Abstract
Despite the identification of JAK mutations and the development of targeted inhibitors, there remain significant unmet needs for patients with myeloproliferative neoplasms. Identification of the myeloproliferative neoplasm populations not currently benefiting from JAK inhibitor therapy highlights the therapeutic deficits still present in this heterogeneous stem cell malignancy. While JAK inhibition has provided significant benefits for patients with intermediate-2 or high-risk myelofibrosis and in patients with polycythemia vera in the second-line setting, JAK inhibitor monotherapy is not approved and not appropriate for all patients with myeloproliferative neoplasms. Continued investigation into additional JAK inhibitors, combination therapy, and novel pathway therapeutics remains key to improving outcomes for all patients with myeloproliferative neoplasms. While therapeutic advances in the JAK inhibitor arena or involving alternative pathways are crucial to improving outcomes in myeloproliferative neoplasms, it is also important to reconsider the role of constitutional symptoms in affected patients as an indication for treatment with agents, such as JAK inhibitors, that can mitigate these debilitating symptoms. In this review, we demonstrate the evolving landscape of clinical investigations that address the important therapeutic needs of patients with myeloproliferative neoplasms.
Introduction
Myeloproliferative neoplasms are a group of myeloid malignancies caused by a hematopoietic stem cell clonal proliferation, the main result of which is primarily either erythrocytosis in polycythemia vera, thrombocytosis in essential thrombocythemia, or progressive cytopenias and splenomegaly in primary myelofibrosis.[1] This group of neoplasms is characterized by a lack of the BCR-ABL fusion protein[2] that is associated with chronic myelogenous leukemia; instead, they are identified with one of three mutually exclusive mutations: JAK2 (Figure),[3] CALR,[4] or MPL.[5] The identification of these mutations has led, through the subsequent development of JAK inhibitors, to changes in diagnostic paradigms,[6,7] prognostication,[8,9] and therapeutic interventions.
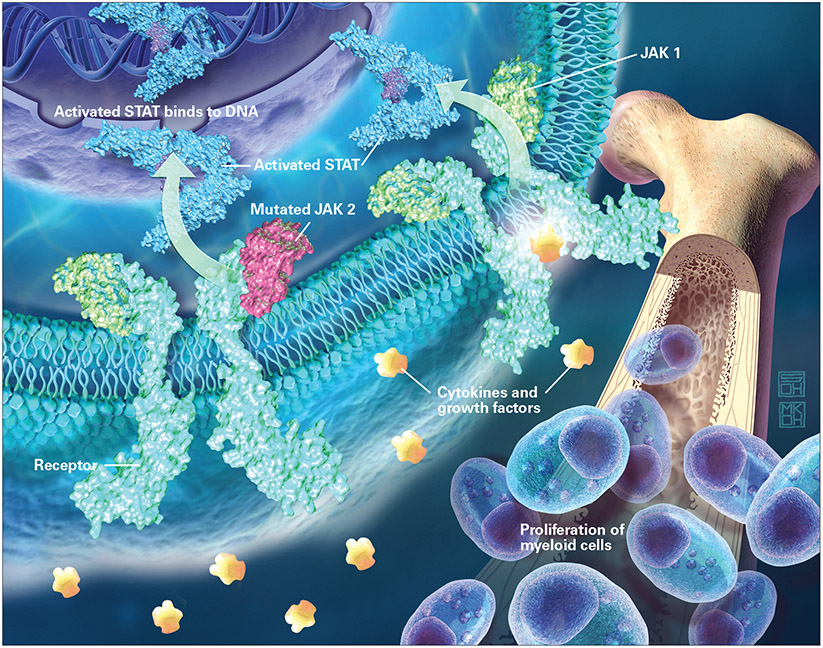
Figure.
Janus-associated kinases (JAKs) bind to receptors at the cell membrane, and when activated, they in turn activate STAT, which translocates to the cell nucleus and there binds to DNA that regulates transcription of selected genes. In the majority of myeloproliferative neoplasms, JAK2 is mutated; this leads to overactive JAK pathway signaling, with a resultant increase in inflammation and progressive myeloproliferation.
