arterial infusion of abciximab was required. Coil loop herniation into the parent arterial lumen happened in a single case. In only one case postprocedural increased hematoma was noted on CT; otherwise no procedural bleeding was observed angiographically.
In the control group, complete or near-complete occlusion of the aneurysms could be achieved in 84 of 87 cases. The other 3 aneurysms partially occluded consisted of a infraclinoid aneurysm, in which reselection of the aneurysm and further coil deployment was not possible, because of the tortuous curvature of the parent artery; a broad-neck aneurysm at the middle cerebral artery bifurcation incorporating the inferior division; and a giant basilar top aneurysm incorporating origins of both posterior cerebral arteries. Procedure-related thromboembolism occurred in 9 cases (10%); in 3 cases intra-arterial infusion of abciximab or urokinase was required. Coil loop herniation into the parent arterial lumen happened in 2 cases. Procedural bleeding was encountered in 3 cases.
Coil Volume and Packing Attenuation
Data regarding the aneurysm volume were available in all 56 aneurysms of the PGLA-coil group and in 77 aneurysms of the bare-coil group. In the PGLA-coil group the aneurysm volumes ranged from 5.20 mm3 to 2540 mm3 (mean ± SD, 222 ± 459 mm3). The volumes of coils deployed ranged from 2.77 mm3 to 471 mm3 (46.5 ± 81.4 mm3). The packing densities ranged from 11.3% to 73.3% (30.9 ± 13.3%).
In the bare-coil group the aneurysm volumes ranged from 5.20 mm3 to 2630 mm3 (181 ± 403 mm3). The volumes of coils deployed ranged from 2.30 mm3 to 421 mm3 (28.4 ± 53.3 mm3). The packing densities ranged from 9.10% to 50.9% (21.4 ± 7.59%).
Mean coil volume deployed and packing attenuation of the PGLA-coil group were significantly higher than those of the bare-coil group (Table 2). Absolute difference in mean packing attenuation was 9.5%.
TABLE 2:
Comparison of volumes of aneurysms and coils deployed and packing densities between the two groups
| PGLA coil(n = 56) | Bare Platinum Coil(n = 77) | P | |
|---|---|---|---|
| Aneurysm volume | 222 ± 459 | 181 ± 403 | .4794* |
| Coil volume | 46.5 ± 81.4 | 28.4 ± 53.3 | .0026* |
| Packing density | 30.9 ± 13.3 | 21.4 ± 7.6 | <0.0001† |
Mann–Whitney test.
Unpaired Student t-test.
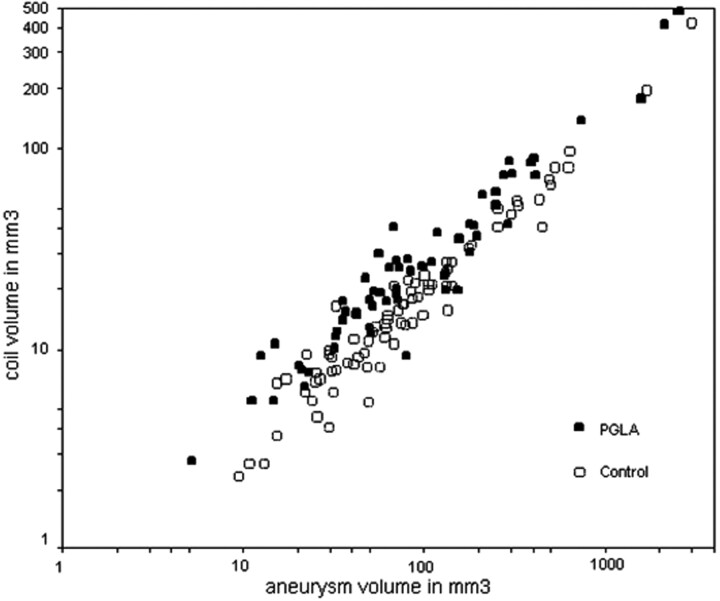
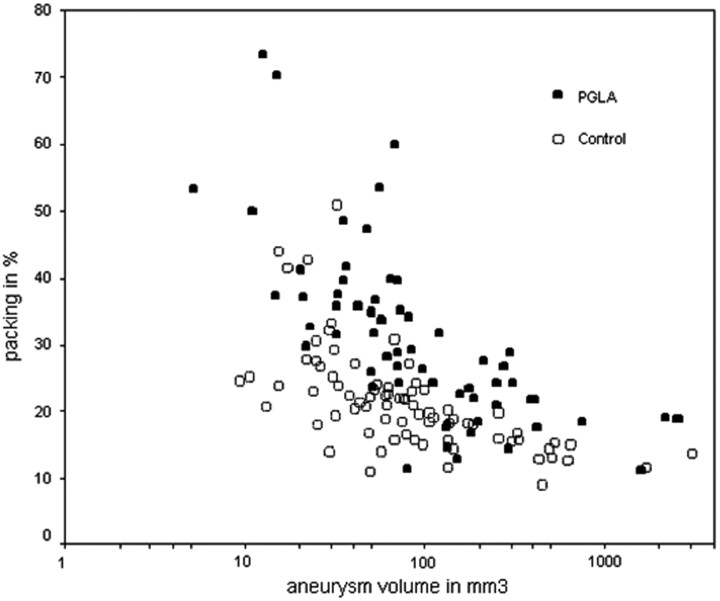
