Abstract
Summary: In the differential diagnosis of midface masses, the nevus of Ota (also called oculodermal melanocytosis) is a rare entity. We present a case of a young white man, who lost his left eye function by progression of a melanocytotic lesion involving the ophthalmic (VI) and maxillary (VII) divisions of the trigeminal nerve. The time course, distribution along the trigeminal nerve, and characteristic MR signal intensities of the lesion, in correlation with the clinical, ophthalmological, and dermatological findings, point to the correct diagnosis.
The nevus of Ota (also called oculodermal melanocytosis) is a dermal melanocytosis, distinguished by an increased number of dermal melanocytes located in the distribution of the ophthalmic (VI) and maxillary (VII) divisions of the trigeminal nerve. Most often, involving only the skin, it manifests with distinct signs such as a slight unilateral hyperpigmentation of the eyelid and the periocular region. In more rare cases, the entire distribution of VI and VII can be involved because the migration of melanoblastic cells from the primitive neural tube to the skin is affected (1–3). Several case reports and review articles describe the clinical aspects of this pathologic entity. However, the imaging findings and neuroradiological work-up, to our knowledge, have not been discussed in the literature so far. In this report, we describe a case of nevus of Ota, with extensive involvement distributed along the VI and VII trigeminal branches, emphasizing the neuroradiological manifestations of the disease and its differential diagnosis.
Case Reports
In November 1995, a 15-year-old white male patient presented for the first time in the department of ophthalmology of our hospital with a more than 8-year progressive exophthalmus of the left eye and a mass in the left oral cavity with a similar time course. Since birth, a blue pigmentation of the periocular skin and nasal part of the inferior sclera was known. Despite the left exophthalmus, the patient was feeling well. Ophthalmologic examination revealed anisocoria with sphincter paresis, a very slight direct light reaction on the left eye, and a slight consensual light reaction on the right side. The right/left intraocular pressure was 9/18 mm Hg, and the visual acuity was 1.25/0.5. The blue left papilla was prominent but vital. There was only a slight motility deficit on the left side. The clinical diagnosis was nevus of Ota with suspected malignant transformation.
CT and MR imaging at this time showed a contrast-enhancing left orbital mass predominantly in the inferolateral orbital cavity, extending to the superior orbital fissure and left cavernous sinus. The round foramen and pterygopalatine fossa were also infiltrated. A distinct round mass was seen in the masticator space (Fig 1). The lacrimal gland, the skin overlying the maxilla, and the temporal muscle also showed an increased contrast enhancement, indicating tumor infiltration. The bony orbit was expanded, the infraorbital canal and the round foramen were enlarged, and the lateral maxillary wall and zygomatic bone were hyperostotic, with partial obliteration of the maxillary sinus. The tumor showed hyperintense signal intensity on T1-weighted and mixed hyper- and hypointense signal intensities on T2-weighted MR images. Partial resection of the mass in the masticator space and biopsy taken from the left infraorbital nevus and the pigmented oral mucosa were performed. Both the resected masticator space mass and the biopsies showed the same histologic patterns consistent with the diagnosis of a pigmented peripheral nerve sheath tumor without mitotic activity. No aspects of malignancy were present.
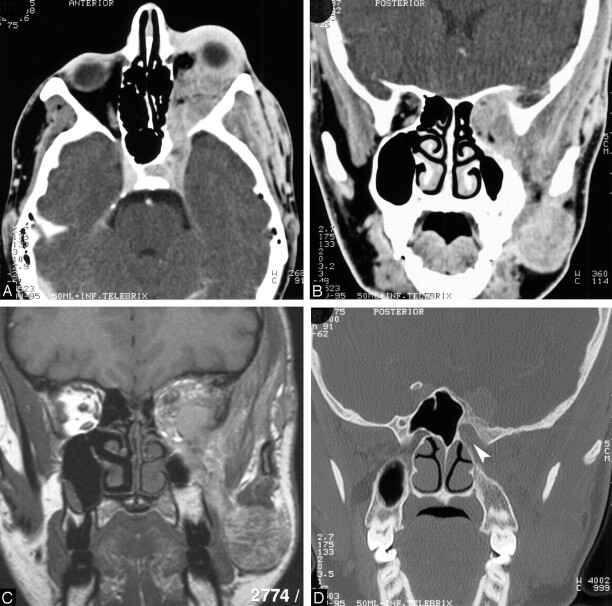
Fig 1.