The COMFORT-1 and COMFORT-2 trials demonstrated that the JAK1/2 inhibitor ruxolitinib, when used in intermediate-2 or high-risk myelofibrosis, reduced spleen size, improved myelofibrosis-related symptom burden, and improved overall survival (OS).[10,11] The success of JAK2 inhibition in myelofibrosis brings into focus new areas of unmet need in the myeloproliferative neoplasm population. The first group with one of these current unmet needs are those patients who are not candidates for JAK inhibition with ruxolitinib. These patients are deemed not to be candidates either because at baseline they have a disease for which ruxolitinib has not been approved, including high-risk polycythemia vera or essential thrombocythemia, which require first-line cytoreductive therapy, or because they have drug-limiting cytopenias that preclude ruxolitinib use.
Another myeloproliferative neoplasm population with an unmet need comprises those patients for whom JAK inhibitor monotherapy is not effective, or is not effective enough. This group includes patients with advanced myeloproliferative neoplasms (accelerated phase, blast phase, or post–myeloproliferative neoplasm acute myeloid leukemia) characterized by poor outcomes and aggressive disease. These patients need the benefit of JAK inhibition to be incorporated into the context of combination therapy for disease management.
Lastly, there remains the unmet need of myeloproliferative neoplasm patients who have a high systemic symptom burden that requires interventions specifically targeting symptom mitigation. In this review, we demonstrate the evolving landscape of clinical investigation as researchers and clinicians attempt to address the important therapeutic needs of patients with myeloproliferative neoplasms.
Frontline Therapy in Polycythemia Vera
Although ruxolitinib has been approved for use in patients with myelofibrosis, this drug has a limited role in patients with polycythemia vera or essential thrombocythemia. Ruxolitinib is approved for management of polycythemia vera only in patients who have failed prior therapy or who are intolerant of hydroxyurea (HU) pharmacotherapy.[12]
For patients with polycythemia vera, management begins with risk stratification based on age and history of thrombosis. Cytoreduction with pharmacotherapy is recommended for high-risk patients. There are two options for frontline cytoreductive therapy in polycythemia vera and essential thrombocythemia: HU, an oral antimetabolite, or interferon-alfa (IFN), a subcutaneous immunotherapy (Table 1).[13]
Table 1.
Studies of Hydroxyurea (HU) and Interferon Alfa (IFN) in Polycythemia Vera (PV) and Essential Thrombocythemia (ET)
| Study | Population Studied |
Sample Size |
Intervention Agent |
Comparison | Response Rates and Outcomes |
|---|---|---|---|---|---|
| Alvarez-Larran et al[14] | PV | 261 | HU | None | HR: 90% CR: 24% PR: 66% |
| Silver[15] | PV | 55 | Recombinant IFN | None | 100% normalization of platelet count by year 2 27/30 had decreased splenomegaly |
| Stauffer Larsen et al[16] | PV and ET | 102 | IFN | None | CHR: 95% (18/19) in ET group, 68% (51/75) in PV group 74% (76/102) decline in JAK allele burden |
| Hansen et al[19] | PV, ET, and MF | 196 | HU | IFN | CHR: 64% with HU, 78.9% with IFN Higher risk of secondary malignancy with HU |
| Huang et al[18] | PV | 136 | HU | IFN | OHR: 70.8% with HU, 70.3% with IFN 5-yr PFS: 47.6% with HU, 75.9% with IFN |
| PROUD-PV[21] (ongoing) | PV | 257 | HU | Ropeginterferon alfa-2b | Results pending |
| MPD-RC[22] (ongoing) | High-risk PV or ET | 75 | HU | Pegylated IFN | ORR: 69% with HU, 81% with pegylated IFN Splenomegaly reduction: 29% with HU, 71% with pegylated IFN Phlebotomy dependence: 0% with HU, 20% with pegylated IFN |
CHR = complete hematologic response; CR = complete response; HR = hematologic response; MF = myelofibrosis; PFS = progression-free survival; PR = partial response; OHR = overall hematologic response; ORR = overall response rate.
