Abstract
Our goal was to establish a contrast injection technique that uses the unique vascular anatomy of the rabbit ear to spare the valuable femoral artery access sites. The central artery of the left ear was cannulated. Contrast injected in a retrograde fashion opacified the right brachiocephalic artery and its branches. The technique can be used in rabbits with the usual bovine-type left common carotid artery origin.
In the past several years, elastase-induced saccular aneurysms in the rabbit have become popular because of their anatomic and histopathologic similarity to human intracranial aneurysms. One drawback of the model is the relative difficulty of a diagnostic follow-up angiogram: femoral catheterization requires a cut-down and frequently results in permanent occlusion of the femoral artery. We decided to establish the feasibility of retrograde ear artery injections and work out details of this new method to promote its use.
Description of the Technique
All animal procedures at the Center for Neurovascular Surgery and Stroke Research were conducted according to protocols approved by the Animal Use and Care Committee of the University of Miami.
Saccular aneurysms were created by transcatheter elastase treatment of the right common carotid artery (CCA) origin. Multiple variations of the technique have been described in the literature (1, 2), but details are beyond the scope of this article.
The animals were weighed, and an injection of 0.01 mg/kg glycopyrrolate (American Regent Laboratories, Shirley, NY) was administered IM into the cranial thigh. A few minutes later, a combination of 5mg/kg xylazine (Xyla-Ject; Phoenix Pharmaceutical, Inc., St. Joseph, MO) and 35 mg/kg ketamine (Ketaset; Fort Dodge Animal Health, Fort Dodge, IA) was injected IM to the opposite side. After 10–15 minutes, the left ear dorsum was carefully shaved. Alcohol was used topically to clean the skin, moisten hair, and also achieve vasodilation. A 0.85 × 25 mm 22-gauge intravenous catheter (PSS Inc., Orlando, FL) was than advanced retrogradely into the central artery of the ear approximately at the distal third, or where the artery diameter allowed safe puncture. The central needle was withdrawn, and the Luer-lock end of the plastic cannula was directly connected to a 5-mL Luer-lock syringe containing undiluted contrast. After quick positioning of the rabbit and the C-arm, a digital subtraction angiography sequence was initiated, followed by prompt and forceful injection of the entire contents of the syringe. If the angiogram quality was sufficient, the cannula was immediately removed and local compression was applied by using a clothes clip over a few gauze layers for 10–15 minutes across the ear. The rabbit was then placed in the recovery cage, where full recovery was rapid. Turnaround time after a short learning period approached 5–10 minutes, so all rabbits in a 12- or 24-animal study could be evaluated within a few hours.
Discussion
Elastase-induced aneurysm models in rabbits are widely used for training and device testing (3). When devices are implanted either into the aneurysm or across its neck, a follow-up angiogram should reveal vascular structures in great detail. Sufficient contrast opacification can be achieved with transcatheter contrast injection via the femoral approach (4, 5).
Unfortunately, the rabbit femoral artery is narrow and very fragile. Every time the femoral artery is accessed via a cut-down, it needs to be ligated at the end of the procedure unless a technically challenging microsurgical closure is performed. The risk of losing the access site is very high. By the time a test device is implanted, one of the femoral arteries is usually rendered inaccessible. This leaves the contralateral artery as the access route for follow-up angiograms. One follow-up is not problematic, but a study requiring multiple follow-up angiograms may run into the risk of occluded vessels. Furthermore, the femoral cut-down and catheterization in our lab could not be performed with IM anesthesia because of the duration of the study. Gas anesthesia resulted in lengthy exchange procedures and long recovery times. It also stressed the respiratory system in animals possibly infected with Pasteurella, resulting in complications and animal losses.
To avoid many of the above problems, intravenous contrast injection is an option used by many researchers (6, 7), but the resulting image quality is inadequate for high-resolution angiograms. Contrast is usually injected via the marginal vein of the ear. The contrast is very much diluted by the time it opacifies the brachiocephalic vessels. The image contrast is poor, and the image quality is frequently further degraded by severe motion artifacts.