15-year-old boy who presented with a more than 8-year progressive exophthalmus of the left eye and a mass in the left oral cavity with a similar time course.
A, Contrast-enhanced axial 4-mm section through the skull base and orbits shows an abnormal strongly enhancing mass infiltrating the left cavernous sinus, superior orbital fissure, and orbit, leading to an exophthalmus and expansion of the superior orbital fissure.
B, Contrast-enhanced coronal 4-mm section reveals the infiltration and expansion of the left superior orbital fissure and the orbit; the masticator space, masseter, and temporal muscles are also infiltrated. The left maxillary sinus and the ethmoidal cells show a hypoplastic pattern.
C, Unenhanced coronal T1-weighted MR image corresponding to B shows short T1 signal intensity characteristics of the lesion.
D, Coronal 1.5 mm high-resolution CT section obtained through the skull base shows infiltration of the opening of the enlarged left round foramen to the pterygopalatine fossa (arrowhead), compared with the contralateral normal round foramen. The major sphenoid wing shows a hyperostotic pattern.
After the first resection, the patient was followed clinically at an ambulatory ophthalmologic institution without any additional therapy. New intermittent visual disturbances appeared on his right eye in 2002, when the patient was again referred to the department of ophthalmology of our hospital. The clinical examination showed a strong progression of the exophthalmus and a nearly complete loss of the left eye function: complete ptosis, fixed position without any motility, minimal light reaction, and slightly increased intraocular pressure (tonometry, 23 mm Hg). The peripapillary pigmentation was unchanged from 1995. On the right eye, the visual acuity (1.25), tonometry (15 mm Hg), and motility were normal. A new MR image revealed a further progression of the known tumor with enlargement of the intraorbital component, leading to a stronger compression and stretching of the optic nerve and progression of the exophthalmus (Fig 2). Further extension to the Meckel’s cave, the infratemporal fossa, the optic canal, the buccinator space, and the middle face was present. The choroidea, the skin of the upper and lower eyelids, and parts of the forehead and cheek were also involved. The short T1 and T2 signal intensity characteristics of this infiltrating process indicated its melanocytic origin. There was no local recurrence of the mass resected from the masticator space during the first operation.
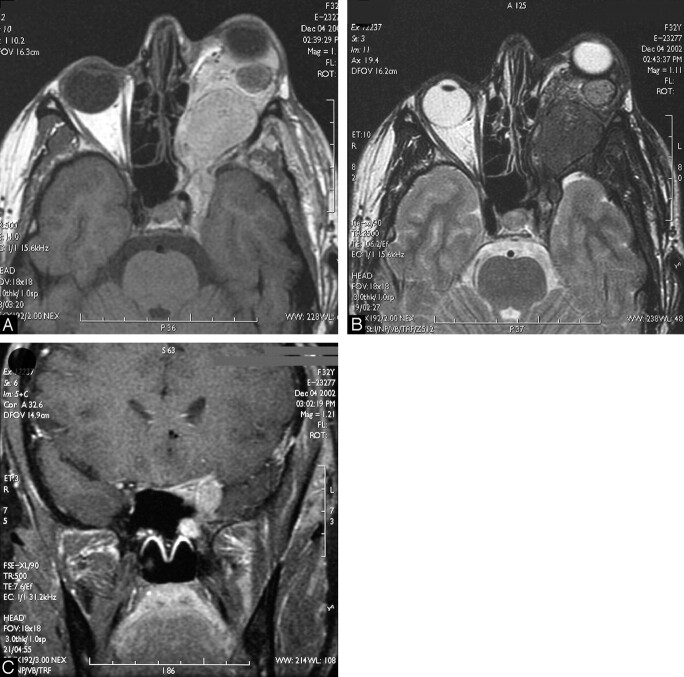
Fig 2.
Eight-year follow-up MR imaging. The now 23-year-old patient returned with a strong progression of the exophthalmus and nearly complete functional loss of the left eye.