A study of 261 polycythemia vera patients treated with HU for a median of 4.4 years demonstrated a high hematologic response rate of 90% (complete responses [CRs], 24%, and partial responses [PRs], 66%) after a median of 4.6 months of therapy. Median OS with HU was 19 years. Survival was not affected by the ability to attain a CR or PR to therapy; however, inability to achieve a white blood cell count response was associated with decreased survival. Intolerance of HU was uncommon, occurring in 13% of patients, and did not affect survival or risk of transformation.[14]
IFN, specifically recombinant IFN (rIFN) has demonstrated response in patients with myeloproliferative neoplasms. A study of 55 patients with polycythemia vera previously treated with phlebotomy with or without HU and followed for a median of 13 years after initiation of rIFN revealed hematologic response with normalization of platelet count (< 400 × 109/L) in all patients by 1 year, despite 75% of participants having thrombocytosis at drug initiation; the study also showed decreased spleen size in 27 of 30 patients who had baseline splenomegaly.[15] During the study, no patients had thrombohemorrhagic events. Long-term retrospective data on rIFN in 102 Danish patients with myeloproliferative neoplasms, with a median follow-up of 42 months, demonstrated high rates of complete hematologic response (in essential thrombocythemia, 95%; in polycythemia vera, 68%), and 74% of patients demonstrated a decline in JAK2 V617F allele burden.[16] Pegylated IFN (PEG) has been shown to increase regulatory T cells and decrease JAK2 allelic burden.[17] This ability to augment the immune system and decrease the allele burden suggests a disease-modifying effect of IFN.
One observational prospective study followed 136 patients with polycythemia vera who received treatment with either IFN or HU.[18] The overall hematologic response rate was similar for the IFN group and the HU group (70.3% and 70.8%, respectively). A molecular response, shown by a decreased JAK2 V617F allele burden, was more common in the IFN group than in the HU group (54.7% vs 19.4%). The IFN group demonstrated decreased phlebotomy needs and greater improvement in systemic symptoms (vasomotor symptoms, distal paresthesia, and erythromelalgia) compared with the HU group. In patients with a JAK2 mutation, better 5-year progression-free survival (PFS) was seen in the IFN group than in the HU group (75.9% vs 47.6%).
One distinct difference between HU and IFN is the associated risk of secondary malignancies. A retrospective review of 196 patients with myeloproliferative neoplasms treated either with HU, IFN, or both revealed a complete hematologic response in 64.4% of those treated with HU and in 78.9% of those treated with IFN.[19] Treatment with HU was associated with a greater risk of secondary malignancies— including acute myeloid leukemia, myelodysplastic syndrome, and skin cancers—compared with IFN (25% vs 8%); however, the patients treated with HU were older than those in the IFN group. The concern for leukemic transformation resulting from treatment with HU has led the European LeukemiaNet (ELN) to caution against using HU in young patients (< 40 years)[13] and has led others to advocate for prioritizing IFN as the treatment of choice for effecting cytoreduction in polycythemia vera.[20]
Two ongoing phase III studies are attempting to identify the ideal upfront therapy for polycythemia vera and essential thrombocythemia. The PROUD-PV study is a phase III randomized controlled trial comparing ropeginterferon alfa-2b with HU in polycythemia vera. Two hundred fifty-seven patients with polycythemia vera have enrolled. Results of the study are awaited. [21] The Myeloproliferative Disorders Research Consortium phase III trial of frontline PEG vs HU is ongoing in patients with high-risk polycythemia vera or essential thombocythemia.[22] Patients with high-risk newly diagnosed polycythemia vera or essential thrombocythemia, with less than 3 months of prior HU therapy, were enrolled and randomized to either HU or PEG. The primary endpoint was complete hematologic response rate based on ELN criteria. Although 168 patients have enrolled, a planned interim analysis of 75 patients was completed. There was a higher average age in the HU group (66 years, compared with 56 years in the PEG group; P < .01). Overall response rate was 69% in the HU group and 81% in the PEG group (P = .6). Reduction in palpable splenomegaly at 12 months was more common in the PEG group (71%, compared with 29% in the HU group), and phlebotomy independence was more common in the HU group (0% phlebotomy use, compared with 20% in the PEG group). Both groups demonstrated reductions in myeloproliferative neoplasm symptoms, as shown by the myeloproliferative neoplasm symptom assessment form (MPN-SAF) total symptom score (TSS). Further and final results are awaited.
Until these phase III studies report their results, the optimal frontline cytoreductive therapy for polycythemia vera and essential thrombocythemia will remain contested. Since the current evidence does not reveal the ideal frontline cytoreductive therapy for polycythemia vera or essential thrombocythemia, we endorse HU or IFN based on patient preference with regard to toxicity profile and ease of administration. We do recommend adherence to the ELN guidelines that advise caution with regard to HU use in younger patients (< 40 years).
