Abstract
BACKGROUND AND PURPOSE: Two large trials indicated that endarterectomy was less beneficial for symptomatic patients with internal carotid artery (ICA) near occlusion than for patients who had severe stenosis without near occlusion. Near occlusions complicate ratio calculations of ICA stenosis and require attention to detail for identification. The goal is to provide diagnostic criteria, illustrate identifying features, estimate accuracy of identification, and assess prognosis for patients with near occlusion.
METHODS:We re-reviewed 1216 patients with severe (≥70%) stenosis on angiography in the North American Symptomatic Carotid Endarterectomy Trial and European Carotid Surgery Trial. One of 5 (n = 262) had 2 or more criteria for near occlusion: (1) delayed cranial arrival of ICA contrast compared with external carotid artery (ECA); (2) intracranial collaterals seen as cross-filling of contralateral vessels or ipsilateral contrast dilution; (3) obvious diameter reduction of ICA compared with opposite ICA; or (4) ICA diameter reduction compared with ipsilateral ECA.
RESULTS: Interrater agreement, sensitivity, and specificity were excellent (0.88, 90.6%, and 93.8%, respectively). By intention to treat, 3-year risks of ipsilateral stroke for medically treated patients with near occlusion was 15.1% versus 10.9% for surgically treated (absolute risk reduction [ARR] = 4.2%; P value = .33). Patients who continued to receive treatment in the medical arm for the trial’s duration had a 3-year risk of 18.3% (ARR = 7.4%; P value = .13). Medically treated patients with severe stenosis but without near occlusion had a 3-year risk of 26.0% versus surgically treated of 8.2% (ARR = 17.8%; P value < .001).
CONCLUSION: It is crucial to identify near occlusions on vascular imaging. Although it still is reasonable to consider endarterectomy for these patients, the benefit is muted.
“Near occlusion” of the cervical internal carotid artery (ICA) is a description of the appearance of partial luminal diameter decrease or virtual luminal collapse of an otherwise normal-appearing artery beyond a prominent carotid bulb stenosis (Fig 1; 1–6). As a stenosis progressively becomes more severe, blood flowing through it at first does so with detectable increase of velocity to maintain volume, flow, and pressure. There is, however, a critical degree of stenosis beyond which further increase in stenosis no longer allows sufficient blood to pass through, and the ICA beyond the stenosis progressively begins to decrease in diameter (Fig 2). The loss of diameter seen in near occlusion, therefore, represents a failure to maintain sufficient flow in the artery beyond an extremely severe stenosis. Ultimately, many cases will progress to complete occlusion (Fig 3). Collateral flow opens up as the stenosis progresses (Fig 4A) and thereby has the potential to obviate a hypoperfusion stroke (5).
Fig 1.
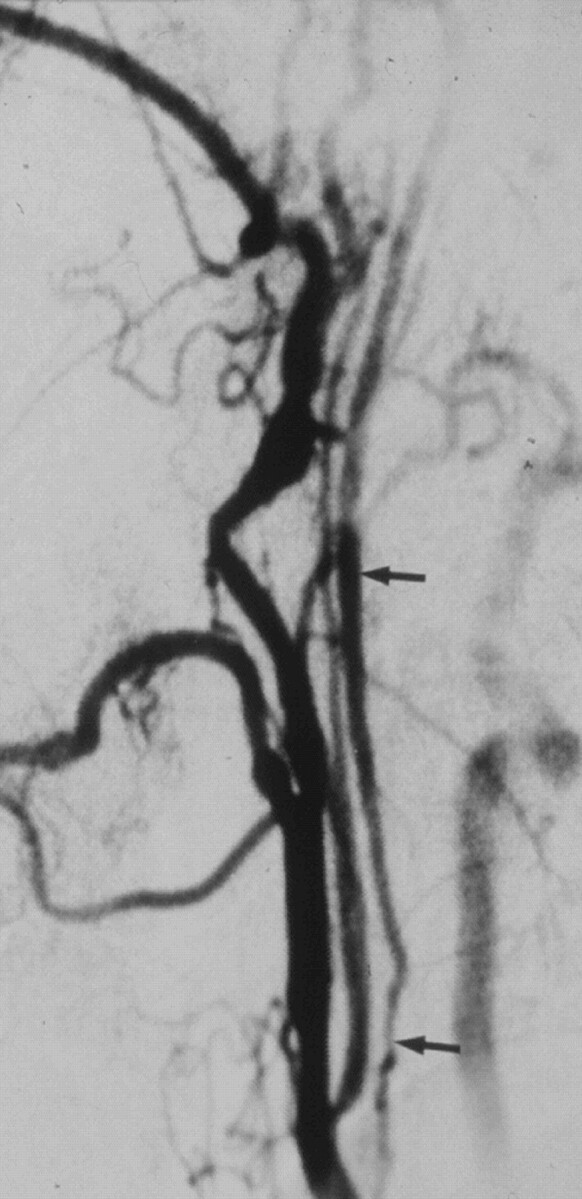
Near occlusion with collapsed lumen. Lateral common carotid angiogram shows the thin collapsed lumen (arrows) of the ICA above a prominent internal carotid stenosis at the carotid bulb (not shown).
Fig 2.
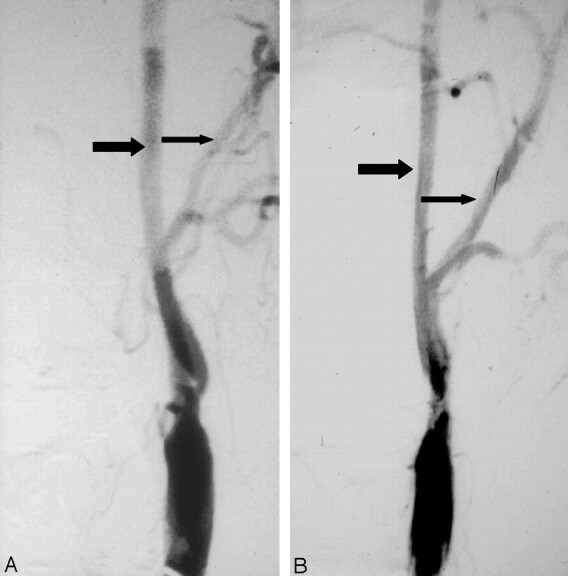
Progression of stenosis to near occlusion.
