Abstract
Ruxolitinib provided clinical benefit for patients with myelofibrosis in clinical trials. However, assessing benefit in community settings remains challenging. This exploratory analysis evaluated results from the phase III COMFORT (Controlled Myelofibrosis Study With Oral JAK inhibitor Treatment)-I trial using practical measures (n = 286). Spleen length alone was insufficient for identifying all patients who received clinical benefit, emphasizing the importance of using multiple myelofibrosis assessment methods in the community setting.
Background:
The phase III COMFORT (Controlled Myelofibrosis Study With Oral JAK inhibitor Treatment)-I and COMFORT-II trials in patients with intermediate-2 or high-risk myelofibrosis (MF) showed that ruxolitinib was superior to placebo and best available therapy, respectively, for improvements in spleen volume, MF-related symptoms, and overall survival (OS). However, patients managed in community settings might not have access to the methods used in the COMFORT trials. In this exploratory analysis we summarize efficacy findings of COMFORT-I using practical, community-oriented measures of patient outcomes.
Patients and Methods:
In this post hoc analysis of data from COMFORT-I we evaluated changes from baseline to week 12 in spleen size (palpable length and volume), patient-reported outcomes (Patient Global Impression of Change; Myelofibrosis Symptom Assessment Form; Patient-Reported Outcomes Measurement System Fatigue Scale), body weight, and serum albumin levels in 5 subgroups of ruxolitinib-treated patients on the basis of week 12 spleen length changes from baseline: (1-4) ≥ 50%, 25% to < 50%, 10% to < 25%, or < 10% reduction; and (5) worsening. OS was evaluated in ruxolitinib-treated patients with week 12 spleen length reductions from baseline ≥ 50%, 25% to < 50%, or < 25% (including worsening).
Results:
In all spleen length subgroups, including patients with worsening spleen length at week 12, ruxolitinib (n = 150) was associated with improvements in spleen volume, patient-reported symptom burden, body weight, and serum albumin levels. Greater reductions in spleen length were associated with prolonged OS.
Conclusion:
A variety of assessment methods beyond palpable spleen length that are easily accessible in the community setting might be useful in evaluating the clinical benefit of ruxolitinib over time in patients with MF.
Keywords: Community hospitals, Janus kinases, Myelofibrosis, Myeloproliferative disorders, Splenomegaly
Introduction
Myelofibrosis (MF) is a chronic, life-shortening myeloproliferative neoplasm (MPN) associated with progressive bone marrow fibrosis and extramedullary hematopoiesis.1,2 MF, particularly at advanced stages, is commonly associated with marked splenomegaly3 and debilitating symptoms attributable to the enlarged spleen itself4,5 and/or circulating levels of proinflammatory cytokines.6 The Janus kinase (JAK)-1/JAK2 inhibitor ruxolitinib provided rapid reductions in spleen volume and symptom burden in patients with intermediate-2 or high-risk MF in 2 pivotal phase III studies: COMFORT (Controlled Myelofibrosis Study With Oral JAK inhibitor Treatment)-I,7 which compared the efficacy and safety of ruxolitinib and placebo, and COMFORT-II,8 which compared ruxolitinib and best available therapy. In both studies, treatment with ruxolitinib was associated with improvements in measures of health-related quality of life9,10 and a survival advantage11,12; overall treatment benefit was maintained with long-term therapy.13,14
In the COMFORT-I study, treatment efficacy was evaluated using magnetic resonance imaging (MRI) or computed tomography (CT) imaging to measure changes in spleen volume and the modified Myelofibrosis Symptom Assessment Form (MFSAF) version 2.0 to assess changes in MF-related symptom burden. However, these assessment methods might not be used in all clinical practices. Spleen size is more routinely assessed using palpation, and the evaluation of symptom burden might not involve a formal questionnaire. In this exploratory analysis, we sought to summarize key efficacy findings of COMFORT-I by using practical, community-oriented measures of spleen size and symptom burden reduction and to analyze the relationship between improvements in these measures and previously reported clinical efficacy in COMFORT-I.
Patients and Methods
Patients and Treatment
Study design and eligibility criteria of the COMFORT-I study (ClinicalTrials.gov identifier NCT00952289) have been described previously.7 Eligible patients were 18 years old or older and had intermediate-2 or high-risk primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF with a life expectancy ≥ 6 months, platelet counts ≥ 100 × 109/L, and palpable spleen length ≥ 5 cm below the left costal margin. Patients were randomized to receive placebo (n = 154) or ruxolitinib (n = 155). The primary end point was the percentage of patients who had a ≥ 35% reduction from baseline in spleen volume, determined using MRI or CT imaging at week 24. The starting dose of ruxolitinib was 15 mg twice daily for patients with a baseline platelet count of 100 to 200 × 109/L and 20 mg twice daily for those with a baseline platelet count > 200 × 109/L. All patients randomized to placebo crossed over to ruxolitinib (median time to crossover, 41 weeks) or discontinued within 3 months of the primary data analysis,11 which occurred when all patients had completed 24 weeks of treatment and half of the patients continuing in the study had completed 36 weeks of treatment.7 Therefore, limited data for patients in the placebo group were available beyond week 48.
This post hoc analysis of the 5-year data cutoff (ie, when all patients reached the 5-year visit or discontinued participation) from COMFORT-I included all patients who had evaluable palpable spleen length data at baseline and week 12. Spleen length changes at week 12 instead of week 24 (the original primary data time point for this study) were evaluated to determine if earlier spleen size changes are associated with long-term patient outcomes. Patients randomized to placebo who crossed over to ruxolitinib before week 12 were excluded from the analysis. In the current analysis, spleen response was solely on the basis of examination using palpation, without a requirement for confirmation using MRI or CT imaging. Investigators determined the edge of the spleen using palpation and measured the distance from the left costal margin to the point of greatest splenic protrusion using a soft ruler.