Myelofibrosis With Cytopenias
The JAK1/2 inhibitor ruxolitinib, when used in intermediate-2 or high-risk myelofibrosis, reduced spleen size, improved the burden of myelofibrosis-related symptoms, and improved OS.[10,11] Inclusion criteria for the phase III trials that led to US Food and Drug Administration approval required that the platelet count be above 100 × 109/L. Common hematologic adverse events were anemia and thrombocytopenia, with anemia commonly seen 8 to 12 weeks after drug initiation, after which time blood counts often returned to baseline. The seriousness of the risk of treatment-induced thrombocytopenia is seen in the fact that recommended starting and maximum ruxolitinib doses are dependent on a patient’s baseline platelet count. For example, the maximum ruxolitinib dose for patients with a platelet count > 100 × 109/L is 25 mg twice daily, while patients with a platelet count < 50 × 109/L have a reduced maximum ruxolitinib dose of 10 mg twice daily.[23] In fact, while not a specific contraindication, ruxolitinib is not recommended for use in patients with platelet counts < 50 × 109/L.[24] Despite dosage modifications, patients receiving ruxolitinib experience cytopenias requiring dose interruptions, which can result in previously ameliorated symptoms returning to pretreatment levels.[25]
Pacritinib, an oral inhibitor of JAK2 and FMS-like tyrosine kinase 3 (FLT3), has been studied in myelofibrosis (Table 2). A phase II study of 400-mg pacritinib daily was completed in 31 patients with myelofibrosis.[26] There were no baseline blood count requirements, and 60% of participants were anemic at enrollment, with hemoglobin levels < 10 g/dL. Similarly, 40% of participants were thrombocytopenic, with platelet counts below 100 × 109/L. The 400-mg dose decreased splenomegaly by ≥ 35% on MRI in 23.5% of evaluable patients and improved myeloproliferative neoplasm–related symptoms (based on a ≥ 50% reduction in the MPN-SAF TSS) in 38.9% at week 24. Common grade 3/4 toxicities included diarrhea, fatigue, anemia, bone pain, and thrombocytopenia.
Table 2.
Alternative JAK Inhibitors in Patients With Cytopenias
| Study | Comparison | Patient Population | Outcome | Toxicities |
|---|---|---|---|---|
| PACRITINIB | ||||
| Phase I/II[26] | None | HGB < 10 g/dL: 60% HGB < 8 g/dL: 20% Platelet count < 100 × 109: 40% Platelet count < 50 × 109: 12% |
Improved splenomegaly Improved constitutional symptoms |
Anemia Diarrhea Fatigue Bone pain thrombocytopenia |
| PERSIST-1 (phase III)[27] | BAT | Myelofibrosis Platelet count < 100 × 109: 30% Platelet count < 50 × 109: 15% |
Splenomegaly reduction at week 24: Pacritinib, 19% BAT, 4.7% MPN-SAF symptom improvement: Pacritinib, 24.5% BAT, 6.5% |
Diarrhea Nausea Vomiting |
| PERSIST-2 (phase III)[28] | BAT | Myelofibrosis Platelet count < 100 × 109 |
35% splenic volume reduction MPN symptom improvement |
|
| MOMELOTINIB | ||||
| Phase I/II[29] | None | Intermediate- to high-risk myelofibrosis | Splenomegaly reduction MPN symptom improvement Anemia response |
Diarrhea Peripheral neuropathy Dizziness Thrombocytopenia |
BAT = best available therapy; HGB = hemoglobin; MPN = myeloproliferative neoplasms; MPN-SAF = Myeloproliferative Neoplasm Symptom Assessment Form.
Two phase III studies of pacritinib in myelofibrosis have been conducted in thrombocytopenic populations. PERSIST-1, a randomized phase III trial of 327 patients with myelofibrosis, compared pacritinib with best available therapy (BAT).[27] Cytopenias did not preclude enrollment (30% of participants had a platelet count < 100 × 109/L, and 15% had a platelet count < 50 × 109/L). The primary endpoint of ≥ 35% reduction in splenomegaly at week 24 was achieved in 19% of patients in the pacritinib arm compared with 4.7% of those in the BAT arm (P < .01). Of patients in the pacritinib arm with platelet counts < 100 × 109/L, 16.7% achieved the primary endpoint, and of those in the pacritinib arm with platelet counts < 50 × 109/L, 22.9% achieved the specified reduction in splenomegaly. The MPN-SAF TSS improved in 24.5% of patients in the pacritinib group, compared with 6.5% of those in the BAT group (P < .01). The most common adverse events associated with pacritinib treatment were diarrhea (grade 3, < 5%) and nausea/vomiting (grade 3, < 1%).