A, Unenhanced T1-weighted MR image of the skull base and orbit reveals a further progression of the tumor with enlargement of the intraorbital component, leading to a stronger compression and stretching of the optic nerve and progression of the exophthalmus.
B, Corresponding T2-weighted section shows the typical short T2 signal intensity characteristics of this infiltrating process, indicating its melanocytic origin.
C, Contrast-enhanced coronal T1-weighted MR image demonstrates involvement of the ophthalmic (VI) and maxillary (VII) divisions of the left trigeminal nerve. The right nervus canalis pterygoidei is easily distinguished. On the left side, the nervus canalis pterygoidei is compressed by the more lateral VII.
A size-reduction operation of the left orbital mass was performed. Postoperatively, there was no change in function of the right eye, but a reduction of the exophthalmus by approximately 10 mm and a decrease of the intraocular pressure (tonometry, 13 mm Hg) were noted. The histopathologic examination revealed a fibromuscular soft tissue with diffuse infiltration by a nevus of Ota with spotlike cellular parts, atypia, and mitosis, but without necrosis. Immunohistochemically, the strong pigmented dendritic cells were positive for S-100, tyrosinase, melan-A, and HMB45 and were of melanocytic origin (4). Because of spotlike mitosis and atypia, a nevus of Ota with progression to at least an atypical cellular nevus bleu with increased malignant potential was diagnosed. A postoperative MR imaging study revealed a two-thirds reduction of the retro-orbital mass with decompression of the intraorbital structures and the optic nerve. Staging for metastasis was not performed.
Discussion
The nevus of Ota and the mongolian spot are 2 pathologic entities of dermal melanocytosis belonging to a group of congenital diseases characterized by an increased number or an unusual site of dermal melanocytes. More frequent in Asians than in whites, the nevus of Ota results from an abnormal migration of melanoblastic cells of the primitive neural tube along the first and second divisions of the trigeminal nerve during embryogenesis. A specific genetic defect underlying this abnormal cell migration has not been identified until now; nevertheless, a familial case of nevus of Ota has been reported (5).
In opposition to the mongolian spot, which is apparent in 90%–100% of neonates of the Mongolian race and disappears at puberty, the nevus of Ota is sometimes not visible at birth, but with increasing age, the degree of hyperpigmentation and the size of the lesion will increase. In a group of 194 patients, 3 variants have been described according to the anatomical distribution of the disease process: In 59.3% of cases, the eye and the skin are preferentially involved; in 34.5%, only the skin; and in 6.2%, only the eye. Teekhasaenee et al (3) reported, “Dermal hyperpigmentation in the combined distribution of the ophthalmic and maxillary divisions of the trigeminal nerve and hyperpigmentation of the nasal or buccal mucosa were closely associated with ocular involvement.” All anatomical structures supplied by the VI and VII cranial nerves can be involved: Ipsilateral sclera, conjunctiva, cornea, iris, fundus, optic nerve papilla, the retroorbital fat, the external eye muscles, the lacrimal gland, the periost of the orbit and the maxillary sinus, the masticator space, the nasal and oral mucosa, even the leptomeninges, and cerebral cortex can show an increased number of melanocytes (1–3). In whites, the nevus of Ota is very rare (incidence, 0.04% [6]). In Chinese, the incidence is about 1–2/1000 (7).
A higher risk for malignant transformation to a melanoma has been reported (8,9). More commonly, uveal (choroidea, iris, and ciliary body) (9–11) followed by retroorbital (12–14), but also intracranial (1, 15) and cutaneous (8), melanomas have been described in association with nevus of Ota.
Ophthalmologic examination of the eye shows a unilateral dark-blue pigmentation of the eyelids, sclera, conjunctiva, cornea, iris, fundus, and the papilla of the optic nerve. Oral and nasal inspections may reveal hyperpigmentation of the nasal and oral mucosa. Exophthalmus, increased intraorbital pressure, and motility disorder are rare, but these conditions are suggestive of involvement of the retroorbital fat and the external eye muscles.