For evaluation of overall survival, patients in the ruxolitinib arm were stratified into 3 mutually exclusive subgroups on the basis of whether they achieved a ≥ 50%, 25% to < 50%, or < 25% (including worsening) reduction from baseline in spleen length at week 12. For evaluation of all other outcomes included in this analysis, patients in the ruxolitinib group were evaluated in 5 mutually exclusive subgroups on the basis of their spleen length status at week 12: (1-4) ≥ 50%, 25% to < 50%, 10% to < 25%, or < 10% reduction from baseline; and (5) patients with worsening spleen length. These subgroups were chosen on the basis of previously published results with similar patient subgroups defined according to improvement in spleen size, which were associated with improvements in overall survival15 and symptoms.9 Because of the limited number of patients in the placebo group who achieved improvements in spleen length, evaluable patients randomized to placebo were not stratified into subgroups.
The COMFORT-I protocol was approved by each participating site’s institutional review board. The study was conducted per the International Conference on Harmonization guidelines for Good Clinical Practice. All patients provided written informed consent.
Assessments and Analyses
In this exploratory analysis we evaluated mean percentage changes from baseline to week 12 in spleen length measured via palpation and its association with mean percentage changes from baseline in spleen volume assessed using MRI or CT imaging at week 12 and subsequent spleen volume assessments.
Patient-reported outcomes were captured using the Patient Global Impression of Change (PGIC), modified MFSAF, and Patient-Reported Outcomes Measurement System (PROMIS) Fatigue Scale questionnaires. The PGIC evaluated patient opinion of treatment benefit with the following response options: “very much improved,” “much improved,” “minimally improved,” “no change,” “minimally worse,” “much worse,” and “very much worse.”16 The patients who reported that their disease was “very much improved” or “much improved” were assessed at week 12. The MFSAF was used to evaluate 7 MF-related symptoms (night sweats, itchiness, abdominal discomfort, pain under ribs, early satiety, muscle or bone pain, and inactivity) on a scale from 0 (absent) to 10 (worst imaginable).7,17 The total symptom score (TSS) was the sum of all symptoms, excluding inactivity. The mean percentage change from baseline to week 12 was assessed for MFSAF TSS and individual symptoms. The PROMIS Fatigue Scale includes 7 items used to measure the frequency or effect of fatigue, each graded on a scale from 1 (never) to 5 (always).18 Mean change from baseline to week 12 was evaluated for the average PROMIS Fatigue Scale scores.
Mean change from baseline to week 12 in body weight and serum albumin levels was evaluated.
Overall survival at the 5-year data cutoff was analyzed using the Kaplan—Meier method.
Results
Overall, 150 of 155 patients randomized to ruxolitinib and 136 of 154 randomized to placebo had spleen length data at baseline and week 12 and were therefore evaluable for this analysis. Patient demographic and disease characteristics at baseline were generally similar between the ruxolitinib subgroups (Table 1).
Table 1.
Demographic and Disease Characterstic at Baseline According to Degree of Treatment Benfit at Week 12 in the Ruxolitinibn Arm
| Ruxolitinib (n = 150) | Placebo (n = 136) |
|||||
|---|---|---|---|---|---|---|
| Reduction From Baseline to Week 12 in Spleen Length | ||||||
| ≥50% (n = 57) |
25% to <50% (n = 42) |
10% to <25% (n = 25) |
<10% (n = 16) | Worsening (n = 10) |
||
| Mean Age (SD), y | 66.2 (8.7) | 67.0 (7.9) | 68.6 (9.3) | 64.6 (9.2) | 67.3 (12.0) | 68.8 (8.0) |
| Female Sex, n (%) | 29 (50.9) | 23 (54.8) | 12 (48.0) | 6 (37.5) | 3 (30.0) | 58 (42.6) |
| IPSS Risk | ||||||
| Intermediate-2 | 24 (42.1) | 16 (38.1) | 8 (32.0) | 7 (43.8) | 7 (70.0) | 47 (34.6) |
| High | 33 (57.9) | 26 (61.9) | 17 (68.0) | 9 (56.3) | 3 (30.0) | 89 (65.4) |
| Blood Transfusion Need, n (%) | 40 (70.2) | 30 (71.4) | 19 (76.0) | 9 (56.3) | 8 (80.0) | 96 (70.6) |
| Mean Body Weight (SD), kg | 72.7 (16.1) | 71.3 (15.8) | 72.3 (20.8) | 72.2 (11.1) | 76.3 (16.7) | 72.0 (13.6) |
| Mean Albumin (SD), mg/dL | 4346 (341.2) | 4295 (326.1) | 4236 (376.3) | 3988 (488.4) | 4360 (419.5) | 4186 (404.1) |
| Mean Hemoglobin (SD), g/dL | 10.8 (2.1) | 10.8 (1.8) | 11.2 (1.9) | 9.9 (1.8) | 11.2 (2.7) | 10.7 (2.1) |
| Mean Platelet Count (SD), × 109/L | 360.1 (215.4) | 326.7 (204.6) | 284.1 (208.9) | 310.4 (189.5) | 253.6 (92.4) | 270.1 (142.5) |
| Platelet Count ≤200 × 109/L, n (%) | 14 (24.6) | 13 (31.0) | 15 (60.0) | 5 (31.3) | 3 (30.0) | 55 (40.4) |
| Mean Spleen Volume (SD), cm3 | 2240.8 (1056.2) | 2831.1 (1077.9) | 3472.0 (1449.6) | 3236.4 (1278.1) | 3004.5 (1192.2) | 2789.8 (1343.3) |
| Mean MFSAF Total Symptom Score (SD) | 15.1 (10.8) | 20.0 (10.3) | 18.5 (10.7) | 22.1 (11.6) | 21.1 (11.8) | 16.7 (11.8) |
| Mean EORTC QLQ-C30 (SD) | ||||||
| Global health status/QoL | 54.1 (24.7) | 52.9 (22.7) | 49.0 (22.3) | 48.7 (21.8) | 52.5 (17.6) | 53.4 (22.3) |
| Fatigue | 47.0 (25.0) | 50.0 (22.9) | 53.7 (26.2) | 55.6 (27.6) | 66.7 (22.8) | 52.8 (25.8) |
Abbreviations: EORTC QLQ-C30 = European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30; IPSS = International Prognostic Scoring System; MFSAF = Myelofibrosis Symptom Assessment Form; QoL = quality of life.