The PERSIST-2 trial was a phase III randomized controlled trial of pacritinib vs BAT in patients with myelofibrosis and thrombocytopenia (platelet count < 100 × 109/L).[28] The co-primary endpoints of the study were 35% splenic volume reduction and 50% improvement in constitutional symptoms, based on the MPN-SAF TSS at 24 weeks. A total of 311 patients were enrolled, and 221 were included in an intention-to-treat analysis at week 24; this analysis demonstrated that the pacritinib arm experienced a significantly greater reduction in splenic volume compared with the BAT arm (18% vs 3%; P < .01). Pacritinib dosed once daily did not improve symptom burden by 50% in a significantly greater number of patients than BAT (25% vs 14%; P = .08); however, patients who received pacritinib twice-daily dosing, instead of daily dosing, did demonstrate significantly improved symptom burden compared with patients in the BAT arm (32% vs 14%; P = .01).
These studies suggest the future use of pacritinib in patients with thrombocytopenia who would not be candidates for JAK inhibition with ruxolitinib, including patients with platelet counts below < 100 × 109/L—and especially those with significant thrombocytopenia (platelet counts < 50 × 109/L).
Another JAK inhibitor showing promise in myelofibrosis patients with cytopenias, specifically those with anemia, is momelotinib, a JAK1/2 inhibitor. A phase II study evaluated the safety and efficacy of twice-daily dosing (momelotinib 200 mg) in 61 myelofibrosis patients with intermediate- or high-risk disease.[29] Splenomegaly reduction on MRI was appreciated at 24 weeks in 45.8% (27/59 participants who were assessed with baseline MRI). Constitutional symptoms were improved. Anemia response based on the International Working Group for Myelofibrosis Research and Treatment 2006 criteria (≥ 8 weeks duration) was appreciated in 45% (18/40); anemia response ≥ 12 weeks was seen in 17% (5/30). The most common hematologic toxicity was thrombocytopenia, seen in 39% of patients (grade 3, in 30%). A phase III randomized controlled trial is underway.
This research, in combination with the pacritinib studies in patients with thrombocytopenia, suggests that in the near future we will be able to select JAK inhibitor therapy in myelofibrosis patients based on a hematologic profile, with ruxolitinib as primary therapy for the majority of patients, and pacritinib and momelotinib preferred in patients with ruxolitinib dose–limiting thrombocytopenia or anemia, respectively.
Myeloproliferative Neoplasm–Accelerated Phase, Myeloproliferative Neoplasm–Blast Phase, and Post–Myeloproliferative Neoplasm Acute Myeloid Leukemia
One population of patients with myeloproliferative neoplasms who have yet to reap the benefits of JAK inhibitor monotherapy are those with myeloproliferative neoplasm–accelerated phase, myeloproliferative neoplasm–blast phase, or post–myeloproliferative neoplasm acute myeloid leukemia. Myeloproliferative neoplasm–blast phase and post–myeloproliferative neoplasm acute myeloid leukemia carry a very poor prognosis, with a median OS between 3 and 6 months (Table 3).[30,31] A small study of 6 patients with myeloproliferative neoplasm–blast phase were treated with ruxolitinib and concurrent induction chemotherapy. Although 2 died in aplasia, 4 demonstrated at least a PR, and 3 were able to undergo allogeneic stem cell transplant. [32] Similarly, a case report of 3 patients with myeloproliferative neoplasm—accelerated phase documented results achieved with combination therapy, with 2 of the patients treated with ruxolitinib plus hypomethylating agents (HMA), and 1 treated with ruxolitinib plus low-dose cytarabine. Each of these patients demonstrated resolution of constitutional symptoms, decreased splenomegaly, and/or stable or improved transfusion requirements.[33]
Table 3.
