Abstract
Current guidelines suggest that polycythemia vera (PV) patients maintain a strict hematocrit less than 45%. However, to date, little is known about the relationship between HCT control and PV-related symptom burden. In this study, PV patient data was analyzed from the CYTO PV trial (n = 224) and the MPN-SAF study cohort (n = 645). No significant differences in symptom burden were seen at the 6 and 12 month follow-up when evaluating prospective hematocrit control in the CYTO PV cohort. Patients in the MPN-SAF cohort with a worst item score of greater than 5/10 on the Myeloproliferative Neoplasm Symptom Total Symptom Score had a significantly lower mean hematocrit (p = .0376). These findings suggest a relationship between traditional aggressive therapy for PV and increased symptom burden with prolonged therapy. Thus, symptom burden should be considered when contemplating the choice of therapy in the second-line setting for PV.
Keywords: Symptom burden, polycythemia vera, quality of life, phlebotomy
Introduction
Polycythemia vera (PV), a Philadelphia chromosome negative myeloproliferative neoplasm, is characterized by bone marrow trilineage hyperplasia with clonal hematopoiesis in the absence of a physiologic stimulus. Although the mechanisms driving disease development remain under investigation, numerous mutations including those within Janus Kinase (JAK) have been associated with cellular proliferation.[1] Thrombotic and hemorrhagic diatheses stemming increased whole blood viscosity, leukostasis, microcirculatory disturbance, platelet dysfunction, and excessive inflammatory cytokines remains a major source of morbidity and mortality.[2] PV patients also suffer from a challenging constellation of disease-related manifestations that significantly compromise quality of life.[3]
Due to the risk of infarction and thrombotic events, current guidelines suggest that PV patients maintain a strict hematocrit (HCT) less than 45%.[4,5] The prospective clinical study “Cytoreductive Therapy in Polycythemia Vera” (CYTO-PV) [6] investigated the impact of rigid HCT control on overall mortality by randomizing patients to strict (HCT maintained less than 45%) or lenient (HCT maintained between 45% and 50%) HCTs. Results identified that strict hematocrit control resulted in a reduced incidence of major thrombosis or cardiovascular mortality and cardiovascular events (1.1 per 100 person-years) in comparison to lenient control (4.4 per 100 person-years).
Independent of the mortality benefits, studies have yet to evaluate the effects of more rigid HCT control on patient symptomatology. Pharmacological reduction frequently utilizes agents such as hydroxyurea, radiophosphorus, busulfan, and pipobroman, all of which may be associated with burdensome toxicity profiles that may compromise patient quality of life.[3] In tandem, recurrent phlebotomy may induce iron deficiency anemia which has been identified outside of PV to induce symptoms such as leg cramps, depression, fatigue, and paresthesias. In contrast, elevated HCT levels may contribute to increased viscosity, leukostasis and microthrombosis, all of which have been identified as contributors to microvascular symptoms such as sexual dysfunction, cognitive impairments, insomnia, and depression.
Recognizing the importance of considering patient quality of life in the employment of therapies, this ad hoc analysis of both the CYTO-PV trial [6] and the Myeloproliferative Neoplasm Symptom Assessment study (MPN SAF) [7] PV patients aims to prospectively evaluate the impact of strict HCT control on patient symptomology.
Materials and methods
Patient data were collected from PV patients enrolled in the CYTO PV trial [6] and the MPN SAF [7] study. As mentioned above, the CYTO PV trial was a prospective, multicenter, randomized, open-label, with blinded end-point investigation assessing the impact of strict HCT control on PV patient outcomes. Recruitment was conducted among adults older than 18 years who met WHO diagnostic criteria of JAK2V617F positive PV. Patients were assigned into either a “strict” HCT arm that maintained at less than 45% or a “lenient” arm where HCT was maintained at 45–50%. Patients were initiated on either hydroxyurea, phlebotomy, or both to ensure maintenance of HCT levels. Phlebotomy was initially performed by removing 250–500 mL every 2 d or twice a week until the target HCT was attained. Alternative therapies included busulfan, pipobroman, interferon, and anti-thrombotic agents. All patients without prior contraindication were initiated on aspirin. Blood counts were monitored on a weekly basis for 6 months.