A, Anterioposterior angiogram shows ulcerated stenosis of the left carotid bulb. The normal ICA (larger arrow) beyond the stenosis is about twice the diameter of the ipsilateral distal ECA (smaller arrow).
B, Anterioposterior angiogram about 1 year later shows progression of stenosis to near occlusion with the left ICA (larger arrow) reduced in diameter in comparison to previous angiogram and close to the diameter of the ECA (smaller arrow).
Fig 3.
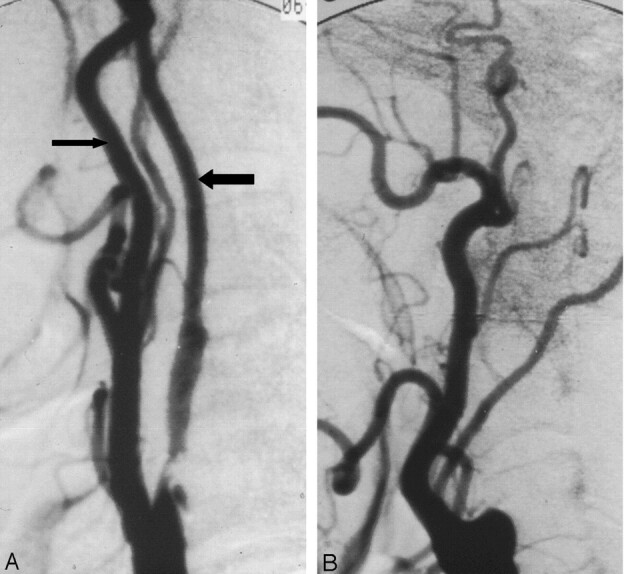
Progression of near occlusion to occlusion.
A, Lateral carotid angiogram shows ulcerated stenosis causing near occlusion with ICA diameter beyond the stenosis (larger arrow), reduced in diameter to be smaller than the distal ECA diameter (smaller arrow). It should be about twice that diameter.
B, Lateral carotid angiogram of the same carotid as A about 8 months later shows that the near occlusion has progressed to occlusion.
Fig 4.
Collaterals (cross-filling and dilution) and late arrival of ICA to the head.
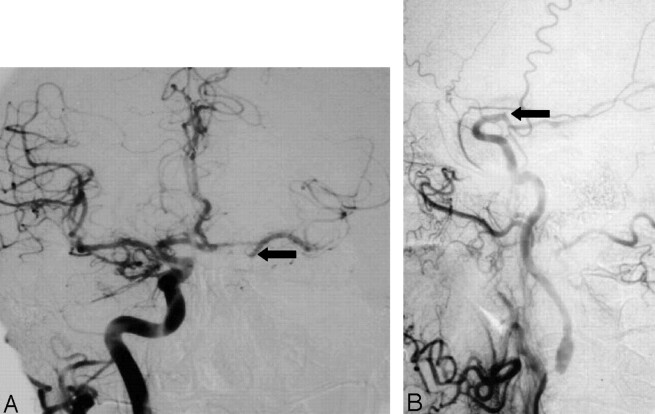
A, Anterioposterior right carotid angiogram, head view, contralateral to left near occlusion shows exuberant cross-filling to left middle cerebral branches and even slightly down the supraclinoid carotid (arrow) to supply the anterior choroidal artery.
B, Lateral left carotid angiogram, late arterial neck and head view, shows delay in ICA filling intracranially, and abrupt dilution contrast in the supraclinoid carotid (arrow) above the origin of a fetal-type posterior cerebral artery. This dilution indirectly demonstrates the excellent collateral already shown in A, not opacified here. It also shows the supraclinoid carotid site of the “watershed” between the right carotid source and the extremely severe left carotid stenosis.
The degree of ICA stenosis from an angiogram is most commonly calculated as the ratio of the diameter at the point of greatest narrowing and the diameter of the distal ICA, well beyond the bulb and the diseased segment, where the walls are parallel (3). If an unrecognized reduced luminal diameter beyond a stenosis is measured and used in the ratio formula, the arithmetic will yield a fallacious stenosis percentage that greatly underestimates the degree of stenosis (2, 3). Because of this, recognition of near occlusion requires attention to detail so that the percent stenosis ratio calculation will not be done. Other methods of calculating stenosis have been used (7). A major collapse (Fig 1) of the normal ICA beyond a critical stenosis is well recognized and has been referred to by terms such as “pseudoocclusion,” “incomplete occlusion,” “near occlusion,” “subtotal occlusion,” “string sign,” “slim sign,” “small distal ICA,” or “poststenotic narrowing” (1–6). All are descriptions of an obvious reduction of the artery diameter; however, loss of the distal ICA diameter to a more modest level (Figs 3A and 5A) is not as well recognized (2, 3).
Fig 5.
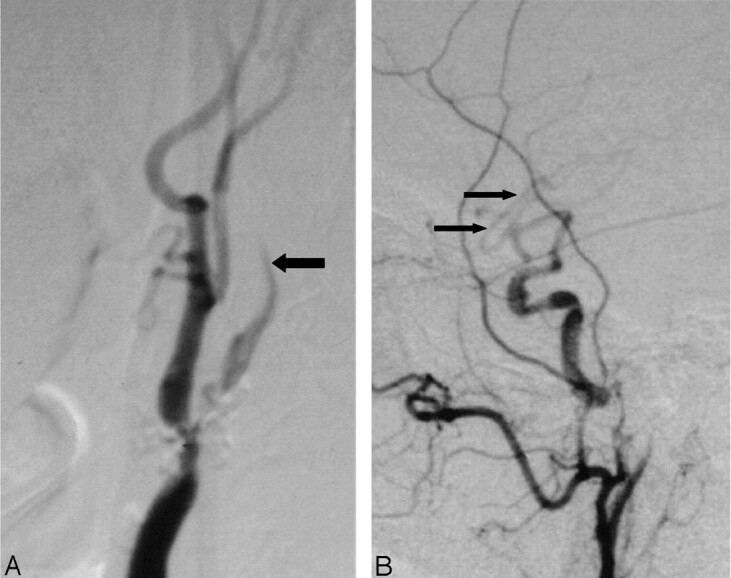
Delayed ICA filling and intracranial dilution from collaterals.