A linear relationship was found between the aneurysm volumes and the coil volumes in either group (Fig 4; Table 3). Overall larger volumes of coils were deployed in the PGLA-coil group than in the bare-coil group over 3 logs of the aneurysm volumes. The PGLA-coil group also showed superiority over the bare-coil group in terms of the packing attenuation (Fig 5).
Fig 4.
Relationship between coil volumes and aneurysm volumes. Graph shows a linear relationship between the aneurysm volumes and the coil volumes in either group. Coil volume and aneurysm volume are expressed in logarithmic scales. Overall spots of the PGLA-coil group are located higher than those of control group.
TABLE 3:
Linear regression analyses for logs of aneurysm volumes and logs of coil volumes
| Parameter | PGLA Coil | Bare Platinum Coil |
|---|---|---|
| Pearson r | 0.9563 (95% CI: 0.9280, 0.9737) | 0.9684 (95% CI: 0.9506, 0.9798) |
| r2 | 0.9146 | 0.9377 |
| Slope | 0.7223 (95% CI: 0.7064, 0.7383) | 0.6451 (95% CI: 0.6296, 0.6605) |
Fig 5.
Relationship between packing densities and aneurysm volumes. Graph shows an inverse relationship between the aneurysm volumes and the packing densities in either group. Aneurysm volume is expressed in a logarithmic scale. Overall spots of the PGLA-coil group are located higher than those of control group.
Within the PGLA-coil group, the coated coils accounted for half or more of total length of coils detached in 30 aneurysms (1–16 coils; 3 cm–395 cm); they accounted for less than half of total length of coils detached in 26 aneurysms (1–5 coils; 3 cm–140 cm). When we divided the PGLA coil group into 4 subgroups according to percent length of PGLA coils detached—that is, (total length of PGLA coils detached into an aneurysm)/(total length of coils detached into an aneurysm) ×100—significant differences in packing densities were found among subgroups (Table 4). When percentage length of PGLA coils was <25%, mean packing attenuation in this subgroup was 19.0 ± 4.5%, similar to that of bare-coil group (21.4 ± 7.6%) and significantly lower that those in the other subgroups. A plateau of mean packing densities around 30% was noted in the subgroups, where percentage lengths of PGLA coils were 25% or more, without significant differences among them.
TABLE 4:
Differences of packing densities in subgroups according to percentage length of PGLA coils
| % Length | n | Mean Packing Density | P* |
|---|---|---|---|
| <25 | 5 | 19.0 ± 4.5 | NA |
| 21 | 30.1 ± 11.7 | .0118 | |
| 17 | 34.5 ± 15.1 | .0293 | |
| 13 | 32.9 − 13.9 | .0093 |
Mann–Whitney U-test, compared with the first group (ie, % length <25).