The MPN-SAF study represented the largest collection of PV-related symptoms to date.[7,8] This study demonstrated that patients suffer from increased symptom burden compared with age-matched controls. Since the creation of this study, study investigators have continued to accrue patient data. Symptom burden data used for this analysis were collected among 645 international PV patients in languages including Albanian, Czech, Chinese, Danish, Dutch, English, French, German, Italian, Japanese, Romanian, Spanish, and Swedish. Patients were prospectively accrued at the time of an office visit. Common therapy among MPN-SAF PV study participants included phlebotomy, hydroxyurea, splenectomy, P32, busulfan, interferon, piporoman, and anti-thrombotic agents.
Data collected from the two cohorts included demographics, disease-related variables, and assessment of disease-related symptom burden. Symptom burden was assessed via the Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score (TSS or MPN-10). The MPN-10 assessment includes a single item from the Brief Fatigue Inventory (BFI) [9] along with nine others specific to MPN-related symptoms including fevers, weight loss, early satiety, abdominal discomfort, inactivity, night sweats, pruritus, problems with concentration, and bone pain. Symptoms were scored on a 0 (absent) to 10 (worst imaginable) scale as per previously published scoring algorithms.[7]
Descriptive statistics were used to summarize demographic and survey data. When evaluating demographics, disease variables, and symptom burden data, results were obtained at the time of the symptom assessment for the MPN-SAF Cohort and at the pre-randomization assessment for the CYTO PV cohort. For the CYTO PV cohort, symptom burden was also assessed at months 6 and 12 in the study. Mean values for individual MPN-10 item scores and the total sum score were then compared by frequencies. Analysis of variance (ANOVA) F-Test was used to analyze continuous data and Chi-square test was used to analyze frequency data. Symptom score changes at 6 and 12 months were compared between HCT assignment group (strict versus lenient) in the CYTO PV cohort by Student’s t-test. Mean hematocrit was compared among patients in the MPN-SAF cohort by low (MPN-10 total sum score less than 20 or any individual symptom score greater than or equal to 5) or high symptom burden (MPN-10 total sum score less than or equal to 20 or any individual symptom score greater than 5).[10] Statistical significance was designated as p < .05. Statistical analysis was conducted using SAS version 9.3 software (SAS Inc., Cary, NC).
Results
CYTO PV cohort
Baseline characteristics and symptom burden
A total of 224 of 365 individuals in the CYTO PV cohort were included in the analysis due to availability of symptom burden data. Mean age (64.7 years) and gender (58% male) were typical of the disease. HCT prior to randomization was elevated (48.0%) with 72.3% of patients demonstrating a baseline HCT greater than 45%. Similarly, initial mean hemoglobin and platelet count tended to be elevated at baseline (15.5 g/dL and 414 × 109/L, respectively). Prior thrombosis (25%) and prior hemorrhage (4.5%) were observed in a minority of patients. In comparing baseline demographics and disease control between the CYTO-PV and MPN-SAF cohort, mean age, HCT, hemoglobin, and platelets were significantly higher at baseline in the CYTO-PV cohort (for all p < .05, Table 1). The mean baseline MPN-10 sum score was 18.3/100. Overall, no significant differences were observed for baseline symptom score items between the CYTO PV cohort and the MPN-SAF Cohort except for the item of worst 24-h fatigue (Table 2).
Table 1.