Just as right vertebral (VA) and CCA angiograms can be performed by using forceful right brachial artery injection in humans, we realized that injection into the surprisingly strong central artery of the left ear quickly fills the left external and CCAs and then continues contrast flow toward the lowest resistance results in retrograde flow to the parent vessel of the left CCA. Because rabbits usually have some variation of the bovine-type origin of the left CCA, the next opacified vessel is the right brachiocephalic artery (BCA) and its derivatives (Fig 1).
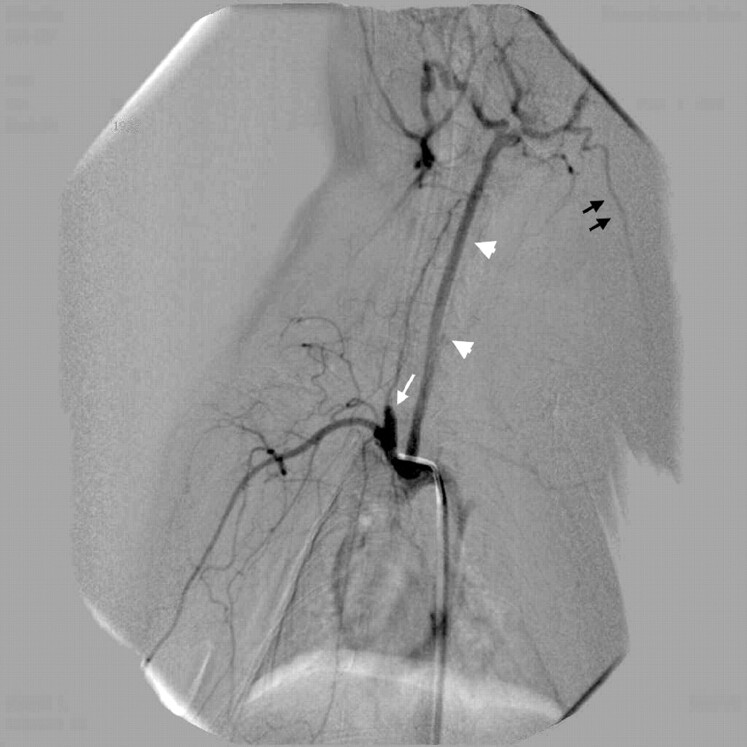
Fig 1.
Vascular anatomy of the central artery of the ear, left antero-oblique view. Transcatheter contrast injection opacifies the BCA with the elastase-treated right CCA origin forming an aneurysm sac (white arrow) and the left CCA (white arrowheads) with its branches. The central artery of the ear (black arrows) originates from the external carotid artery territory as one of its major branches.
Interim follow-up angiograms were of excellent quality when using the left ear injection method, if the anatomy was favorable. Contrast opacification was strong, and the duration of the injection bolus was long enough that good-quality subtraction could be performed at a rate of 6 frames per second without significant motion artifacts, despite the excessive breathing motion of the rabbits. Fig 2A shows retrograde ear injection of a rabbit 3 months after stent placement and coiling of an elastase-induced saccular aneurysm of the BCA. The same rabbit underwent a 6-month follow-up angiogram by using a 4F diagnostic catheter via the left femoral artery. Although the magnifications used in the 2 studies differ, the superior contrast opacification of the ear injection is obvious. The fact that the proximal markers of the stent are positioned at the orifice of the BCA is notable. To avoid mechanical damage to the investigational device, we were reluctant to seek a more invasive catheter tip position.
Fig 2.