Clinical Outcome and Radiologic Follow-Up Results
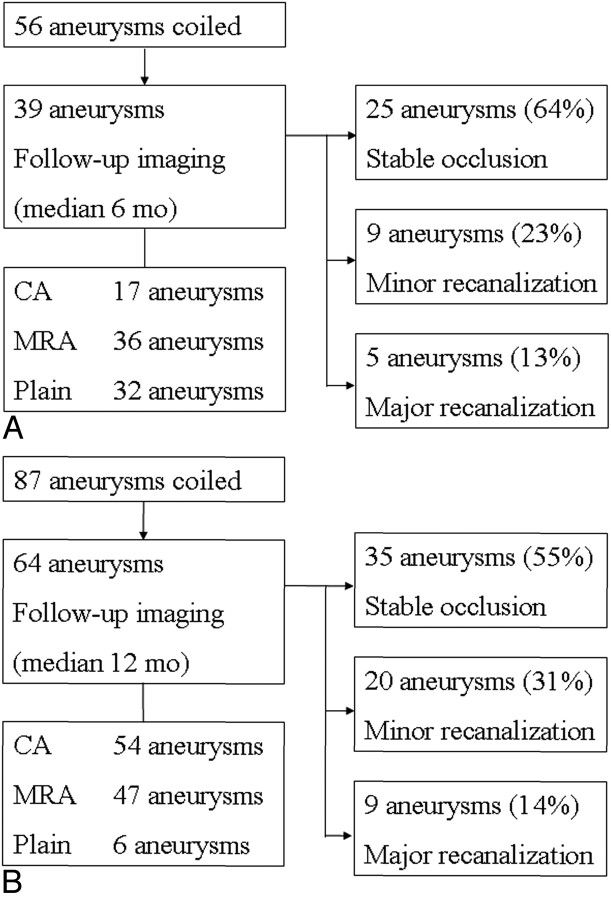
Excellent management outcome was attained in 44 cases (86%) in the PGLA-coil group; there were 2 fatalities (3.9%; one due to thromboembolism and rebleeding and the other due to pneumothorax). Radiologic follow-up evaluation after at least 1-month postembolization was performed in 39 aneurysms (70%). For follow-up evaluation conventional angiography was performed in 17 aneurysms, 3D MRA in 36, and conventional radiography in 32. Mean follow-up interval was 6.2 ± 2.2 months postembolization (median, 6 months; range, 1–12 months). Among these, recanalization was observed in 14 aneurysms (major recanalization in 5 [13%] and minor recanalization in 9 [23%]). Repeat embolization was performed for all the cases of aneurysms showing major recanalization 1 month to 10 months after postembolization (Fig 6A).
Fig 6.
Paths of follow-up in PGLA-coil group (A) and bare-coil group (B). There are no significant differences in the recanalization rates between two groups. CA, conventional angiography.
In the bare-coil group, 70 patients (90%) showed excellent clinical outcome; there were 2 fatalities (2.6%; one due to initial brain damage from bleeding and the other due to cardiac arrhythmia). Follow-up radiologic evaluation after at least 1 month postembolization was available in 64 aneurysms (74%). For follow-up evaluation, conventional angiography was performed in 54 aneurysms, 3D MRA in 47, and plain radiography in 6. Mean follow-up interval was 11.9 ± 4.7 months postembolization (median, 12 months; range, 1–24 months). Recanalization was found in 29 aneurysms (major recanalization in 9 [14%] and minor recanalization in 20 [31%]). Repeat embolization was performed in 6 cases of aneurysms showing major recanalization 8 months to 21 months after postembolization (Fig 6B).
Difference of major recanalization rates between the 2 groups was not significant (P = 1.000, Fisher’s exact test). The total observation period was 247 and 746 aneurysm-months in the PGLA-coil group and in the bare-coil group, respectively. Thus, by using the concept of person-time incidence rate, incidence of major recanalization was calculated to be 2.0/100 aneurysm-months (95% CI: 0.2501, 3.799) in the PGLA-coil group and 1.2/100 aneurysm-months (95% CI: 0.4183, 1.995) in the bare-coil group, and the difference was not significant (P = .3481).