At week 12, 38.0% of evaluable patients (57 of 150) achieved a ≥ 50% reduction in spleen length (measured via palpation), 28.0% (42 of 150) achieved a 25% to < 50% reduction, 16.7% (25 of 150) a 10% to < 25% reduction, and 10.7% (16 of 150) a < 10% reduction; a worsening in spleen length occurred in 6.7% (10 of 150) of evaluable patients. We next analyzed whether there was an association between reduction in spleen length using palpation (assessed by physicians) and spleen volume according to MRI/CT imaging. Subgroups of patients who had a reduction in spleen length with ruxolitinib therapy at week 12 also had corresponding reductions in spleen volume (Figure 1A). However, patients treated with ruxolitinib who experienced worsening spleen length assessed using palpation at week 12 had a reduction in splenomegaly on the basis of spleen volume measurement (Figure 1A). All ruxolitinib subgroups, including those with worsening spleen length at week 12, had mean reductions in spleen volume at week 12 that were maintained over time (Figure 1B). In comparison, patients treated with placebo had mean increases in palpable spleen length (Figure 1A) and spleen volume (Figure 1B).
Figure 1.
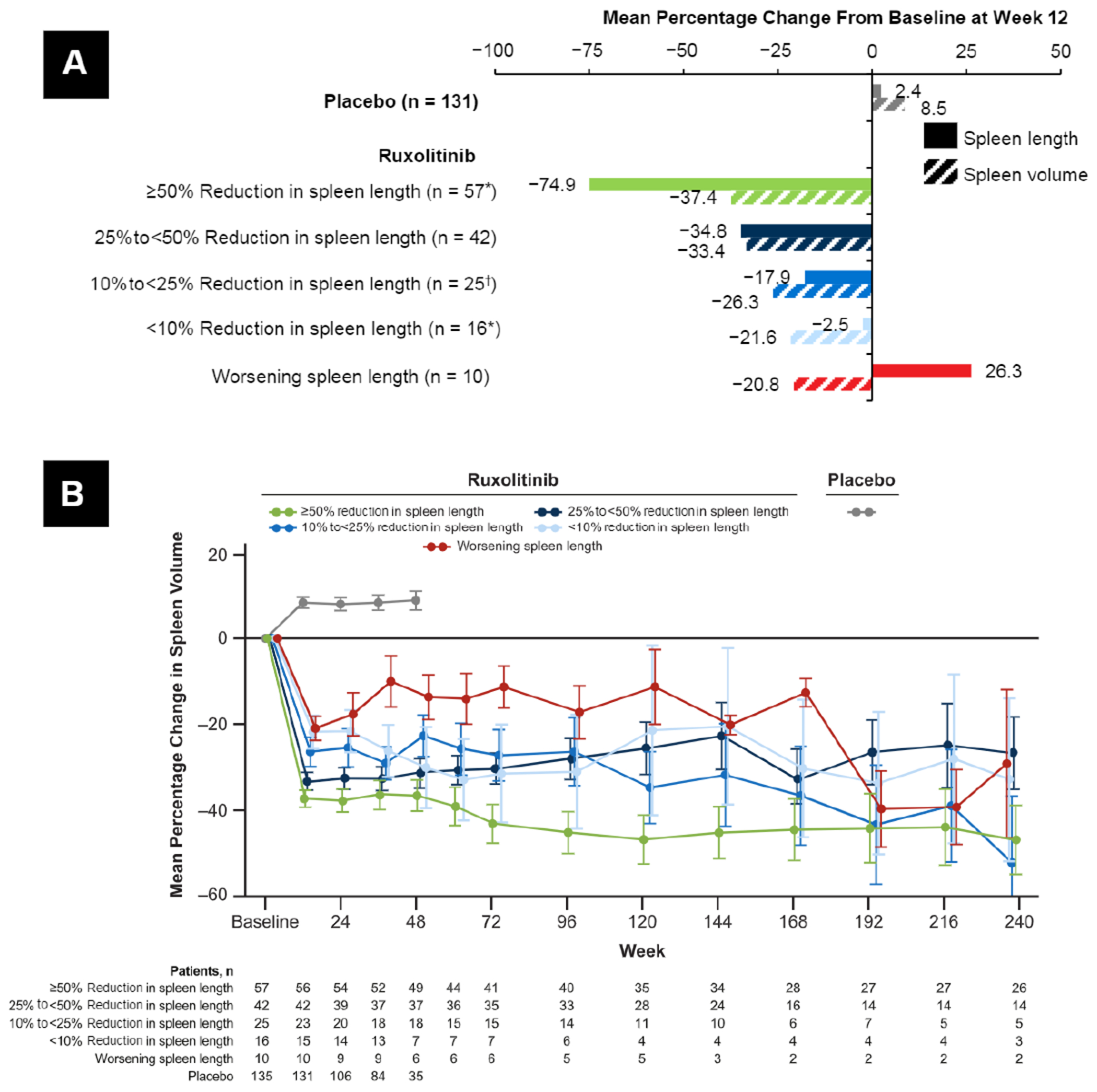
Change From Baseline in Spleen Size. (A) Mean Percentage Change From Baseline to Week 12 in Spleen Length and Spleen Volume. (B) Mean (SE) Percentage Change From Baseline in Spleen Volume Over Time *1 Patient Did Not Have an Evaluable Spleen Volume Assessement and Was Excluded From the Spleen Volume Calculation. †2 patients Did Not Have Evaluable Spleen Volume Assessments and Were Excluded From the Spleen Volume Calculation
Treatment with ruxolitinib was associated with improvements in patient-reported symptom burden. Compared with placebo, greater proportions of patients in all ruxolitinib subgroups stratified according to change in spleen length (measured via palpation), including those with worsening spleen length, reported that their disease condition was much improved or very much improved on the PGIC from baseline to week 12 (Figure 2). Similarly, all ruxolitinib subgroups had mean improvements from baseline to week 12 in the MFSAF TSS and most individual symptoms (Figure 3), as well as the PROMIS Fatigue Scale score (Figure 4), including patients with worsening spleen length.