Disease characteristics of PV patients by cohort. Values for the CYTO PV cohort are prior to study randomization. For the MPN-SAF cohort, values are calculated at the same time as the one time symptom burden assessment.
| CYTO PV cohort (N = 224) |
MPN-SAF cohort (N = 645) |
Total (N = 869) |
p Value | |
|---|---|---|---|---|
| Current age | ||||
| Mean (SD) | 64.7 (12.0) | 62.6 (13.1) | 63.1 (12.9) | .029a |
| Range | (29.6–87.8) | (22.0–91.0) | (22.0–91.0) | |
| Sex | ||||
| F (%) | 93 (41.5) | 295 (45.8) | 388 (44.7) | NS |
| Prior thrombosis | ||||
| Yes (%) | 56 (25.0) | 163 (25.3) | 219 (25.2) | NS |
| Prior hemorrhage | ||||
| Yes (%) | 10 (4.5) | 32 (5.0) | 42 (4.8) | NS |
| Hematocrit | ||||
| Mean (SD) | 48.0 (4.6) | 44.9 (7.2) | 45.7 (6.8) | <.001a |
| Range | (38.1–66.0) | (24.0–74.0) | (24.0–74.0) | |
| Hematocrit >45% (%) | 162 (72.3) | 246 (38.1) | 408 (47.0) | <.001b |
| Hemoglobin | ||||
| Mean (SD)c | 15.5 (1.7) | 15.0 (2.4) | 15.1 (2.3) | .005a |
| Rangec | (11.7–22.0) | (8.0–24.5) | (8.0–24.5) | |
| White blood celld | ||||
| Mean (SD) | 9.3 (4.3) | 9.2 (6.0) | 9.2 (5.6) | NS |
| Plateletse | ||||
| Mean (SD) | 414.0 (173.3) | 367.8 (201.6) | 379.7 (195.7) | .002a |
ANOVA F-test.
Chi-square.
Hemoglobin units of g/dL.
White blood cell count in units of cells ×109 per liter (L).
Platelet noted in units of platelets ×109/L.
Table 2.
Comparison of MPN-10 baseline symptom items and total score for PV patients by cohort.
| MPN-SAF Survey Item | Cyto PV cohort (N = 224) |
MPN-SAF cohort (N = 645) |
Total (N = 869) |
p Value (adjusted) | ||
|---|---|---|---|---|---|---|
| Mean (SD) | Adjusted mean (std err)a | Mean (SD) | Adjusted mean (std err)a | Mean (SD) | ||
| WORST fatigue (BFI) | 3.1 (2.8) | 3.1 (0.21) | 3.9 (2.9) | 3.9 (0.11) | 3.7 (2.9) | .004b |
| Early satiety | 2.0 (2.7) | 2.0 (0.33) | 2.2 (2.7) | 2.2 (0.11) | 2.2 (2.7) | NS |
| Abdominal discomfort | 1.6 (2.1) | 1.7 (0.28) | 1.5 (2.3) | 1.5 (0.09) | 1.6 (2.2) | NS |
| Inactivity | 1.6 (2.4) | 1.8 (0.33) | 2.1 (2.7) | 2.1 (0.11) | 2.1 (2.6) | NS |
| Concentration | 2.0 (2.6) | 2.0 (0.33) | 2.2 (2.6) | 2.2 (0.11) | 2.2 (2.6) | NS |
| Night sweats | 1.8 (2.7) | 2.1 (0.34) | 1.9 (2.7) | 1.9 (0.11) | 1.9 (2.7) | NS |
| Itching | 2.7 (3.2) | 2.8 (0.38) | 2.5 (3.0) | 2.5 (0.12) | 2.6 (3.0) | NS |
| Bone pain | 1.9 (2.9) | 1.9 (0.35) | 2.0 (2.8) | 2.0 (0.11) | 2.0 (2.8) | NS |
| Fever | 0.4 (1.1) | 0.4 (0.14) | 0.4 (1.1) | 0.4 (0.05) | 0.4 (1.1) | NS |
| Weight loss | 0.7 (1.5) | 0.8 (0.26) | 1.1 (2.2) | 1.1 (0.08) | 1.1 (2.1) | NS |
| MPN-SAF TSS | 18.3 (15.7) | 19.4 (2.0) | 19.9 (16.3) | 19.9 (0.64) | 19.7 (16.2) | NS |
Adjusted mean values estimated from multiple regression model which included the following covariates: age, baseline hematocrit, platelets, PV risk category, cohort, and interaction effect of hematocrit and cohort.