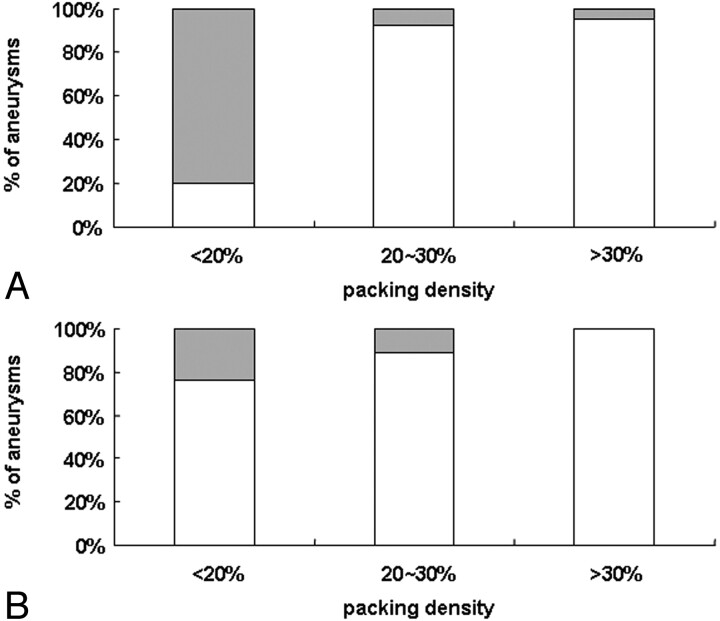
Recanalization rates according to packing densities are shown in Fig 7. In either group, there was a significant or borderline significant tendency to less recanalization rate with greater packing attenuation.
Fig 7.
Recanalization rate according to packing attenuation group in PGLA-coil group (A, P = .0016) and bare-coil group (B, P = .0837). Gray bars represent percentages of aneurysms showing recanalization and white bars those showing stable occlusion at follow-up imaging. With increasing packing densities less percentage of aneurysms become recanalized in either group (χ2 test for trend).
Among 5 cases showing major recanalization in the PGLA-coated coil group, packing densities were <20% in 3 [Table 5]. On the other hand, percentage lengths of PGLA-coated coils deployed were >50% in 3 cases.
TABLE 5:
Cases showing major recanalization in the PGLA-coated coil group
| Patient No./Age (y)/Sex | H-H | Loc. | Size (mm) | PD (%) | % PGLA | Interval (mo) |
|---|---|---|---|---|---|---|
| 13/66/F | 0 | ICA | 10.4 × 7.8 × 7.0 | 29.0 | 70 | 5 |
| 15/35/M | I | ACoA | 7.6 × 6.1 × 5.4 | 17.7 | 37 | 6 |
| 24/62/F | 0 | ACoA | 9.3 × 6.2 × 6.5 | 18.6 | 28 | 6 |
| 36/63/M | 0 | BA | 15.0 × 8.6 × 6.2 | 17.7 | 59 | 4 |
| 42/47/F | 0 | ICA | 5.2 × 5.0 × 5.3 | 35.2 | 100 | 1 |
Note.—H-H indicates Hunt and Hess grade; Loc., location of aneurysm; Size, size of aneurysm; PD, packing density; % PGLA, % length of PGLA coils among total coils deployed; Interval, interval from coil embolization to diagnosis of aneurysm recanalization.
Recanalization rates according to percentage length of PGLA-coated coils deployed are shown in Table 6. The group in which 50% or more of PGLA-coated coils were deployed in length showed a similar result in terms of major recanalization but had an inferior result in terms of total percentage of recanalizations (P = .0173). When 75% is applied as the dividing criterion, the differences were not statistically significant.
TABLE 6:
Recanalization rate according to percentage length of PGLA coils deployed
| Criterion | n* | Major Recanal,No. (%) | P† | Total Recanal,No. (%) | P† | ||
|---|---|---|---|---|---|---|---|
| Below | 17/26 | 2 (12) | 2 (12) | ||||
| 50% | 1.000 | 0.0173 | |||||
| Above | 22/30 | 3 (14) | 11 (50) | ||||
| Below | 28/43 | 4 (14) | 8 (29) | ||||
| 75% | 1.000 | 0.2293 | |||||
| Above | 9/13 | 1 (11) | 5 (56) | ||||
Number of cases with follow-up imaging studies/number of total cases.