Figure 2.
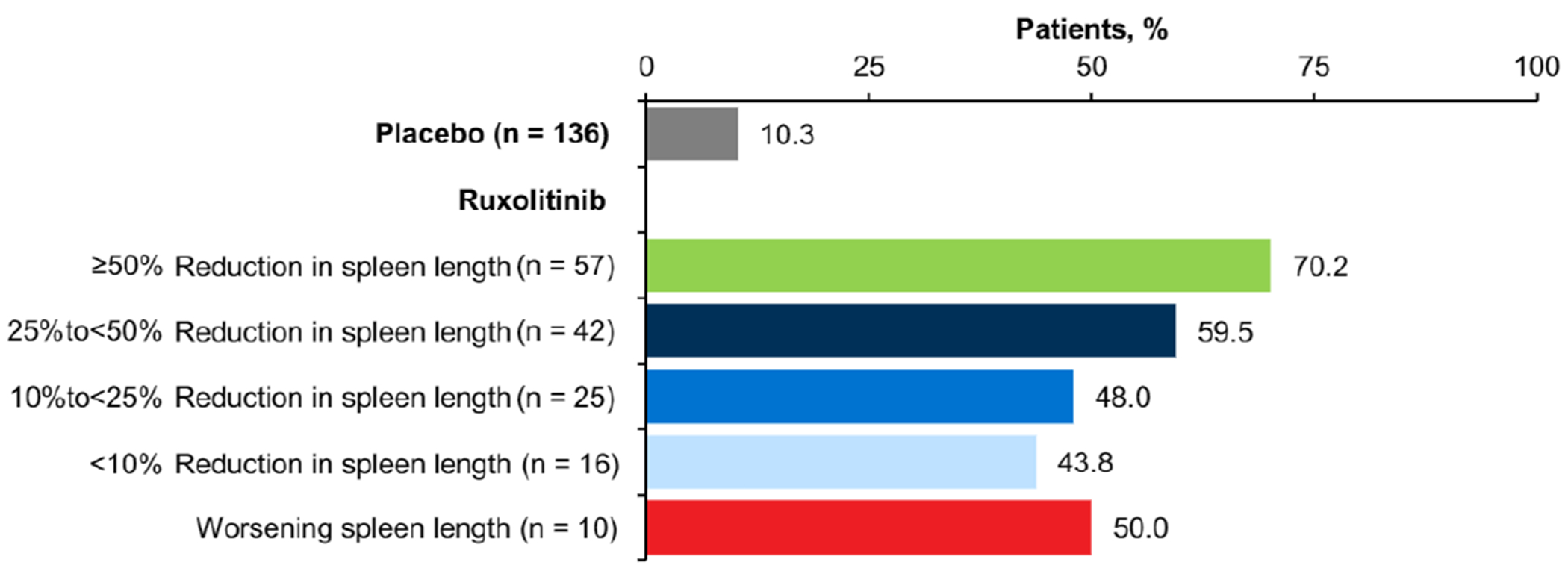
Proportion of Patients Reporting “Much Improved” or “Very Much Improved” on the PGIC at Week 12
Abbreviation: PGIC = Patient Global Impression of Change
Figure 3.
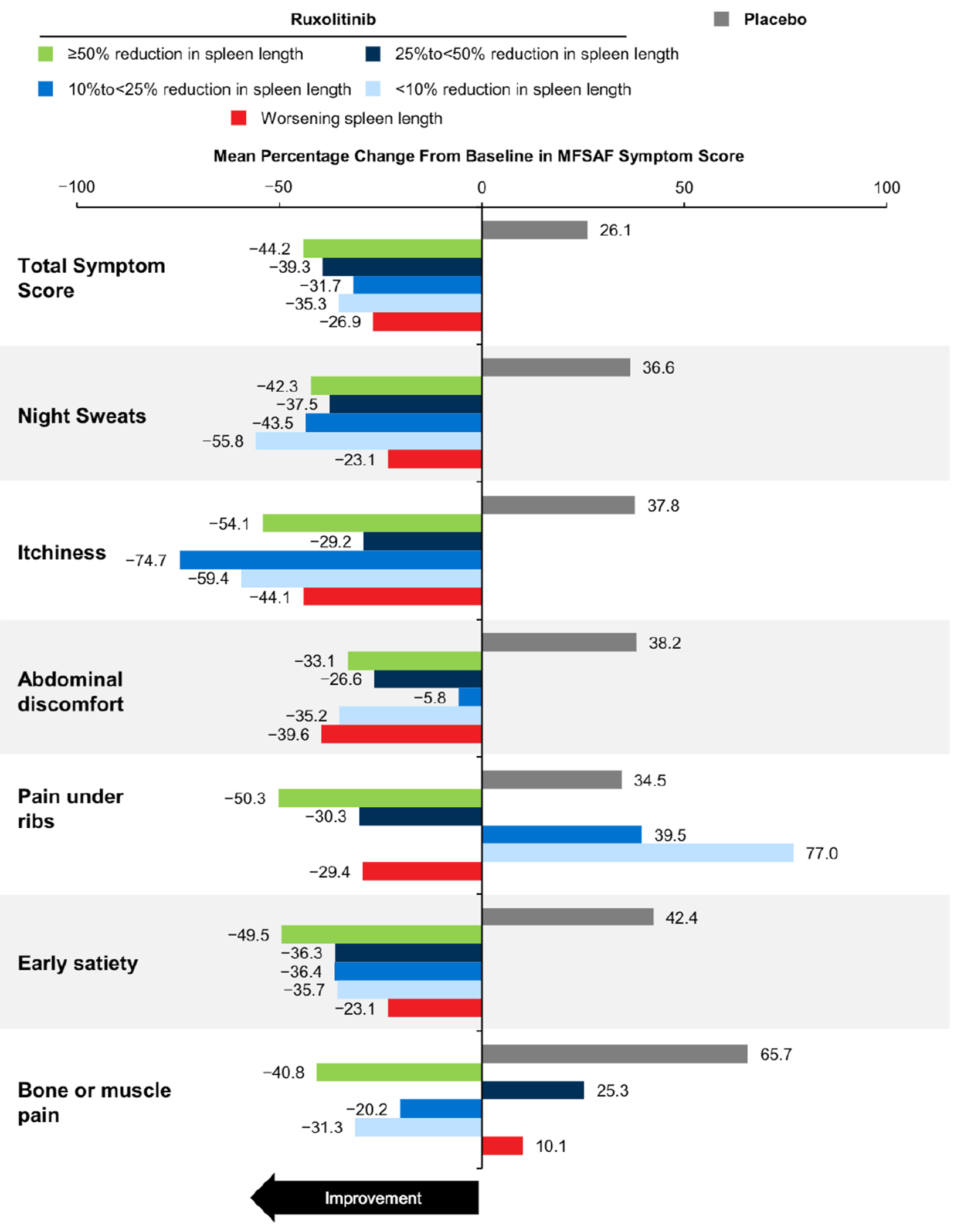
Mean Percentage Change From Baseline to Week 12 MFSAF TSS and Individual Symptom Score. The Number of Evaluable patients in Each Group Ranged as Follows. Ruxolitinib: ≥ 50% Reduction, 40 to 53; 25% to < 50% Reduction, 34 to 41; 10% to < 25% Reduction,14 to 21;<10% Reduction, 9 to 15; Worsening , 6 to 9; Placebo: 97 to 129
Abbreviations: MFSAF = Myelofibrosis Symptom Assessment Form; TSS = total symptom score.