A, Lateral carotid angiogram (neck view) shows delayed ICA filling (larger arrow) beyond the nearly occluded bulb stenosis.
B, There is dilution of contrast of middle cerebral branches (small arrows) intracranially, implying inflow of unopacified blood from circle of Willis collateral sources.
Clear evidence of the increasing risk of stroke and increasing benefit from carotid endarterectomy (CE) with increasing degrees of ICA stenosis emerged from the large symptomatic randomized trials (8, 9). The short-term prognosis of patients with near occlusion was first reported from the North American Symptomatic Carotid Endarterectomy Trial (NASCET; 10). When the ICA was nearly occluded by the stenosing plaque, it was observed that the risk of stroke with medical treatment alone was less, the perioperative surgical risk was not increased, and the benefit from endarterectomy was reduced, compared with the other patients in the severe category (10). A study from the European Carotid Surgery Trial (ECST) did not report any benefit from endarterectomy (11). Marginal results were reported from the pooled data of the Carotid Endarterectomy Trialists’ Collaboration (12). The primary aim of the present study was to set down angiographic criteria and provide illustrations for the identification of near occlusion, to help practitioners to apply them in routine practice. The secondary aim was to report the risk of ipsilateral stroke, in the territory of the near occlusion, as demonstration of the importance of identifying near occlusion.
Subjects and Methods
Angiograms showing moderate (50%–69%) or severe (≥ 70%) stenosis in the NASCET and the ECST were carefully re-reviewed (A.J.F., P.M.R.), by using the criteria below to identify the presence of near occlusion. Consensus agreement was reached in every case. Details of the methods from the trials have been published elsewhere (13, 14). In brief, both trials were designed to determine the efficacy of CE in patients with symptomatic ICA stenosis. Patients were enrolled if they had a transient ischemic attack (TIA) or nondisabling ischemic stroke within 180 days of randomization and an angiographic stenosis in the ipsilateral ICA. Eligible patients were randomized to carotid endarteterctomy plus best medical care or to best medical care alone. Patients were examined by a neurologist at prespecified intervals during follow-up, and outcome events were centrally reviewed.
Criteria for the Identification of Near Occlusion
Although near occlusion has been recognized by its trademark loss of luminal diameter, the recognition of less profound loss of diameter for the diagnosis of near occlusion, to our knowledge, is original. No comprehensive set of diagnostic criteria has appeared in the literature, and, as such, the present article is the first to describe 4 angiographic criteria for near occlusion:
Delayed Time of Contrast Arrival. Delayed arrival of contrast material filling the ICA and its branches in the head, compared with the arrival of contrast material filling the external carotid artery (ECA; primarily its main continuation branch, the internal maxillary artery) and scalp branches over the head (Figs 4 and 5).
Evidence of Collaterals. (A) Direct evidence as positive demonstration of intracranial collaterals by sustained cross-filling of vessels with angiographic contrast material via anterior (Fig 4A) or posterior communicating artery or external carotid collaterals (usually ophthalmic); (B) indirect evidence as demonstration of intracranial collaterals by contrast dilution in the branches of the injected vessels due to unopacified blood from collaterals, mainly via anterior or posterior communicating artery (Figs 4B and 5B).
ICA-to-ICA Comparison of Diameter Reduction. Obvious diameter reduction of the ipsilateral distal cervical ICA in comparison to the opposite ICA (Fig 6).
ICA-to-ECA Comparison of Diameter Reduction. Obvious reduction of the ipsilateral distal ICA in comparison to the distal ECA, beyond facial and occipital artery origins, (Figs 2B, 3A, and 7) at a similar level to the observed ICA. A number of centimeters beyond the carotid bifurcation the ECA is a vessel normally much smaller than the ICA, on the order of half its diameter. A reduction of the ICA to a diameter similar to or narrower than the ECA, therefore, indicates that the ICA is reduced to about half or less of its original diameter. Because cross-sectional area is proportional to the radius squared, the cross-sectional area of the artery is reduced to 25% or less of the original cross-sectional area.
Fig 6.
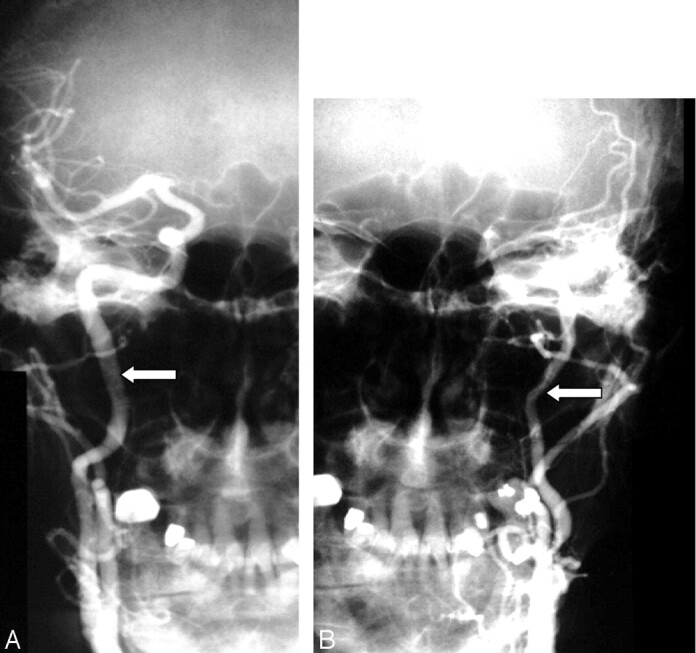
Decreased diameter of the ipsilateral ICA compared with the normal opposite carotid artery.