Fisher exact test.
Discussion
Our study showed feasibility of PGLA-coated coils in humans as an embolic material for intracranial aneurysms with comparable procedural success rate and complication rate, compared with bare platinum coils. Furthermore, higher volumes of coils could be deployed into the aneurysms and, as a result, higher packing densities could be achieved with them.
We also documented a linear relationship between aneurysm volumes and coil volumes in either group with the r value of 0.9563 and 0.9684, respectively (Table 2 and Fig 3). This means that log (coil volume)/log (aneurysm volume) equals a constant when we attain total or near-total occlusion of intracranial aneurysms with coil embolization. From this formula, we can predict amount of coils necessary to achieve complete or near-complete aneurysmal occlusion with coil embolization. Meanwhile, the slopes were significantly different (stiffer in the PGLA-coil group), considering 95% confidence intervals, between the 2 groups.
Many studies have shown a significant correlation between packing densities and stability of embolized aneurysms (12–14, 16, 20); however, the true significance of packing attenuation remains to be proved, especially when PGLA-coated coils are employed for treatment of aneurysms. In our series, the PGLA-coated coil group did not show the superior stability of embolized aneurysms even though higher packing densities could be achieved. Some factors can be considered to explain these results. First PGLA, accounting for 70% of the coil volume, is a bioabsorbable polymer that is actually absorbed as time goes by. In the previous experimental study (7) the polymer was visible at 14 days after embolization and was essentially gone at 3 months. We suppose that the coils are, at least, not different from or may be inferior to the bare platinum coils in terms of recanalization, because the polymer is absorbed and may provide potential spaces for coil compaction and, as a result, aneurysm recanalization. Total or near-total occlusion had been achieved in all 5 aneurysms showing major recanalization in the PGLA-coil group.
Murayama et al (7) reported in an experimental study that the aneurysms treated with PGLA-coated coils showed more extensive area of organized thrombus and thicker neck tissue compared with GDC-treated aneurysms. These findings need to be confirmed in humans and our study revealed that the new coil system is far from perfect and at most has a similar efficacy in comparison with bare platinum coils in terms of aneurysmal recanalization. Further study with more cases and longer follow-up data are required to ascertain our results.
In our study, the decisive defect in coil embolization (ie, delayed aneurysmal recanalization) could not be overcome by PGLA-coated coils. Coil embolization for intracranial aneurysm is not perfect for the time being. We believe other solutions should be sought in the field of endovascular aneurysm treatment.
Our study has limitations in that this is, in itself, a retrospective study with historical controls. First of all, there can be a sample selection bias. We tried to overcome this limitation by including all the cases satisfying the inclusion criteria in the control group. Regarding the PGLA coil group, there was no specific selection criteria for patients or for aneurysms treated with PGLA coils. The baseline characteristics showed no significant differences between 2 groups, as shown in Table 1.
The second limitation is lack of a uniform follow-up on all patients. Follow-up data of the shorter period was available in the PGLA-coil group and thereby the smaller number of cases in the group underwent conventional angiography in light of our follow-up protocol. Even during the shorter follow-up period, however, similar rates of recanalization were found in the PGLA coil group.
Conclusion
We present that short-term outcome of PGLA-coated coil embolization for intracranial aneurysms is not superior to that with bare platinum coils in terms of aneurysmal recanalization, despite deployment of higher coil volumes and attainment of higher packing densities in the new coil system group. Further study is required to determine whether there is benefit or harm in using PGLA-coated coils for intracranial aneurysms.