Figure 4.
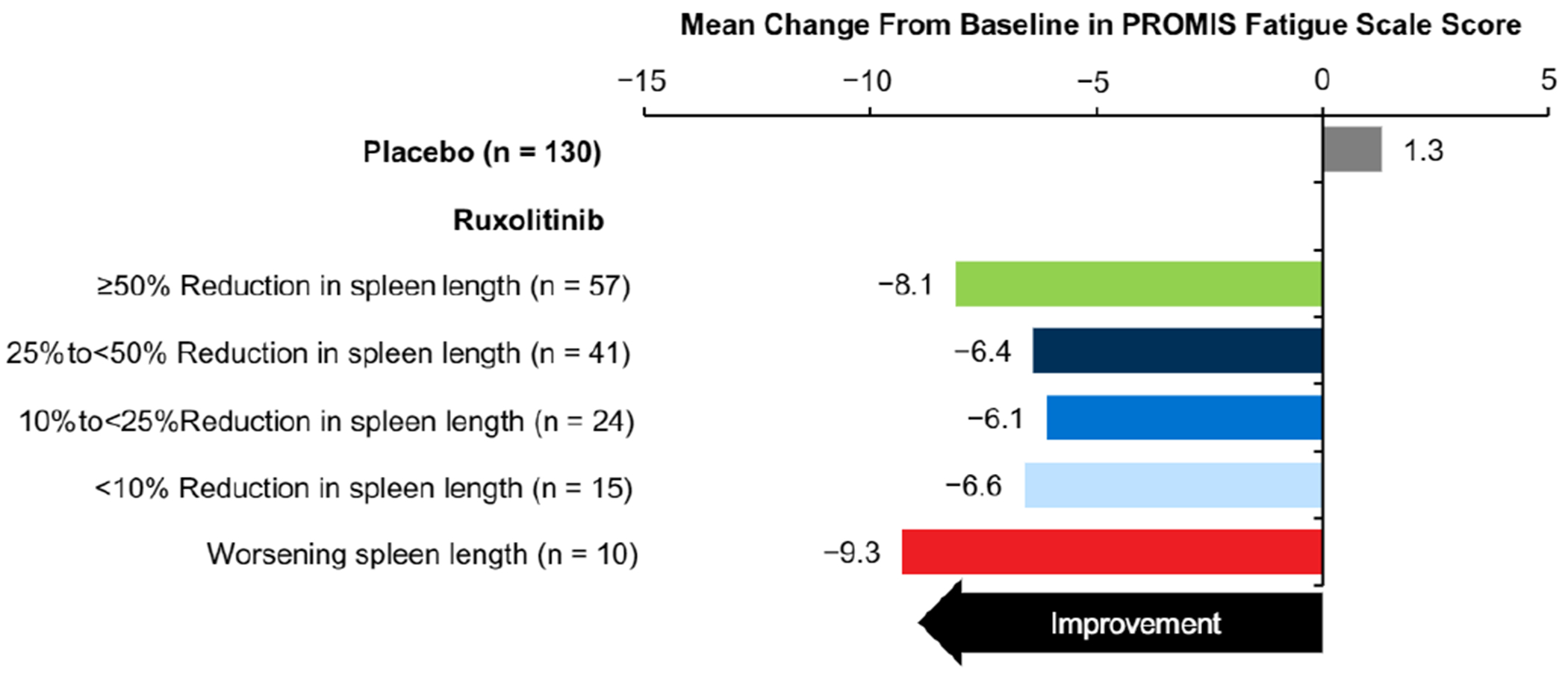
Mean Change From Baseline to Week 12 in PROMIS Fatigue Scale Score
Abbreviation: PROMIS = Patient-Reported Outcomes Measurement System.
Mean body weight (Figure 5A) and serum albumin levels (Figure 5B) improved for all ruxolitinib subgroups between baseline and week 12, whereas patients in the placebo group experienced worsening during this period.
Figure 5.