A, Anterioposterior right carotid angiogram shows the diameter of the normal ICA (arrow).
B, Anterioposterior left carotid angiogram done with the same magnification (same position of radiograph tube and image intensifier; same field of view) as the right in A showing decreased diameter of the left ICA (arrow).
Fig 7.
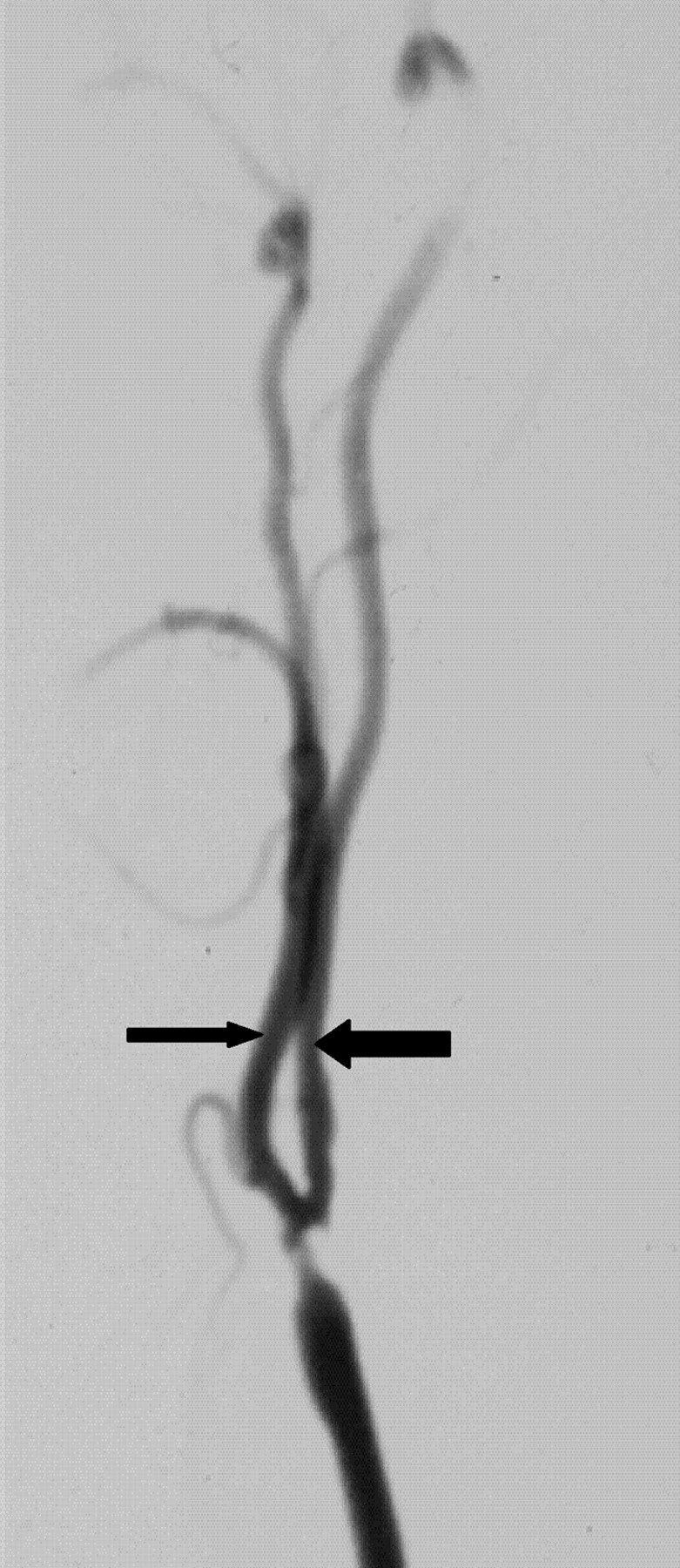
Reduced ICA diameter compared with ECA. ICA diameter (larger arrow) is slightly less than distal ECA (small arrow), implying gross reduction of expected diameter. This implies that the ICA is much narrower than it should be.
Application of Near Occlusion Criteria
Most patients with near occlusion in the NASCET were identified from the onset of the trial (10). Others from the NASCET and all from the ECST were identified from the subsequent angiographic re-review, including some who were brought forward for consideration by using a ratio between the ICA and common carotid arteries (15). Recognition of 2 or more of the criteria was used to categorize the prominent stenosis as near occlusion. Definite delay in filling of ICA to the head and visible collaterals (criteria 1 and 2) led to certain consideration of near occlusion. Decreased diameter of the normal ICA beyond a stenosis (criteria 3 and 4) was subject to interpretation when subtle and bearing in mind the variability of carotid bifurcation anatomy (16). Nearly occluded arteries were further subdivided visually by noting whether they had a tiny threadlike lumen (or “string sign”) (10) or had a more normal-looking, though reduced, lumen.
Interrater Agreement and Accuracy of Identifying Near Occlusion
Consistency in the differentiation between subtle cases of near occlusion and moderate stenosis was studied as a separate exercise. Sixteen patients recognized at the NASCET central office as having criteria for near occlusion had been erroneously enrolled into NASCET as moderate stenosis by participating sites after 1991, on the bais of the ratio calculation. Each of these sites had overlooked that the distal ICA above a prominent stenosis was smaller than expected. They then incorrectly applied the ratio calculation, resulting in percentages corresponding to moderate stenosis. This is a known pitfall of not recognizing near occlusion and inadvertently calculating percent stenosis. Calculation of percent stenosis will be fallacious if the ICA beyond the bulb is too small because of the severity of the disease; this is near occlusion. For the interrater agreement and accuracy study, the selective angiograms of these patients were matched to 16 angiograms of other patients in NASCET showing bona fide moderate stenosis. The matching variables were side of carotid lesion and degree of stenosis. The sum total of 32 angiograms were reviewed independently by 3 neuroradiologists working at the same institution, blinded to all clinical information. The 3 readers were instructed specifically to consider each of the 4 criteria for near occlusion in their evaluation of the angiograms. The intraclass correlation coefficient was used to estimate interrater agreement. Sensitivity and specificity in differentiating near occlusion from moderate stenosis, by using various criteria, were estimated by using the primary neuroradiologist reader (A.J.F.) as the reference standard.