CT and MR imaging are necessary to demonstrate tumor extension and the involvement of the retroorbital structures, lacrimal gland, periost of the orbit and maxillary sinus, masticator space, leptomeninges, and eventually the cerebral cortex. Both techniques can accurately evaluate the extension of the disease process and monitor its time course on follow-up examinations. Besides the clinical, dermatologic, and ophthalmologic examinations, MR imaging is the best tool to identify a malignant transformation by follow-up of suspected retroorbital lesions. A fast-growing mass, with changing signal intensity patterns and infiltration of primary uninvolved structures, should be suggestive of malignant transformation. Positron emission tomography (PET) may be useful in detecting malignant transformation of the nevus of Ota, although, to our knowledge, no data are available. The available data of 18F-FDG PET in metastasis of melanocytosis independent melanomas show that the method is useful in detecting recurrent and metastatic disease (American Joint Committee on Cancer [AJCC], stages III and IV). However, for lymph node staging in clinical localized melanoma (AJCC stages I and II), the value of 18F-FDG PET is not established (16–18).
Differential diagnosis includes other orbital masses like capillary hemangioma, lymphangioma, rhabdomyosarcoma, lymphoma, and metastasis, which can be differentiated by the correlation with the clinical, ophthalmological, and imaging findings. The typical short T1 and T2 MR signal intensities suggest the melanocytic origin of the tumor. The unilateral distribution of the nevus of Ota along the VI and VII territory, with possible involvement of the sclera, retroorbital fat, the external eye muscles, the periost of the orbit and the maxillary sinus, nasal and oral mucosa, masticator space, and the leptomeninges leads to the diagnosis. CT demonstrates better involvement of the bone and the paranasal sinuses. Typically, there is a hypertrophy pattern. Thickening of the bony orbital wall, the frontal, ethmoidal and maxillary sinuses, but also the alveolar process, as observed in our case, has been previously reported in the pathologic literature (2).
The treatment and prognosis of the nevus of Ota depend on the severity and extension of the disease. In most cases, where the disease process is limited to hyperpigmented changes involving the eye region, only cosmetic corrections are performed. When the anterior eye chamber is involved (10.3% of cases [3]), the possibility of developing glaucoma requires clinical follow-up. Only in the rare cases of a retroorbital mass impairing the eye function, as in our patient, should debulking of optic nerve and globe be performed. The only report regarding the management of benign masses associated with oculodermal melanocytosis emphasizes surgical therapy over irradiation (2). The higher risk for developing a malignant melanoma justifies close (annual) ophthalmologic controls in all patients. If malignant transformation is suspected, biopsy is indicated for early treatment initiation.
In cases of uveal melanoma, the prognosis depends on its size, location, histologic type, extraocular extension, and metastatic pattern. Because the uveal tract is a highly vascular layer that lacks lymphatic channels, metastasis occurs primarily via hematogenous spread, most commonly to the liver and lungs. Treatment options in uveal melanoma are photocoagulation, ruthenium-106 irradiation, proton irradiation, local resection, or enucleation (11). Uveal melanomas are the most common malignant entity associated with nevus of Ota. Concerning melanomas associated with melanocytosis other than of uveal origin, only few reports have been published. No recurrence or metastasis was reported 2 and 3 years, respectively, after an operation of a primary orbital melanoma in nevus of Ota (13, 14). In 3 of 10 cases of cutaneous melanoma and oculodermal melanocytosis, there was lung or liver metastasis (8). In a review of 95 cases of meningeal melanocytoma and nevus of Ota, after a median follow-up of 46 months, there was a tumor recurrence rate and tumor-related death rate of 26.3% and 10.5%, respectively. These were not statistically significant for different therapeutic modalities (15).
Conclusion
In the differential diagnosis of midface lesions, the nevus of Ota is a rare entity with typical imaging patterns. CT and MR imaging are the best tools to show the midface involvement and follow-up lesions suspected for malignant transformation.