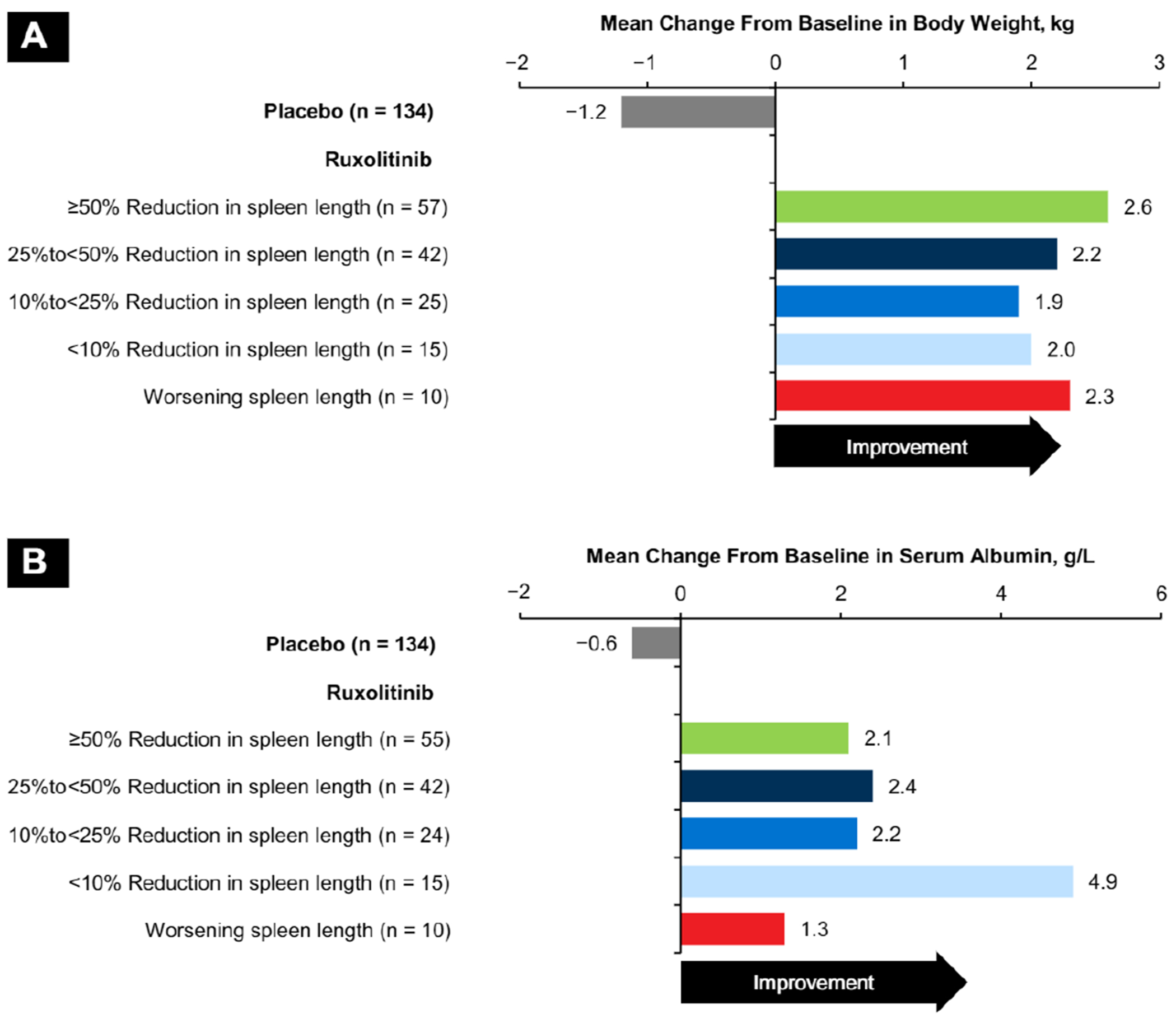
Mean Change From Baseline to Week 12 Metabolic Measures Assessed Measures Were (A) Body Weight and (B) Serum Albumin
Overall survival in the ruxolitinib randomized group at the 5-year data cutoff revealed a trend toward prolonged survival in patients with greater reductions from baseline in spleen length at week 12 (Figure 6).
Figure 6.
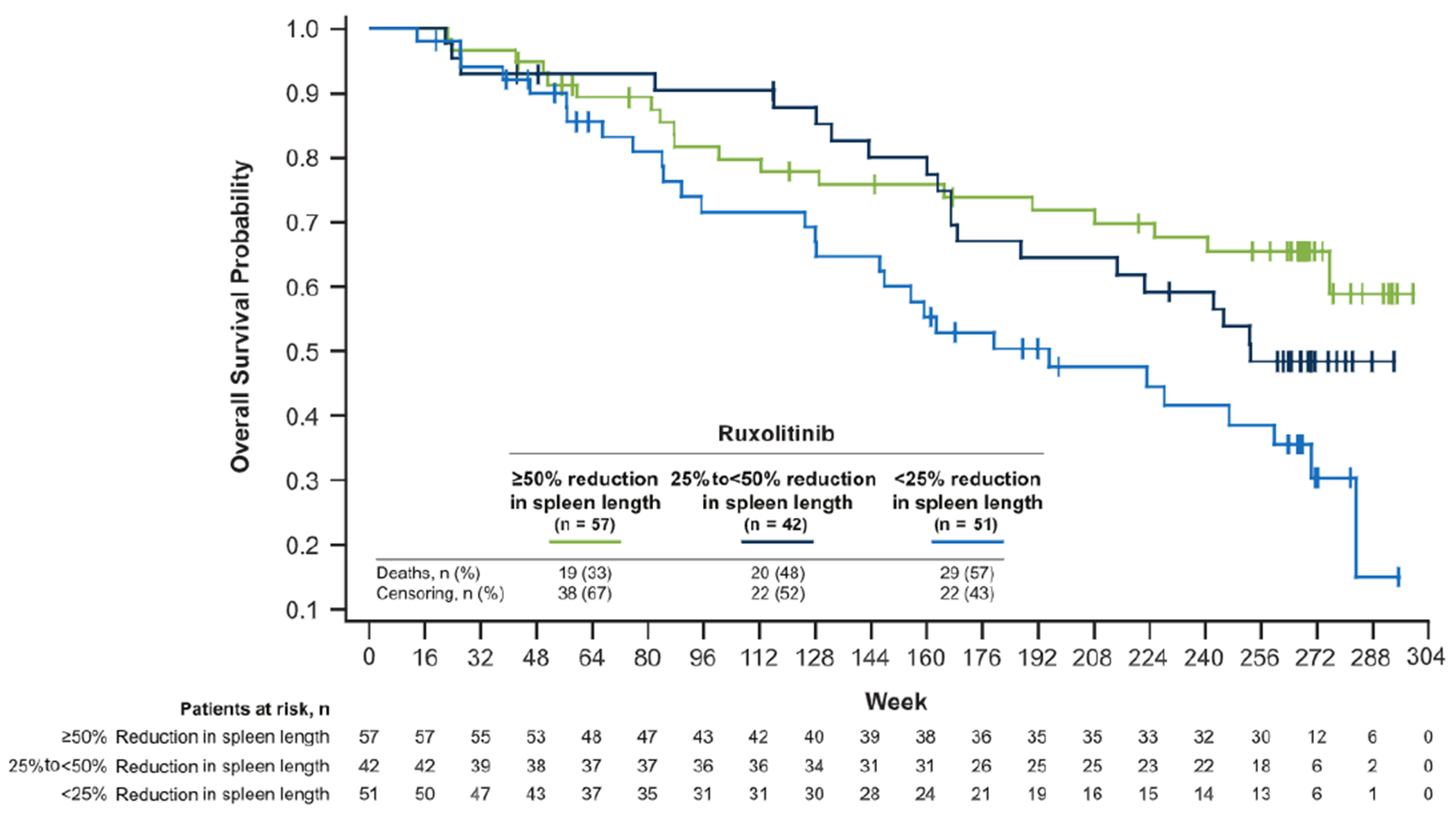
Overall Survial. Patients in the Ruxolitinib Group Were Analyzed in 3 Subgroups on the Basis of Reduction From Baseline in Spleen Length at Week 12: ≥ 50% 25% to 50% , and < 25% (including Woresening Spleen Length)
Discussion
The International Working Group-Myeloproliferative Neoplasms Research and Treatment and European LeukemiaNet criteria for measuring treatment response in patients with MF19 that were used in the COMFORT-I primary analysis7 are important for evaluating the effectiveness of treatment for MF in clinical trials. However, these criteria require the use of advanced assessment methods (eg, imaging techniques, bone marrow analyses) that might not be available in many community-based practices. Furthermore, these consensus-based definitions of response were designed to standardize response assessments in clinical trials, not to measure treatment efficacy in routine clinical practice.19 This exploratory analysis of data from COMFORT-I suggests that changes in spleen length measured using palpation are not necessarily correlated with changes in spleen volume according to MRI/CT imaging among patients with intermediate-2 or high-risk MF treated with ruxolitinib. Indeed, patients treated with ruxolitinib who achieved minimal or no improvement in spleen length at week 12 benefited from ruxolitinib therapy as evidenced by improvements in volumetric response, MF-related symptoms, body weight, and serum albumin levels.
Although a full assessment of MF-related symptoms with a validated tool such as the MFSAF is preferred, outside of clinical trials, extensive or systematic assessments of specific symptoms might not be conducted. In the MPN Landmark survey, 51% of patients with MF reported that their physician only asked how they were feeling, and only 35% reported that their physician asked about a full list of symptoms.20 The PGIC, a tool that evaluates patient opinions on treatment benefit, is the questionnaire that most closely resembles clinical visits in which the physician simply asks how a patient is feeling. In the current analysis, all ruxolitinib subgroups, including those with worsening spleen length, reported disease improvement on the PGIC questionnaire, as well as improvements on the MFSAF TSS and PROMIS Fatigue Scale.