Statistical Analysis of Outcomes
Baseline characteristics of patients were compared between the NASCET and ECST trials by using a χ2 test for comparing 2 proportions. The risks of ipsilateral stroke and absolute risk reductions (ARR), in the territory of the symptomatic ICA were estimated from Kaplan-Meier event-free survival curves. The Kaplan-Meier analyses also counted all strokes (in any territory) and all deaths (from any cause) that occurred during the 30-day perioperative period in surgically treated patients. The logrank test was used to compare surgically treated to medically treated patients. The analyses followed the intention-to-treat principle. On-treatment analyses were also performed, consisting of medically treated patients who remained in their assigned treatment arm, because approximately half of the medically treated NASCET patients had CE after the 1991 NASCET Clinical Alert. At the request of the central office, the suitability to perform endarterectomy was made on a patient-by-patient basis by the participating NASCET site investigators, following the results from phase I of the NASCET (17) and the interim analysis of ECST (14) demonstrating benefit of surgery for patients with severe stenosis and recent ischemic symptoms. In the ECST, 2 patients had surgery, whereas the remainder were not considered for surgery. The occurrence of ICA occlusion subsequent to near occlusion was sought from the ultrasonographic examination of patients only in the NASCET, because this information was not collected in the ECST.
Results
The interrater agreement ranged from 0.46 (good) to 0.83 (excellent) with 3 readers for the individual criteria (Table 1). The diagnosis of near occlusion on the basis of 2 or more criteria yielded the highest level of agreement (0.88). The criterion identifying delayed time of contrast arrival had the poorest sensitivity for diagnosing near occlusion (37.5%), whereas the criterion ICA to ECA comparison of diameter reduction had the highest (84.4%). The specificities for the individual criteria ranged from 90.6% to 93.8%. The presence of 2 or more criteria had the highest level of accuracy for diagnosing near occlusion (sensitivity, 90.6%; specificity, 93.8%). Diagnosing near occlusion by using only the combination of presence of ICA to contralateral ICA or ICA to ipsilateral ECA diameter reduction also achieved high sensitivity (90.6%) and specificity (90.6%).
TABLE 1:
Interrater agreement and sensitivity and specificity in differentiating near occlusion from moderate stenosis using various criteria and combinations
| Criteria | Interrater Agreement | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| Delayed time of contrast arrival | 0.46 | 37.5 | 93.8 |
| Direct or indirect evidence of collaterals | 0.71 | 75.0 | 93.8 |
| ICA to ICA comparison of diameter reduction | 0.75 | 75.0 | 90.6 |
| ICA to ECA comparison of diameter reduction | 0.83 | 84.4 | 93.8 |
| One criterion present | 0.76 | 90.6 | 84.4 |
| Two or more criteria present | 0.88 | 90.6 | 93.8 |
| Three or more criteria present | 0.72 | 59.4 | 96.9 |
| Four criteria present | 0.61 | 31.3 | 96.9 |
| ICA to ICA or ICA to ECA comparison of diameter reduction | 0.84 | 90.6 | 90.6 |
| ICA to ICA and ICA to ECA comparison of diameter reduction | 0.78 | 68.8 | 93.8 |
Note.—ICA indicates internal carotid artery; ECA, external carotid artery.
Using the near occlusion criteria, of 1216 patients in both trials who had severe stenosis of the symptomatic ICA, 137 of the 662 (20.7%) NASCET and 125 of 554 (22.6%) ECST had sufficient evidence for near occlusion. Of the 262 (137 + 125) patients with near occlusion, only 16 (6.1%) were identified as having a threadlike residual lumen of the ICA lumen beyond the bulb stenosis. Because the small number of patients was insufficient to be analyzed separately and none of these patients had a stroke during follow-up, the 16 patients were analyzed together with the others.
Baseline patient characteristics for the 2 trials are shown in Table 2. Because there was no difference in baseline variables between medically and surgically treated patients as a result of the trials’ randomization process, the frequencies of baseline patient characteristics in each trial are presented in aggregate form. Comparing the two trials, the age of the near occlusion patients in the ECST was significantly lower. There was also a lower prevalence of diabetes, ischemic heart disease, and hyperlipidemia. Significantly more patients in the ECST had surgery beyond 4 weeks of the last ischemic event. Smoking in the past year and the presence of an ulcerated carotid artery plaque were more prevalent in the ECST.
TABLE 2:
Comparison of baseline characteristics for patients with near occlusion (% of group)
| Patient Characteristic | NASCET ITT (N = 137) | ECST ITT (N = 125) | P Value | Medical ITT (N = 114) | Medical On- Treatment* (N = 78) |
|---|---|---|---|---|---|
| Assigned to carotid endarterectomy | 51.1 | 62.4 | — | — | |
| Age ≥65 y† | 53.3 | 36.8 | 0.007 | 48.2 | 44.9 |
| Male sex | 79.6 | 82.4 | 0.56 | 78.1 | 82.1 |
| Hemispheric TIA or stroke in the past 180 d‡ | 67.1 | 72.0 | 0.39 | 74.6 | 82.0 |
| Last ischemic event to randomization >4 wk | 43.1 | 60.0 | 0.006 | 54.4 | 55.1 |
| History of: | |||||
| Treated hypertension | 46.7 | 43.2 | 0.57 | 45.6 | 44.9 |
| Diabetes mellitus | 16.8 | 9.6 | 0.09 | 18.4 | 19.2 |
| Myocardial infarction or angina | 32.1 | 19.2 | 0.02 | 28.9 | 24.4 |
| Intermittent claudication | 12.4 | 18.4 | 0.18 | 14.9 | 15.4 |
| Hyperlipidemia | 26.3 | 10.4 | 0.001 | 21.9 | 17.9 |
| Smoking in the past year | 48.2 | 64.8 | 0.007 | 55.3 | 53.8 |
| Irregular/ulcerated carotid artery plaque§ | 38.7 | 73.6 | <0.001 | 55.3 | 59.0 |
Note.—ITT indicates intention-to-treat; TIA, transient ischemic attack.