References
- 1.Balmaceda CM, Fetell MR, O’Brien JL, Housepian EH. Nevus of Ota and leptomeningeal melanocytic lesions. Neurology 1993;43:381–386 [DOI] [PubMed] [Google Scholar]
- 2.Koca MR, Rummelt V, Fahlbusch R, Naumann GO. Orbital, osseous, meningeal and cerebral findings in oculodermal melanocytosis (nevus of Ota). Clinico-histopathologic correlation in 2 patients [in German]. Klin Monatsbl Augenheilkd 1992;200:665–670 [DOI] [PubMed] [Google Scholar]
- 3.Teekhasaenee C, Ritch R, Rutnin U, Leelawongs N. Ocular findings in oculodermal melanocytosis. Arch Ophthalmol 1990;108:1114–1120 [DOI] [PubMed] [Google Scholar]
- 4.Xu X, Chu AY, Pasha TL, Elder DE, Zhang PJ. Immunoprofile of MITF, tyrosinase, melan-A, and MAGE-1 in HMB45-negative melanomas. Am J Surg Pathol 2002;26:82–87 [DOI] [PubMed] [Google Scholar]
- 5.Trese MT, Pettit TH, Foos RY, Hofbauer J. Familial nevus of Ota. Ann Ophthalmol 1981;13:855–857 [PubMed] [Google Scholar]
- 6.Gonder JR, Ezell PC, Shields JA, Augsburger JJ. Ocular melanocytosis: a study to determine the prevalence rate of ocular melanocytosis. Ophthalmology 1982;89:950–952 [PubMed] [Google Scholar]
- 7.Sun CC, Lu YC, Lee EF, Nakagawa H. Naevus fusco-caeruleus zygomaticus. Br J Dermatol 1987;117:545–553 [DOI] [PubMed] [Google Scholar]
- 8.Patel BC, Egan CA, Lucius RW, Gerwels JW, Mamalis N, Anderson RL. Cutaneous malignant melanoma and oculodermal melanocytosis (nevus of Ota): report of a case and review of the literature. J Am Acad Dermatol 1998;38(5 Pt 2):862–865 [DOI] [PubMed] [Google Scholar]
- 9.Terheyden P, Rickert S, Kampgen E, et al. Nevus of Ota and choroid melanoma [in German]. Hautarzt 2001;52:803–806 [DOI] [PubMed] [Google Scholar]
- 10.Singh M, Kaur B, Annuar NM. Malignant melanoma of the choroid in a naevus of Ota. Br J Ophthalmol 1988;72:131–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grin JM, Grant-Kels JM, Grin CM, Berke A, Kels BD. Ocular melanomas and melanocytic lesions of the eye. J Am Acad Dermatol 1998;38:716–730 [DOI] [PubMed] [Google Scholar]
- 12.Dutton JJ, Anderson RL, Schelper RL, Purcell JJ, Tse DT. Orbital malignant melanoma and oculodermal melanocytosis: report of two cases and review of the literature. Ophthalmology 1984;91:497–507 [DOI] [PubMed] [Google Scholar]
- 13.Rice CD, Brown HH. Primary orbital melanoma associated with orbital melanocytosis. Arch Ophthalmol 1990;108:1130–1134 [DOI] [PubMed] [Google Scholar]
- 14.Koranyi K, Slowik F, Hajda M, Banfalvi T. Primary orbital melanoma associated with oculodermal melanocytosis. Orbit 2000;19:21–30 [DOI] [PubMed] [Google Scholar]
- 15.Rahimi-Movaghar V, Karimi M. Meningeal melanocytoma of the brain and oculodermal melanocytosis (nevus of Ota): case report and literature review. Surg Neurol 2003;59:200–210 [DOI] [PubMed] [Google Scholar]
- 16.Wagner JD, Schauwecker D, Davidson D, et al. Prospective study of fluorodeoxyglucose-positron emission tomography imaging of lymph node basins in melanoma patients undergoing sentinel node biopsy. J Clin Oncol 1999;17:1508–1515 [DOI] [PubMed] [Google Scholar]
- 17.Crippa F, Leutner M, Belli F, et al. Which kinds of lymph node metastases can FDG PET detect? A clinical study in melanoma. J Nucl Med 2000;41:1491–1494 [PubMed] [Google Scholar]
- 18.Wagner JD. PET detection of melanoma metastases in lymph nodes. J Nucl Med 2003;44:486. [PubMed] [Google Scholar]