Constitutional symptoms such as weight loss have been associated with increased risk of mortality in patients with MF,21 and a previous analysis of COMFORT-I data suggested that increases in body weight were associated with a survival advantage.22 Results from the current analysis agree with a previous exploratory analysis of COMFORT-I data that reported improvements in body weight, total cholesterol, and serum albumin between baseline and week 24 irrespective of improvements in spleen volume and MFSAF TSS.23 The results presented in this report provide additional evidence suggesting that ruxolitinib might help abate the metabolic and/or nutritional concerns associated with MF independent of improvements in palpable spleen length.
Improvements in palpable spleen length might be correlated with overall survival in patients treated with ruxolitinib. Long-term follow up data from patients who received ruxolitinib for MF and associated splenomegaly indicated that patient subgroups with greater reductions in palpable spleen length had prolonged survival.15 A similar trend was observed in the current analysis, in agreement with these findings.
Conclusion
Collectively, results from this exploratory analysis suggest that change from baseline in spleen length alone is not sufficient for identifying all patients who receive clinical benefit from ruxolitinib. Rather, additional assessment methods, which are practical and easily accessible in the community setting, such as patient-reported symptom severity, body weight, and serum albumin levels, might be useful in evaluating and monitoring the clinical benefit of ruxolitinib over time in patients with MF. These findings underscore the importance of using a variety of assessment methods when monitoring response to treatment in patients with MF.
Clinical Practice Points.
Ruxolitinib treatment was associated with clinical benefit in patients with intermediate-2 or high-risk primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF in the COMFORT clinical trials.7–14
However, clinical trials often use tools that are either not available or impractical in community settings (eg, MRI or CT imaging for spleen assessments; extensive patient-reported outcomes questionnaires), making it difficult to identify patients who adequately respond to treatment.
This exploratory analysis of data from the phase III COMFORT-I trial showed that greater reductions in spleen length were associated with longer overall survival; however, measurements of spleen length alone were not sufficient to identify all patients who received clinical benefit from ruxolitinib.
Even among patients with worsening spleen length, ruxolitinib treatment was associated with improvement in several practical measures of clinical benefit that are available in community settings, including global impressions of treatment benefit, symptom severity, body weight, and serum albumin levels.
Physicians managing patients with MF in community settings should use multiple assessment methods to determine whether ruxolitinib treatment provides benefit in individual patients.