Anova F-test.
HCT control and symptom burden
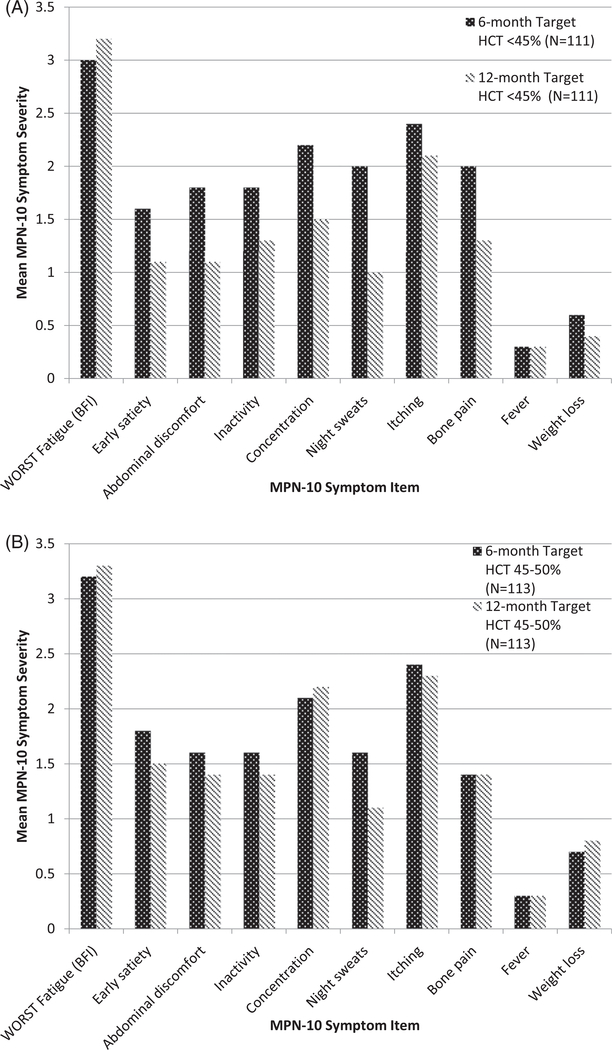
Results of the CYTO PV study were analyzed to evaluate whether prospective symptom burden changes were observed when controlling for HCT levels. Individual MPN-SAF items scores were evaluated after 6 and 12 months of prospective HCT control (Figure 1). There were no significant differences in symptoms scores at the 6-month and 12-month analysis between the lenient and strict HCT control. The mean MPN-10 sum score for the 6-month follow up was 18.4/100 (SD = 16.3) for those with a target hematocrit <45% and 17.4/100 (SD = 15.1) for those with a hematocrit between 45 and 50%. Similarly, the mean MPN-10 sum score for the 12-month follow up was 13.3 (SD = 12.6) for those with a target hematocrit <45% and 15.7 (SD = 16.2) for those with a hematocrit between 45 and 50%.
Figure 1.
CYTO-PV study prospective comparison of treatment arms for strict versus lenient hematocrit control at 6 months and twelve months (N = 224). (A) Comparison of the six and twelve month scores for individuals randomized to lower hematocrit control. (B) Six and 12 month symptom scores among participants randomized to elevated hematocrit goal.
MPN-SAF cohort
Baseline characteristics and symptom burden
Among the MPN cohort, 645 PV patients were included in the analysis. Mean age and gender for this cohort was similar to the CYTO PV cohort (age 62.6 and 46% female, respectively), Mean hemoglobin level was 15.0 g/dL among 645 MPN-SAF PV patients. Among the MPN-SAF cohort, 38.1% of patients had a hematocrit greater than 45%. Mean hemoglobin and platelet count tended to be elevated at baseline (15.0 g/dL and 367.8 × 109/L, respectively). Prior thrombosis (25.3%) and prior hemorrhage (5.0%) were observed in a minority of patients similar to the CYTO PV cohort. Mean MPN-10 score was 19.9/100.