Medically treated patients who remained on medical therapy for the duration of the trial. There were no statistically significant different patient characteristics in comparison to the Medical ITT.
Mean age of patients: NASCET ITT, 64.0 years; ECST ITT, 61.4 years; P value = .01. Mean age of patients: Medical ITT, 63.3 years; Medical On-Treatment, 63.6 years.
Hemispheric, not ocular only events.
Ipsilateral to the territory of the last ischemic event.
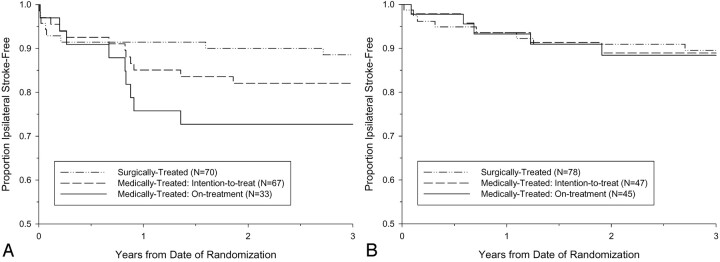
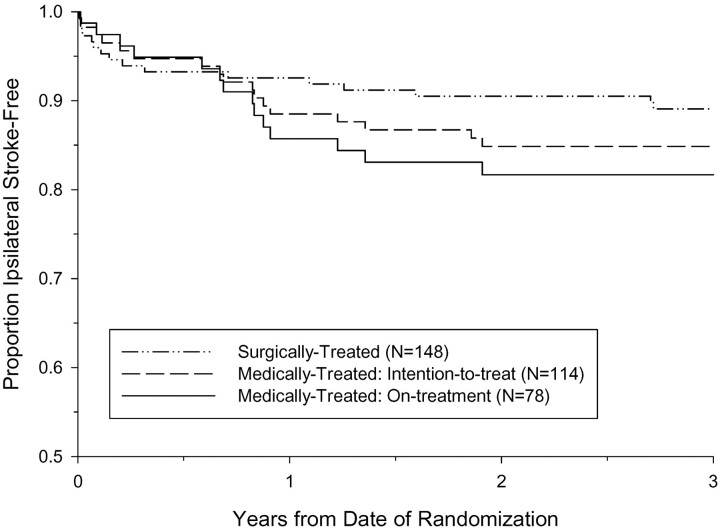
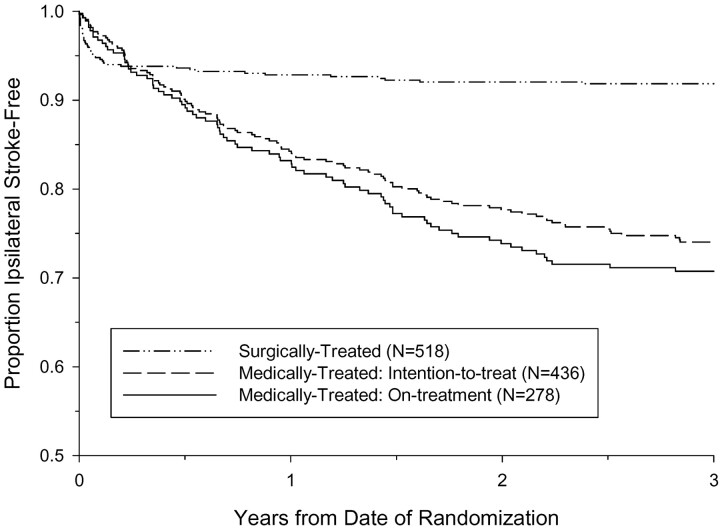
A total of 12 ipsilateral strokes occurred within 3 years after randomization among 67 medically treated near occlusion NASCET patients, and 5 ipsilateral strokes occurred among 47 medically treated near occlusion ECST patients. The Kaplan-Meier ipsilateral stroke-free survival curves are shown in Figs 8A and 8B. The 3-year intention-to-treat risk of ipsilateral stroke in medically treated ECST patients was 11.1%, compared with 17.9% in medically treated NASCET patients (absolute difference, −6.8%; P value = .31). The 3-year risks of ipsilateral stroke in surgically treated patients were similar between the trials, 11.4% in the NASCET and 10.5% in the ECST. Pooling the individual data from the two trials, the 3-year intention-to-treat risk of ipsilateral stroke among 114 medically treated patients was 15.1%, compared to 10.9% among 148 surgically treated patients (ARR, 4.2%; logrank P value = .33; Fig 9). Compared with patients with severe stenosis but without near occlusion, the corresponding 3-year risks were 26.0% and 8.2% for medically and surgically treated patients, respectively (ARR, 17.8%; logrank P value < .001; Fig 10).
Fig 8.
Kaplan-Meier event-free survival from ipsilateral stroke for patients with near occlusion.
A, The 3-year intention-to-treat risk of ipsilateral stroke in NASCET patients was 17.9% for medically treated and 11.4% for surgically treated. The 3-year risk of ipsilateral stroke for medically treated NASCET patients who continued to receive medical therapy throughout the duration of the trial (on-treatment) was 27.3%.
B, The 3-year intention-to-treat risk of ipsilateral stroke in ECST patients was 11.1% for medically treated and 10.5% for surgically treated. The 3-year risk of ipsilateral stroke for medically treated ECST patients who continued to receive medical therapy throughout the duration of the trial (on-treatment) was 11.6%.
Fig 9.
Kaplan-Meier event-free survival from ipsilateral stroke for patients with near occlusion, pooled data from the NASCET and ECST. The 3-year intention-to-treat risk of ipsilateral stroke in was 15.1% for medically treated and 10.9% for surgically treated. The 3-year risk of ipsilateral stroke for medically treated patients who continued to receive medical therapy throughout the duration of the trial (on-treatment) was 18.3%.
Fig 10.