Treatments for Myeloproliferative Neoplasm–Blast Phase (MPN-BP), Myeloproliferative Neoplasm–Accelerated Phase (MPN-AP), and Post–Myeloproliferative Neoplasm Acute Myeloid Leukemia (MPN-AML)
| Study | Population | Sample Size | Dosing | Outcomes | Toxicities |
|---|---|---|---|---|---|
| Phase I[35] | MPN-AP MPN-BP |
21 | Ruxolitinib twice daily, 28-day cycle Decitabine 20 mg/m2 IV on days 1–5 |
CR or CRi: 7/21 | Anemia, leukopenia, thrombocytopenia |
| Phase I/II[36] | MPN-AML | 12 | Ruxolitinib twice daily, 28- to 42-day cycle Decitabine 20 mg/m2 on days 1–5 |
ORR: 5/21 | Grade 3/4 thrombocytopenia, neutropenia |
| Case series[32] | MPN-AP MPN-BP |
6 | Ruxolitinib Concurrent induction chemotherapy |
4 PRs (including relapse, 3 bridges to allo SCT) 2 died |
|
| Case series[33] | MPN-AP | 3 | Ruxolitinib + hypomethylating agent Ruxolitinib + cytarabine |
Improved constitutional symptoms Reduced splenomegaly |
Allo = allogeneic; CR = complete response; CRi = complete response with incomplete blood count recovery; ORR = overall response rate; PR = partial response; SCT = stem cell transplant.
A study of 39 patients with myelofibrosis investigated treatment with JAK inhibition in combination with HMA.[34] Patients received ruxolitinib for 3 cycles, and then the HMA 5-azacytidine was added to each subsequent cycle (25 mg/m2 on days 1–5). Objective response was noted in 69% of enrolled patients (27). A decrease in splenomegaly of ≥ 50% by palpation at any time over the course of the study was achieved in 79% (23/29) of patients with baseline splenomegaly, and 26% of these splenic reductions occurred after initiation of combination therapy. Two patients progressed to acute myeloid leukemia. Grade 3/4 toxicities included anemia (61%); thrombocytopenia (27%); and, less commonly, fatigue, nausea, and pneumonia.
Two studies recently presented in abstract form combine ruxolitinib with the HMA decitabine in the treatment of advanced myeloproliferative neoplasms. One study included 21 patients with myeloproliferative neoplasm–accelerated phase or –blast phase who had a prior diagnosis of essential thrombocythemia, polycythemia vera, or myelofibrosis; a proportion of the patients had been previously treated (29% had received prior ruxolitinib, and 24% had received prior decitabine).[35] A standard dose-escalation design combined ruxolitinib (10 mg to 50 mg orally twice daily) with decitabine (20 mg/m2 intravenously on days 1–5 of each cycle) in a 28-day cycle. Seven patients (33%) achieved a CR or CR with incomplete blood count recovery. Nine patients died during the study or follow-up. Median OS was 10.4 months. The most common grade 3/4 nonhematologic adverse event was infection. Although uncommon, grade 3/4 hematologic adverse events occurred, including anemia, leukopenia, and thrombocytopenia. The most common reasons for patients ending study treatment were adverse events (33%) and disease progression (22%). A phase II study is planned.
Similarly, a phase I study of ruxolitinib and decitabine in post–myeloproliferative neoplasm acute myeloid leukemia enrolled 12 patients.[36] Combination therapy included continuous ruxolitinib (10 to 50 mg orally twice daily), with a cycle length of 4 to 6 weeks, concurrently with decitabine (20 mg/m2 on days 1–5 of each cycle). Ten patients had post–myeloproliferative neoplasm acute myeloid leukemia, one had acute myeloid leukemia post–myelodysplastic syndrome (with a JAK2 mutation), and one had de novo acute myeloid leukemia. Five of the patients were myeloproliferative neoplasm treatment–naive, three had received HU, and two had been heavily pretreated for myelofibrosis. In the patients with post–myeloproliferative neoplasm acute myeloid leukemia, the overall response rate was 42%, with responses including CR, initial CR without blood count recovery and subsequent relapse, and PR followed by allogeneic stem cell transplant. Grade 3/4 adverse events were uncommon but included thrombocytopenia and neutropenia. No dose-limiting toxicity was noted.
Additional studies are needed in this population, and we suggest clinical trial enrollment for patients with myeloproliferative neoplasm–accelerated phase, myeloproliferative neoplasm–blast phase, or post–myeloproliferative neoplasm acute myeloid leukemia. In patients who are not able to enroll in a clinical trial, it would be reasonable to consider JAK inhibition in combination with HMA, although the optimal dosing and scheduling of this combination is not known at this time.