HCT control and symptom burden
Mean hematocrit was compared between individuals using MPN-10 symptom score cutoffs which have been identified as markers of high symptom burden (Table 3). In this analysis, individuals who met the criteria of a MPN-10 individual item score of greater than 5/10 had a significantly lower mean hematocrit (HCT = 44.9%) than those who did not meet this cutoff (HCT = 46.7%). A second cutoff of a MPN-10 total score of greater than or equal to 20 was analyzed; however, this cutoff did not meet statistical significance.
Table 3.
Evaluation of mean hematocrit among individuals in the MPN-SAF cohort with (A) a worst individual MPN-SAF score of >5/10 and ≥5/10 or (B) sum MPN 10 score of <20/100 and ≥20/100.
| (A) | ||
| Hematocrit | Worst MPN-10 symptom item score severity of ≤5/10 (n = 165) | Worst MPN-10 symptom item score severity of >5/10 (n = 171) |
| Mean (SD) | 46.7% (8.0%)* | 44.9% (8.1%)* |
| Range | 32.0–73.0% | 30.0–74.0% |
| (B) | ||
| Hematocrit | Cumulative MPN-10 score severity of < 20/100 (n = 194) | Cumulative MPN-10 score severity of ≥20/100 (n = 142) |
| Mean (SD) | 46.5% (8.2%)** | 44.8% (7.9%)** |
| Range | 30.0–73.0% | 32.0–74.0% |
p = .0376 via ANOVA F-test.
p = .0592 via ANOVA F-test.
Phlebotomy and symptom burden
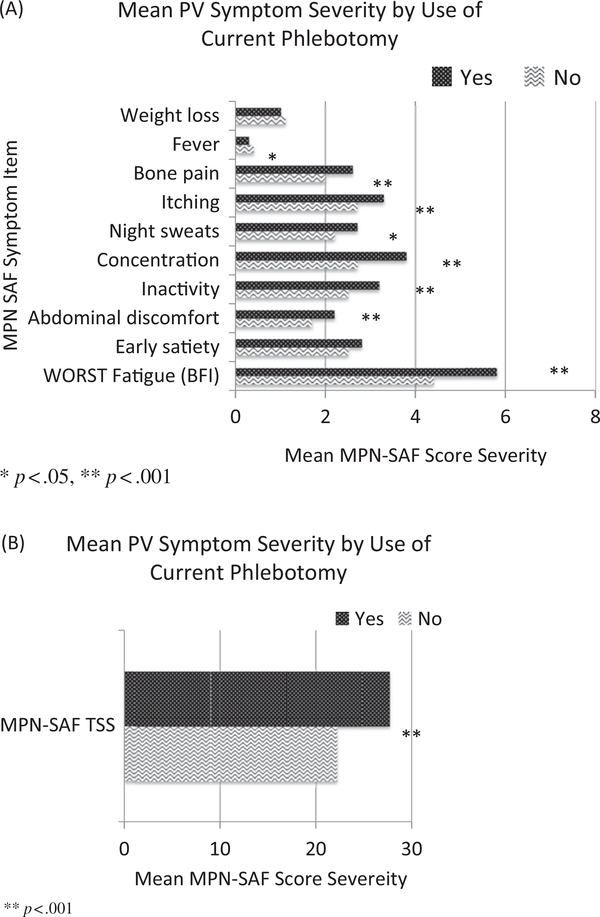
In order to observe trends between phlebotomy and symptom burden in the MPN-SAF cohort, individual MPN 10 item scores and total sum score were compared between patients undergoing phlebotomy and alternative therapy (Figure 2). In these patients, mean items scores for worst fatigue (5.8 versus 4.4, p < .001), abdominal discomfort (2.2 versus 1.7, p < .001), inactivity (3.2 versus 2.5, p < .001), concentration (3.8 versus 2.7, p < .001), night sweats (2.7 versus 2.2, p = .02), pruritus (3.3 versus 2.7, p < .001), and bone pain (2.6 versus 2.0, p < .001) were significantly more severe among patients receiving phlebotomy compared with patients undergoing observation or alternative treatments. Fever was significantly more severe among those not receiving phlebotomy (0.3 versus 0.4, p = .03). Evaluation of MPN 10 total sum symptom score was significantly more severe in the individuals receiving phlebotomy (27.7 versus 22.2, p < .001).