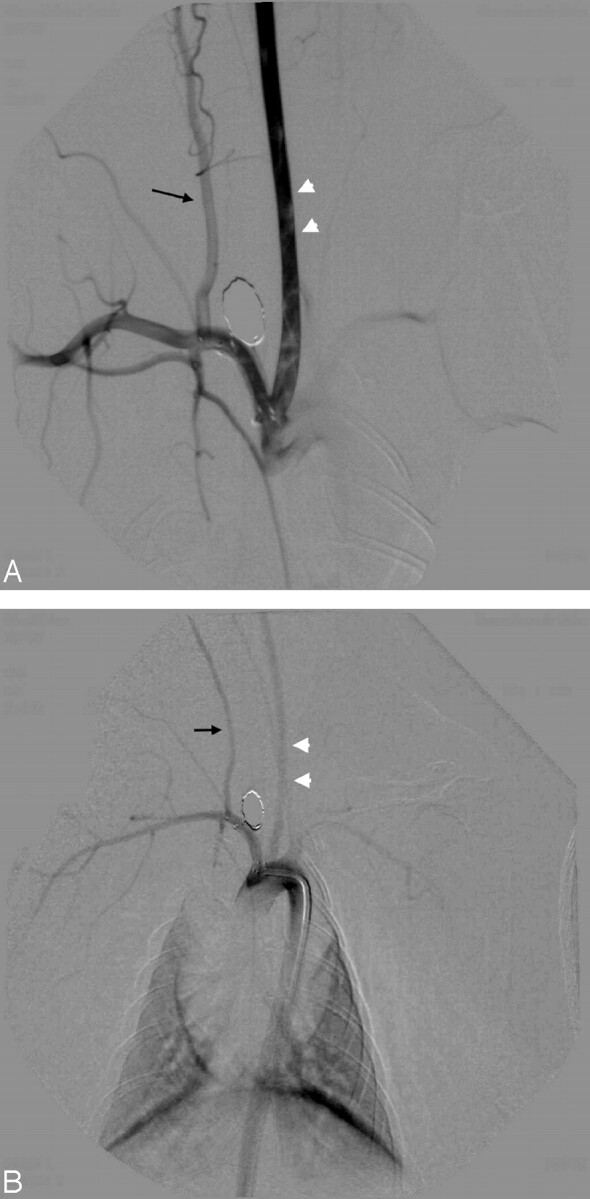
A, Left ear injection 3 months after stent implant and coiling of the elastase-induced aneurysm densely opacifies the left CCA (white arrowheads) and the BCA along with its branches. Notice the excellent opacification of the right VA (black arrow) and its derivative muscular branches. This angiogram was performed by hand injection of 5 mL of undiluted Visipaque via a 21-gauge plastic venous cannule into the left central ear artery.
B, Aortogram in the same animal at 6 months opacifies the same vessels, but to a lesser degree. Both the left CCA (white arrowheads) and the right VA (black arrow) are less opacified when compared with the ear injection technique. Note the nonvisualization of the muscular branches of the right VA. This angiogram was performed by hand injection of 5 mL of undiluted Visipaque via a 4F diagnostic catheter without sideholes.
We tested the new technique in 2 groups of rabbits in 2 separate studies. In the first study, 10/17 animals had a left CCA origin that yielded good opacification of the right BCA. In the second study, 4/6 animals had such an anatomy. When the left CCA originated directly from the aortic arch, or as a common origin with the BCA but with an unfavorable angulation, the contrast ended up opacifying the distal aortic arch only but not the BCA (Fig 3). According to a recent personal communication (D.F. Kallmes, “Vascular anatomic variation in rabbits,” 2005), study of a large group of animals suggests that approximately 70%–80% of New Zealand white rabbits may have favorable anatomy for retrograde ear injection. Preselection of animals can be achieved by performing test injection before creation of an aneurysm and excluding animals with unfavorable anatomy.
Fig 3.