Additional Pharmacologic Therapies for Myeloproliferative Neoplasms
Additional pharmacologic therapies for myeloproliferative neoplasms include cellular pathway inhibition beyond the JAK-STAT pathway. The telomerase reverse transcriptase inhibitor imetelstat has been investigated in a small pilot study of 33 patients with myelofibrosis, and has proven to be active in approximately 20% of patients; the main adverse effect is myelosuppression.[37] The PI3K/AKT/mTOR pathway has proven to be constitutively active in myeloproliferative neoplasms and a targetable pathway for management of these entities.[38,39] Similarly, histone deacetylase inhibitors have been studied in myelofibrosis, polycythemia vera, and essential thrombocythemia,[40,41] and have proven to be active and able to reduce the JAK2 allele burden in a small proportion of patients, suggesting a disease-modifying effect. Combination studies with ruxolitinib are ongoing. Other pathways of interest in the management of myeloproliferative neoplasms include hedgehog pathway signaling, for which there are promising preclinical data but no benefit yet identified in clinical trials,[42,43] and antifibrotic agents, which are in ongoing phase II studies in combination with ruxolitinib.
Nonpharmacologic Interventions
Patients with myeloproliferative neoplasms have a significant symptom burden. While ruxolitinib has yielded improvements in symptoms, many symptoms persist; moreover, not all patients are candidates for JAK inhibition. Improved understanding of the etiology and optimal management of constitutional symptoms is needed.
The impact of constitutional symptoms on quality of life is not only seen in high-risk disease. The presence of systemic symptoms is included in the Dynamic International Prognostic Scoring System–Plus (DIPSS-Plus), which estimates survival for patients with myelofibrosis (Table 4).[44] The approval of JAK inhibitors that can address both splenomegaly and constitutional symptoms raises the issue of whether to treat patients with JAK inhibitors specifically for relief of constitutional symptoms. This proposed paradigm shift elevates patient experience to a level where it is considered similarly to quantitative laboratory values and physical examination findings.[45]
Table 4.
Dynamic International Prognostic Scoring System–Plus (DIPSS-Plus) for Myelofibrosis[44]
| Risk Factor | 1 Point | 2 Points |
|---|---|---|
| Age | > 65 yr | |
| Constitutional symptoms | Present | |
| Hemoglobin | < 10 g/dL | |
| Leukocyte count | > 25 × 109/L | |
| Circulating blasts | > 1% | |
| Platelet count | < 100 × 109/L | |
| Red blood cell transfusion need | Yes | |
| Unfavorable karyotype | Yes | |
| Risk Group | DIPSS-Plus Score |
Median Overall Survival |
| Low risk | 0 | 180 mo |
| Intermediate-1 risk | 1 | 80 mo |
| Intermediate-2 risk | 2 or 3 | 35 mo |
| High risk | ≥ 4 | 16 mo |
One double-blind randomized controlled trial evaluated constitutional symptoms in 110 patients with polycythemia vera who transitioned from HU to JAK inhibition.[46] Patients who were receiving a stable dose of HU were enrolled in the trial and were randomized to ruxolitinib 10 mg daily or continuation of HU. Symptoms were assessed at baseline and while receiving study therapy. The primary endpoint was ≥ 50% improvement in symptoms at week 16. This was achieved by 43% of the ruxolitinib group and, surprisingly, by 29% of the HU group; these results were not statistically significant. The only symptom to demonstrate statistically significant improvement between the two arms was pruritus, which was significantly more diminished in the ruxolitinib arm.
The CYTO-PV study of 365 patients with polycythemia vera demonstrated that a restrictive goal hematocrit (HCT) of < 45% produced reduced risk of major thrombosis, cardiovascular events, and cardiovascular mortality compared with a lenient strategy (with a goal HCT of 45% to 50%).[47] The CYTO-PV and MPN-SAF study cohorts were analyzed based on patient-reported outcomes.[48] No difference in symptom scores were noted between the strict and lenient HCT groups in the CYTO-PV study. The MPN-SAF study analysis revealed that patients with lower mean HCT (< 45%) or active phlebotomy were more likely to have a worse MPN-10 individual item score than either those being managed with a lenient HCT goal or those not using phlebotomy. This study shed light on the question of how to view the effect that traditional treatments, such as pursuit of iron deficiency in polycythemia vera patients, have on symptom burden.