Figure 2.
Evaluation of phlebotomy on symptom burden for individuals in the MPN-SAF cohort. Phlebotomy (yes/no) was compared by (A) individual MPN-10 symptom items and (B) the MPN-10 total scores. *p < .05, **p < .001.
Discussion
This secondary analysis among two large cohorts of PV patients suggests that there may be an impact of HCT control on symptom burden. The previous CYTO PV study demonstrated that individuals with a HCT goal of 45–50% experienced an overall four times increased risk of death from cardiovascular events as compared with their counterparts with a HCT goal of less than 45%. Thus, CYTO PV trial answered a pivotal question of optimal HCT targets in the prevention of PV related morbidity and mortality. It is notable that the authors of the study hypothesized that the increased HCT would be non-inferior for the prevention of vascular events but superior in terms of symptom improvement. Over the year of prospective follow-up among patients in the CYTO PV study, no changes in symptom burden were observed between those undergoing strict or lenient HCT control. This lack of effect is likely due to the variable and often extended amount of time required to develop iron deficiency and the variable use of phlebotomy in both treatment groups to keep HCT in the respective range. Among the MPN-SAF cohort, severe symptom burden assessed via a symptom burden cutoff of an individual MPN-10 symptom item of greater than 5/10 was associated with a significantly lower mean HCT. It is notable that the CYTO-PV cohort also had significantly higher baseline HCT, hemoglobin, and platelets than in the MPN-SAF cohort, which is likely a result of the longer treatment duration and more consistent treatment intensity among PV patients in the MPN-SAF cohort.
Despite having patient data from two large cohorts and a comprehensive symptom assessment, limitations of this study included the inability to control for treatment type (i.e. phlebotomy versus pharmacologic therapy). Additionally, the lack of data available regarding underlying iron stores or from related indicators such as mean corpuscular volume or serum ferritin inhibited us from being able to assess for iron deficiency. Future studies should evaluate whether symptom burden alterations exist among PV patients with confirmed iron deficiency.
The symptom burden observed in PV patients is multifactorial, with the greatest contribution being due to cytokine dysfunction, splenomegaly, and microthrombosis stemming from hyperviscosity. However, the results of this analysis suggest that there is an additional contributing factor, which is that of therapy-related effects. At the time of the CYTO-PV and MPN-SAF cohort data collection, the primary treatments of PV included toxic agents and phlebotomy. The pharmacological interventions for PV include hydroxyurea, interferon, radiophosphorus, busulfan, and pipobroman, all of which may be associated with burdensome toxicity profiles. Worsened symptom burden in those undergoing aggressive treatment of PV is consistent with a recent analysis by Geyer et al. [11] evaluating symptom burden among 1224 PV patients. This analysis found that patients who were undergoing treatment to target adequate HCT control with hydroxyurea and phlebotomy demonstrated a bothersome and severe symptom burden profile that was significantly higher than those not receiving these interventions.