Kaplan-Meier event-free survival from ipsilateral stroke for patients with severe stenosis but without near occlusion, pooled data from the NASCET and ECST. The 3-year intention-to-treat risk of ipsilateral stroke in was 26.0% for medically treated and 8.2% for surgically treated. The 3-year risk of ipsilateral stroke for medically treated patients who continued to receive medical therapy throughout the duration of the trial (on-treatment) was 29.3%.
Of the 67 medically treated NASCET patients, 33 remained on-treatment throughout the trial, while 45 of 47 patients in the ECST remained on-treatment. Among the 33 medically on-treatment NASCET patients, 19 had follow-up sonography, either because of a TIA or stroke or annual follow-up. An occlusion of the ICA was suggested in 5 of 19 (26.3%) patients between 30 days and 3.0 years (median = 1.2 years). No apparent differences were observed in the baseline patient characteristics between the 114 (67 + 47) medically treated patients in the intention-to-treat analysis and the 78 (33 + 45) medically treated patients remaining in the on-treatment analysis (Table 1). The 3-year on-treatment risks of ipsilateral stroke for medically treated patients were 27.3% in the NASCET and 11.6% in the ECST. The pooled 3-year on-treatment risk of ipsilateral stroke for medically treated patients was 18.3%, compared with 10.9% among surgically treated patients (ARR = 7.4%, logrank P value = 0.13, Fig 9). Compared with patients with severe stenosis but without near occlusion, the corresponding 3-year risks were 29.3% and 8.2% for medically and surgically treated patients, respectively (21.1%, logrank P value < .001, Fig 10).
Discussion
In the present study, the definition of near occlusion has been explained in detail, identifying a greater number of near occlusion cases than has previously been recognized. For patients entered into NASCET and ECST, those categorized as near occlusion represented 21.5% of those considered to have severe stenosis. In effect, 1/5 patients with severe stenosis met 2 or more prespecified criteria of near occlusion. Seeking the subtleties of partially collapsed ICAs prevents the fallacious calculation of percentage stenosis as a ratio by using an artifactually reduced ICA diameter.
In recent years, an important refinement of the interpretation of the less-profound appearance of near occlusion without a threadlike collapsed lumen evolved to avoid fallacious entry of patients with <70% stenosis in the continuation of phase II of the NASCET. The first study of near occlusion from the NASCET had 106 patients (10). The present study reported on 137 patients from the NASCET, in addition to 125 patients from the ECST, identified by using the same criteria and the same central readers. The combined results showed a low risk of stroke in the medically treated patients with near occlusion. A widespread clinical impression that near occlusions require urgent surgery is not borne out for this group. The low medical risk provides further evidence that most strokes arising from a stenosed artery are not hemodynamic but embolic. With near occlusion there is relative protection from emboli by the decrease in the arterial diameter and the contribution to the affected hemisphere from the ipsilateral carotid circulation. Angiographically, this is visualized by the late arrival of contrast filling the ICA and reaching the brain and by the presence of good collateral circulation. The previous clinical impression that surgery is especially hazardous in these patients was also not supported, as the perioperative surgical risk was similar to that of severe stenosis. Although near occlusions have been considered “critical” stenoses and visually represent the worst degree of carotid stenosis, it is paradoxical that they are critical only in the perception of the artery’s appearance and not critical from a stroke outcome point of view.
The agreement study showed that the 4 criteria can be applied by independent skilled observers with excellent agreement and accuracy. An institutional bias may have contributed to the high interobserver agreement, because the 3 readers worked at the same hospital at the time of the study. On the other hand, it is not surprising that interobserver agreement was not perfect, especially the interpretation of delayed time of contrast arrival. The lower agreement was partly due to the inability to review full angiographic series, because only limited selected images were available. This limitation was unavoidable because the angiographic films sent to the NASCET central office were chosen at the discretion of the participating site investigator to show clearly the stenosis for the patient’s entry into the trial. This meant that looking at only one image from a series, an interpretation needed to be made of less filling of ICA branches compared with branches of the ECA at a similar level on the same image. In clinical practice, where a complete angiographic series is available, an opportunity exists to review angiographic filling on a more dynamic image-by-image basis. This means that there should be better agreement to assess delayed ICA branch filling in a clinical setting than reported in the present article from the available archive of films.
Higher agreement and accuracy of diagnosing near occlusion was achieved with ICA to contralateral ICA and ICA to ECA comparisons, because complete series were not as crucial to the process of interpretation. The observed decrease in agreement with an increasing number of criteria present can be explained by the fact that it is more difficult for 3 readers to agree on the presence of all 4 criteria than it is to agree on the presence of any 2 of the 4. The observed decrease in sensitivity and increase in specificity, because the number of criteria present increased, is indicative of the increased stringency for ruling in a near occlusion. The results also show excellent interrater agreement and excellent accuracy when using only the combination of ICA to ICA or ICA to ECA comparison for diagnosing near occlusion. This has direct application for the contemporary identification of near occlusion on the basis of CT and MR angiography, where assessments of arrival time and collaterals are not currently possible.
In making artery-to-artery diameter comparisons, the existence of anatomic variations of the arteries requires attention. For example, an ICA that supplies both anterior cerebral arteries, ipsilateral middle cerebral artery, and a fetal-type posterior cerebral artery will carry more blood and be larger than an ICA that supplies only the middle cerebral artery. In addition, an ECA can enlarge with time if recruited as a collateral or as a feeder to a fistula or a vascular malformation. The present study does not proscribe absolute numerical threshold diameters for these vessels. Rather, each criterion is applied in relation to the others for an overall, interpretative diagnosis. Minor differences of ICA are not declared as near occlusion. Because contemporary cross-sectional imaging, CT angiography, and MR angiography are calibrated in real millimeters, it may be possible in the future to incorporate absolute measures of diameters and anatomic variations into the criteria for near occlusion.