Such an approach might encourage optimal ruxolitinib treatment practices, leading to better clinical outcomes in some patients.
Acknowledgments
M.D. Anderson received a cancer center support grant from the National Cancer Institute of the National Institutes of Health (P30 CA016672). The COMFORT-I clinical trial (ClinicalTrials.gov identifier, NCT00952289) was sponsored by Incyte Corporation. Writing assistance was provided by Cory Pfeiffenberger, PhD (Complete Healthcare Communications, LLC [Chadds Ford, PA, a CHC Group Company]) and Roland Tacke, PhD, of Evidence Scientific Solutions, whose work was funded by Incyte Corporation. This work was supported by research funding from Incyte Corporation.
Disclosure
C.B.M.’s institution has received clinical trial support from Incyte Corporation, Novartis, Gilead, and Celgene; C.B.M. received honoraria and served on a speakers bureau and as a consultant for Incyte Corporation and served as a consultant and on a speakers bureau for Novartis. R.S.K. served on advisory committees for and received research funding from Incyte Corporation, was a consultant and advisor for CTI BioPharma and Celgene, and served on a speakers bureau for Novartis. R.A.M. received research funding from Incyte Corporation, CTI, Gilead Sciences, Genentech, Eli Lilly, Promedior, NS Pharma, Sanofi, and Celgene and has served as a consultant for Novartis and ARIAD. W.S. and M.M. are employees and stockholders of Incyte Corporation. S.V. participated in advisory boards for Incyte Corporation and received research support for the conduct of clinical studies from Incyte Corporation, Roche, AstraZeneca, Lilly Oncology, Geron, NS Pharma, Bristol-Myers Squibb, Celgene, Gilead, Seattle Genetics, Promedior, CTI BioPharma Corp, Galena BioPharma, Pfizer, and Genentech.
Footnotes
Presented, in part, at the 56th Annual Meeting of the American Society of Hematology (abstract 3202).
References
- 1.Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia 2008; 22:14–22. [DOI] [PubMed] [Google Scholar]
- 2.Gregory SA, Mesa RA, Hoffman R, Shammo JM. Clinical and laboratory features of myelofibrosis and limitations of current therapies. Clin Adv Hematol Oncol 2011; 9:1–16. [PubMed] [Google Scholar]
- 3.Tefferi A, Lasho TL, Jimma T, et al. One thousand patients with primary myelofibrosis: the Mayo Clinic experience. Mayo Clin Proc 2012; 87:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mesa R, Miller CB, Thyne M, et al. Myeloproliferative neoplasms (MPNs) have a significant impact on patients’ overall health and productivity: the MPN Landmark survey. BMC Cancer 2016; 16:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mesa RA, Shields A, Hare T, et al. Progressive burden of myelofibrosis in untreated patients: assessment of patient-reported outcomes in patients randomized to placebo in the COMFORT-I study. Leuk Res 2013; 37:911–6. [DOI] [PubMed] [Google Scholar]
- 6.Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independendy prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol 2011; 29:1356–63. [DOI] [PubMed] [Google Scholar]
- 7.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012; 366:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 2012; 366:787–98. [DOI] [PubMed] [Google Scholar]
- 9.Mesa RA, Gotlib J, Gupta V, et al. Effect of ruxolitinib therapy on myelofibrosis-related symptoms and other patient-reported outcomes in COMFORT-I: a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2013; 31:1285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison CN, Mesa RA, Kiladjian JJ, et al. Health-related quality of life and symptoms in patients with myelofibrosis treated with ruxolitinib versus best available therapy. Br J Haematol 2013; 162:229–39. [DOI] [PubMed] [Google Scholar]
- 11.Verstovsek S, Mesa RA, Gotlib J, et al. Efficacy, safety, and survival with ruxolitinib in patients with myelofibrosis: results of a median 3-year follow-up of COMFORT-I. Haematologica 2015; 100:479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cervantes F, Vannucchi AM, Kiladjian JJ, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood 2013; 122:4047–53. [DOI] [PubMed] [Google Scholar]
- 13.Harrison CN, Vannucchi AM, Kiladjian JJ, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia 2016; 30:1701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta V, Verstovsek S, Mesa RA, et al. Long-term outcomes of ruxolitinib therapy in patients with myelofibrosis: 5-year update from COMFORT-I. J Clin Oncol 2016; 34 (abstract 7012). [Google Scholar]
- 15.Verstovsek S, Kantarjian HM, Estrov Z, et al. Long-term outcomes of 107 patients with myelofibrosis receiving JAK1/JAK2 inhibitor ruxolitinib: survival advantage in comparison to matched historical controls. Blood 2012; 120:1202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005; 113:9–19. [DOI] [PubMed] [Google Scholar]
- 17.Mesa RA, Kantarjian H, Tefferi A, et al. Evaluating the serial use of the Myelofibrosis Symptom Assessment Form for measuring symptomatic improvement: performance in 87 myelofibrosis patients on a JAK1 and JAK2 inhibitor (INCB018424) clinical trial. Cancer 2011; 117:4869–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol 2010; 63:1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tefferi A, Cervantes F, Mesa R, et al. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood 2013; 122:1395–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mesa R, Miller C, Thyne M, et al. Differences in treatment goals and perception of symptom burden between patients with MPNs and hematologists/oncologists in the United States: findings from the MPN landmark survey. Cancer 2017; 123:449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood 2009; 113:2895–901. [DOI] [PubMed] [Google Scholar]
- 22.Mesa RA, Verstovsek S, Gupta V, et al. Improvement in weight and total cholesterol and their association with survival in ruxolitinib-treated patients with myelofibrosis from COMFORT-I. Blood 2015; 120 (abstract 1733). [Google Scholar]
- 23.Mesa RA, Verstovsek S, Gupta V, et al. Effects of ruxolitinib treatment on metabolic and nutritional parameters in patients with myelofibrosis from COMFORT-I. Clin Lymphoma Myeloma Leuk 2015; 15:214–21, e211. [DOI] [PMC free article] [PubMed] [Google Scholar]