We can conclude from our lack of significant symptom burden seen in the strict hematocrit control group in the CYTO PV study that there is a lead time by which phlebotomy would result in symptomatic iron deficiency in PV patients. Given that each unit of blood phlebotomized contains approximately 200 mg of elemental iron,[12] phlebotomies preformed every 8 weeks would lead to the removal of 1.4 g of iron over the course of 12 months. Given that the total body iron content is approximately 1.5–6 g,[13] it would take approximately this same interval of time for the patient with normal iron stores to develop symptomatic iron deficiency. It is known that PV patients with normal hemoglobin levels undergoing phlebotomies have been found to have a high rate of symptoms associated with iron deficiency including restless leg syndrome (29.6% among PV patients and 10% in the general community).[14]
This analysis represents a timely assessment of the impact of HCT control on symptom burden given the FDA approval of JAK2 inhibitors in 2014 as an alternative therapeutic agent in those who are intolerant or resistant to hydroxyurea [15] based on the results of the RESPONSE trail which demonstrated that JAK2-directed therapy improves spleen size, hematocrit control, and symptom burden among PV patients.[16] The current treatment paradigm in PV is that treatment should be driven by assessment of thrombotic risk.[17] However, we suggest that symptom burden be assessed and taken into strong consideration when contemplating the choice of therapy in the second-line setting for PV. If significant symptom burden is found, we encourage clinicians to discuss the risks and benefits to JAK2 inhibitor therapy with patients and that this therapy be given priority over other therapies that are associated with worsened symptom burden if appropriate.[11] Future trials should consider JAK2 inhibitor therapy among individuals with severe symptom burden in the first line setting.[18]
Footnotes
Potential conflict of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article at http://dx.doi.org/10.1080/10428194.2016.1246733.
References
- [1].Vainchenker W, Delhommeau F, Constantinescu SN, et al. New mutations and pathogenesis of myeloproliferative neoplasms. Blood. 2011;118:1723–1735. [DOI] [PubMed] [Google Scholar]
- [2].Landolfi R, Cipriani MC, Novarese L. Thrombosis and bleeding in polycythemia vera and essential thrombocythemia: pathogenetic mechanisms and prevention. Best Pract Res Clin Haematol. 2006;19: 617–633. [DOI] [PubMed] [Google Scholar]
- [3].Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer. 2007;109:68–76. [DOI] [PubMed] [Google Scholar]
- [4].Spivak JL. Polycythemia vera: myths, mechanisms, and management. Blood. 2002;100:4272–4290. [DOI] [PubMed] [Google Scholar]
- [5].Passamonti F How I treat polycythemia vera. Blood. 2012;120:275–284. [DOI] [PubMed] [Google Scholar]
- [6].Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368:22–33. [DOI] [PubMed] [Google Scholar]
- [7].Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30:4098–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118:401–408. [DOI] [PubMed] [Google Scholar]
- [9].Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. [DOI] [PubMed] [Google Scholar]
- [10].Scherber R, Dueck A, Mesa R. Symptoms, risk classification, and spleen size in JAK2 inhibitor naive myelofibrosis: implications for JAK2 inhibitor treatment. EHA Learning Center; 2016. a132894. [Google Scholar]
- [11].Geyer H, Scherber R, Kosiorek H, et al. Symptomatic profiles of patients with polycythemia vera: implications of inadequately controlled disease. J Clin Oncol. 2016;34:151–159. [DOI] [PubMed] [Google Scholar]
- [12].Porter JB. Practical management of iron overload. Br J Haematol. 2001;115:239–252. [DOI] [PubMed] [Google Scholar]
- [13].Hunt JR, Zito CA, Johnson LK. Body iron excretion by healthy men and women. Am J Clin Nutr. 2009;89:1792–1798. [DOI] [PubMed] [Google Scholar]
- [14].Tobiasson M, Alyass B, Soderlund S, et al. High prevalence of restless legs syndrome among patients with polycytemia vera treated with venesectio. Med Oncol. 2010;27:105–107. [DOI] [PubMed] [Google Scholar]
- [15].FDA news release. FDA approves Jakafi to treat patients with a chronic type of bone marrow disease. 2014. [Internet]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm425677.htm.
- [16].Geyer HL, Mesa RA. Therapy for myeloproliferative neoplasms: when, which agent, and how? Blood. 2014;124:3529–3537. [DOI] [PubMed] [Google Scholar]
- [17].Barbui T, Finazzi G. Therapy for polycythemia vera and essential thrombocythemia is driven by the cardiovascular risk. Semin Thromb Hemost. 2007;33:321–329. [DOI] [PubMed] [Google Scholar]
- [18].Verstovsek S, Passamonti F, Rambaldi A, et al. A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 Inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea. Cancer. 2014;120:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]