References
- 1.Vinuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475–482 [DOI] [PubMed] [Google Scholar]
- 2.Byrne JV, Sohn MJ, Molyneux AJ, Chir B. Five-year experience in using coil embolization for ruptured intracranial aneurysms: outcomes and incidence of late rebleeding. J Neurosurg 1999;90:656–663 [DOI] [PubMed] [Google Scholar]
- 3.International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a radomised trial. Lancet 2002;360:1267–1274 [DOI] [PubMed] [Google Scholar]
- 4.Murayama Y, Nien YL, Duckwiler G, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years’ experience. J Neurosurg 2003;98:959–966 [DOI] [PubMed] [Google Scholar]
- 5.Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003;34:1398–1403 [DOI] [PubMed] [Google Scholar]
- 6.Abrahams JM, Diamond SL, Hurst RW, et al. Topic review: surface modifications enhancing biological activity of Guglielmi detachable coils in treating intracranial aneurysms. Surg Neurol 2000;54:34–41 [DOI] [PubMed] [Google Scholar]
- 7.Murayama Y, Tateshima S, Gonzalez NR, Vinuela F. Matrix and bioabsorbable polymeric coils accelerate healing of intracranial aneurysms: long-term experimental study. Stroke 2003;34:2031–2037 [DOI] [PubMed] [Google Scholar]
- 8.Raymond J, Roy D, Leblanc P, et al. Endovascular treatment of intracranial aneurysms with radioactive coils: initial clinical experience. Stroke 2003;34:2801–2806 [DOI] [PubMed] [Google Scholar]
- 9.Cloft HJ, Kallmes DF. Aneurysm packing with HydroCoil embolic system versus platinum coils: initial clinical experience. AJNR Am J Neuroradiol 2004;25:60–62 [PMC free article] [PubMed] [Google Scholar]
- 10.Murayama Y, Vinuela F, Tateshima S, et al. Bioabsorbable polymeric material coils for embolization of intracranial aneurysms: a preliminary experimental study. J Neurosurg 2001;94:454–463 [DOI] [PubMed] [Google Scholar]
- 11.Murayama Y, Vinuela F, Tateshima S, et al. Cellular response of bioabsorbable polymeric material and Guglielmi detachable coil in experimental aneurysms. Stroke 2002;33:1120–1128 [DOI] [PubMed] [Google Scholar]
- 12.Satoh H, Matsubara S, Hondoh H, Nagahiro S. Intracranial aneurysm embolization using interlocking detachable coils: correlation between volume embolization rate and coil compaction. Intervent Neuroradiol 1997;3 (suppl 2):125–128 [DOI] [PubMed] [Google Scholar]
- 13.Uchiyama N, Kida S, Nomura M, et al. Significance of volume embolization ratio as a predictor of recanalization on endovascular treatment of cerebral aneurysms with Guglielmi detachable coils. Intervent Neuroradiol 2000;6 (suppl 1):59–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawanabe Y, Sadato A, Taki W, Hashimoto N. Endovascular occlusion of intracranial aneurysms with Guglielmi detachable coils: correlation between coil packing density and coil compaction. Acta Neurochir (Wien) 2001;143:451–455 [DOI] [PubMed] [Google Scholar]
- 15.Kang HS, Han MH, Kwon BJ, et al. Postoperative 3D angiography in intracranial aneurysms. AJNR Am J Neuroradiol 2004;25:1463–1469 [PMC free article] [PubMed] [Google Scholar]
- 16.Sluzewski M, van Rooij WJ, Slob MJ, et al. Relation between aneurysm volume, packing, and compaction in 145 cerebral aneurysms treated with coils. Radiology 2004;231:653–658 [DOI] [PubMed] [Google Scholar]
- 17.Kwon BJ, Han MH, Oh CW, et al. Anatomical and clinical outcomes after endovascular treatment for unruptured cerebral aneurysms: a single center experience. Intervent Neuroradiol 2002;8:367–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwon BJ, Han MH, Oh CW, et al. Procedure-related haemorrhage in embolisation of intracranial aneurysms with Guglielmi detachable coils. Neuroradiology 2003;45:562–569 [DOI] [PubMed] [Google Scholar]
- 19.Park SW, Han MH, Cha SH, et al. PC-based 3D reconstruction of MR angiography in evaluation of intracranial aneurysms. Intervent Neuroradiol 2002;8:169–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamatani S, Ito Y, Abe H, et al. Evaluation of the stability of aneurysms after embolization using detachable coils: correlation between stability of aneurysms and embolized volume of aneurysms. AJNR Am J Neuroradiol 2002;23:762–767 [PMC free article] [PubMed] [Google Scholar]