Discrepant results regarding the usefulness of endarterectomy for patients with near occlusion arose from the individual trials. The NASCET results lead to the conclusion that the benefit of surgery to prevent stroke for patients with near occlusion was perceived but was less than the benefit of surgery in patients with severe stenosis but without near occlusion (10). From the ECST, however, the conclusion was that patients with near occlusion had no benefit from endarterectomy (11). The side-by-side comparison of the 2 trials in the present study (Figs 8A, -B) indicated that the primary reason for the discrepant results may be the much lower risk of ipsilateral stroke for on-treatment medically treated patients in ECST (11.6%) than in NASCET (27.3%). The risks for surgically treated patients were similar (10.5% vs 11.4%, respectively). The lower medical stroke risk in ECST may have been due to the lower prevalence of some risk factors, such as old age, diabetes, ischemic heart disease, and hyperlipidemia.
Neither the NASCET nor the ECST, on their own, had sufficient numbers of patients to assess with statistical certainty the effect of surgery in patients with near occlusion. The present study was intended to provide greater statistical strength by using a consistent definition of near occlusion and a method for reconciling discrepancies between the 2 trials. Unfortunately, the pooled data do not lead to any steadfast conclusions about the benefit of endarterectomy, as would have been preferred. The small sample size, low event rates, and fact that approximately half of the patients with severe stenosis randomized to the NASCET medical arm underwent endarterectomy following the 1991 publications makes it difficult to come to a stronger conclusion from the pooled data.
Conclusions
In summary, carotid near occlusions complicate the calculation of ICA stenosis by a ratio by using the “normal” distal ICA beyond the bulb as denominator. The outcome results strongly suggest that endarterectomy is of less value for patients with near occlusion than for patients with severe stenosis but without near occlusion. It is crucial to identify these near occlusions on imaging because they differ from other severe stenoses not just in appearance, but also for clinical outcome expectations from treatment. Although it still is reasonable to consider endarterectomy for patients with near occlusion, both the physician and the patient must be aware that the benefit will be muted.
Acknowledgments
We thank the participating physicians, surgeons, coordinators, and patients of the NASCET and of the ECST, who made the gathering of these data possible. We especially thank Drs. David Pelz and Irene Gulka, who kindly read angiograms for the interrater reliability study.
Footnotes
The National Institute of Neurological Disorders and Stroke supported the NASCET (grant R01-NS-24456). The UK Medical Research Council funded the ECST (grant G116/112) and Dr. Rothwell (senior clinical fellowship). Dr. Eliasziw was supported by the Alberta Heritage Foundation for Medical Research.
The findings reported in this article were presented in part at the 33rd and 40th annual meetings of the American Society of Neuroradiology Annual Meetings, May 23–27, 1995 and May 13–17, 2002.
References
- 1.Berman SS, Devine JJ, Erodes LS, Hunter GC. Distinguishing carotid artery pseudo-occlusion with color flow Doppler. Stroke 1995;26:434–438 [DOI] [PubMed] [Google Scholar]
- 2.Dix JE, McNulty BJ, Kalimes DF. Frequency and significance of a small distal ICA in carotid stenosis. AJNR Am J Neuroradiol 1998;19:1215–1218 [PMC free article] [PubMed] [Google Scholar]
- 3.Fox AJ. How to measure carotid stenosis. Radiology 1993;186:316–318 [DOI] [PubMed] [Google Scholar]
- 4.Gabrielsen TO, Seeger JF, Knake JE, et al. The nearly occluded internal carotid artery: a diagnostic trap. Radiology 1981;138:611–618 [DOI] [PubMed] [Google Scholar]
- 5.Henderson R, Eliasziw M, Fox AJ, et al. The importance of angiographically defined collateral circulation in patients with severe carotid stenosis. Stroke 2000;31:128–132 [DOI] [PubMed] [Google Scholar]
- 6.Lee DH, Gao F, Rankin RN, et al. Duplex and color Doppler flow sonography of occlusion and near occlusion of the carotid artery. AJNR Am J Neuroradiol 1996;17:1267–1274 [PMC free article] [PubMed] [Google Scholar]
- 7.Rothwell PM, Gibson RJ, Slattery J, et al. Equivalence of measurements of carotid stenosis: a comparison of three methods on 1001 angiograms. Stroke 1994;25:2435–2439 [DOI] [PubMed] [Google Scholar]
- 8.North American Symptomatic Carotid Endarterectomy Trialists’ Collaborative Group. The final results of the NASCET trial. N Engl J Med 1998;339:1415–1425 [DOI] [PubMed] [Google Scholar]
- 9.European Carotid Surgery Trialists’ Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998;351:1379–1387 [PubMed] [Google Scholar]
- 10.Morgenstern LB, Fox AJ, Sharpe B, et al. The risks and benefits of carotid endarterectomy in patients with near-occlusion of the carotid artery. Neurology 1997;48:911–915 [DOI] [PubMed] [Google Scholar]
- 11.Rothwell PM, Gutnikov SA, Warlow CP. Reanalysis of the final results of the European Carotid Surgery Trial. Stroke 2003;34:514–523 [DOI] [PubMed] [Google Scholar]
- 12.Rothwell PM, Eliasziw M, Gutnikov SA, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 2003;361:107–116 [DOI] [PubMed] [Google Scholar]
- 13.Steering Committee of NASCET. North American Symptomatic Carotid Endarterectomy Trial: methods, patient characteristics, and progress. Stroke 1991;22:711–720 [DOI] [PubMed] [Google Scholar]
- 14.European Carotid Surgery Trialists’ Collaborative Group. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. Lancet 1991;337:1235–1242 [PubMed] [Google Scholar]
- 15.Rothwell PM, Warlow CP. Low risk of ischemic stroke in patients with reduced internal carotid artery lumen diameter distal to severe symptomatic carotid stenosis: cerebral protection due to low poststenotic flow? Stroke 2000;31:622–630 [DOI] [PubMed] [Google Scholar]
- 16.Schulz UGR, Rothwell PM. Major variation in carotid bifurcation anatomy: a possible risk factor for plaque development? Stroke 2001;32:2522–2529 [DOI] [PubMed] [Google Scholar]
- 17.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445–453 [DOI] [PubMed] [Google Scholar]