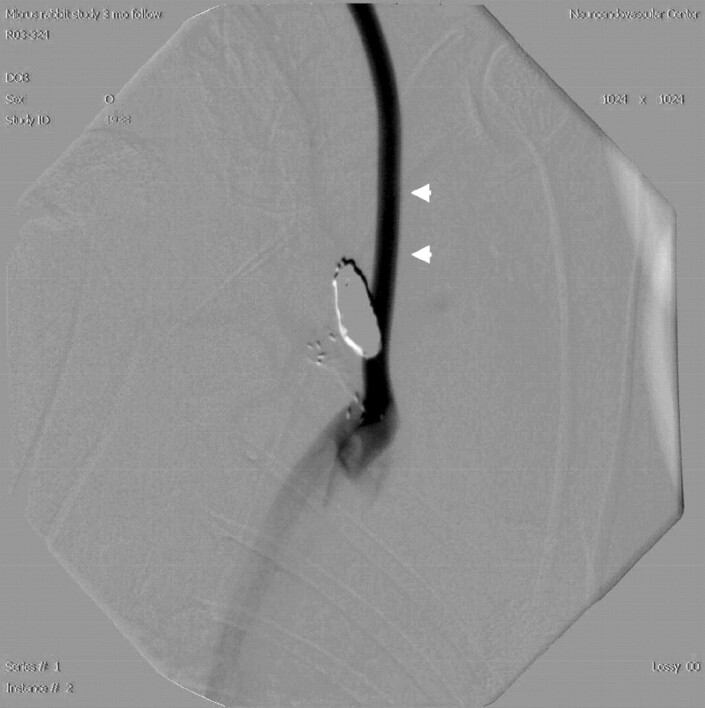
This angiogram represents the unfavorable human-type left CCA origin directly from the aortic arch. Contrast injected via the left central ear artery densely opacifies the left CCA (white arrowheads) but flushes caudally by the flow in the aortic arch. Injection parameters were similar to those described in Fig 2B.
Acknowledgments
We wish to thank Dr. Ajay K. Wakhloo for his support. We also thank Ann Marie Paciorek and Dr. Mark Harrigan for their invaluable contribution. Finally, without the encouragement of Dr. David F. Kallmes this article might have never been published.
Footnotes
The study was partially supported by Cordis Neurovascular, a J&J Company, Miami Lakes, FL, and by Micrus Corporation, Sunnyvale, CA.
References
- 1.Miskolczi L, Guterman LR, Flaherty JD, Hopkins LN. Saccular aneurysm induction by elastase digestion of the arterial wall: a new animal model. Neurosurgery 1998;43:595–600; discussion 600–601 [DOI] [PubMed] [Google Scholar]
- 2.Cloft HJ, Altes TA, Marx WF, et al. Endovascular creation of an in vivo bifurcation aneurysm model in rabbits. Radiology 1999;213:223–228 [DOI] [PubMed] [Google Scholar]
- 3.Kallmes DF, Helm GA, Hudson SB, et al. Histologic evaluation of platinum coil embolization in an aneurysm model in rabbits. Radiology 1999;213:217–222 [DOI] [PubMed] [Google Scholar]
- 4.Thiex R, Hans FJ, Krings T, et al. Haemorrhagic tracheal necrosis as a lethal complication of an aneurysm model in rabbits via endoluminal incubation with elastase. Acta Neurochir (Wien) 2004;146:285–289; discussion 289 [DOI] [PubMed] [Google Scholar]
- 5.Kallmes DF, Fujiwara NH, Yuen D, et al. A collagen-based coil for embolization of saccular aneurysms in a New Zealand White rabbit model. AJNR Am J Neuroradiol 2003;24:591–596 [PMC free article] [PubMed] [Google Scholar]
- 6.Hoh BL, Rabinov JD, Pryor JC, Ogilvy CS. A modified technique for using elastase to create saccular aneurysms in animals that histologically and hemodynamically resemble aneurysms in human. Acta Neurochir (Wien) 2004;146:705–711 [DOI] [PubMed] [Google Scholar]
- 7.Doerfler A, Becker W, Wanke I, et al. Multimodal imaging in the elastase-induced aneurysm model in rabbits: a comparative study using serial DSA, MRA and CTA. Rofo Fortschr Geb Rönsgenstr Neuen Bildgeb Verfahr 2004;176:590–596 [DOI] [PubMed] [Google Scholar]