Understanding the impact of symptoms on patients with myeloproliferative neoplasms and appreciating that symptoms can be consistently measured and improved should remain a focus of treatment. An analysis of 695 patients with myelofibrosis revealed an association between patient-reported single worst symptom, or total MPN-SAF TSS, and higher DIPSS-Plus prognostic risk score.[49] Worse symptoms were associated with female gender, splenomegaly > 15 cm, higher white blood cell count, and presence of peripheral blasts. The study concluded that significant symptoms correlate with clinical characteristics that suggest symptom-based criteria for JAK inhibition therapy.
The foregoing studies highlight the complexity of the relationships between disease severity, symptom burden, and management approach. In our view, it would be reasonable to consider initiation of JAK inhibition for patients with high or debilitating symptom burden despite a low DIPSS risk score.
Patients with malignancy are at risk for significant psychological burdens, in addition to physical symptoms. One study assessed myeloproliferative neoplasm patients’ perception of antidepressant prescribing. One hundred seventeen participants with myeloproliferative neoplasms were questioned about their willingness to accept an antidepressant from a hematologist or psychiatrist.[50] The majority of participants were willing to accept an antidepressant (63%), and 58% said they would be willing to accept that antidepressant from a hematologist. A quarter of participants (26%) indicated that they would not accept any treatment for depression.
Future considerations include nonpharmacologic interventions aimed at symptom management. Our group is investigating psychiatric interventions, as well as dietary modifications, that can ameliorate symptoms of myeloproliferative neoplasms.
Conclusion
Improved understanding of pathophysiology has led to therapeutic advances for patients with intermediate-2 and high-risk myelofibrosis, in the form of JAK inhibition. However, there are still unanswered questions and unmet needs in certain populations of patients with myeloproliferative neoplasms that are being addressed in ongoing research. Researchers are working to better understand the importance of upfront therapy in essential thrombocythemia and polycythemia vera, and to understand the impact of allele burden on disease outcomes. The two phase III studies comparing HU and IFN in these populations should shed light on this issue. Additionally, while JAK inhibition has benefitted many patients with myelofibrosis, the hematologic toxicities of ruxolitinib limit its use in some patients with baseline or progressive anemia or thrombocytopenia. The development of alternative JAK inhibitors, such as pacritinib and momelotinib, suggest a future ability to tailor JAK inhibition to a particular subset of patients with myelofibrosis based on hematologic parameters. Ongoing efforts to harness the clinical benefit of JAK inhibition in combination with HMA or induction chemotherapy may yield improved efficacy in the aggressive myeloproliferative neoplasm populations (those with myeloproliferative neoplasm–accelerated phase, myeloproliferative neoplasm–blast phase, or post–myeloproliferative neoplasm acute myeloid leukemia). Lastly, regardless of the evolution of therapeutic interventions that continue to show promise for disease control, further research is needed in the area of symptom mitigation, including nonpharmacologic interventions that target fatigue and the psychological impact of chronic malignancy.
KEY POINTS.
Further development of JAK inhibitor therapy is necessary in order to address the hematologic toxicities that can preclude use of ruxolitinib in patients with myeloproliferative neoplasms.
Investigations of combination therapy that incorporate JAK inhibition are necessary in order to achieve improved outcomes in patients with advanced or aggressive myeloproliferative neoplasms.
Constitutional symptoms associated with myeloproliferative neoplasms are a significant burden to patients, and treatments—either pharmacologic or nonpharmacologic—are needed to address the pervasive unmet need for symptom amelioration.
Footnotes
Financial Disclosure: Dr. Mesa serves as a consultant to Ariad, Galena, and Novartis; he receives research support from Celgene, CTI BioPharma, Gilead, Incyte, and Promedior. Dr. Padrnos has no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
REFERENCES
- 1.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–97. [DOI] [PubMed] [Google Scholar]
- 2.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–302. [DOI] [PubMed] [Google Scholar]
- 3.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–90. [DOI] [PubMed] [Google Scholar]
- 4.Rotunno G, Mannarelli C, Guglielmelli P, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood. 2014;123:1552–5. [DOI] [PubMed] [Google Scholar]
- 5.Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108:3472–6. [DOI] [PubMed] [Google Scholar]
- 6.Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2015 update on diagnosis, risk-stratification and management. Am J Hematol. 2015;90:162–73. [DOI] [PubMed] [Google Scholar]
- 7.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405. [DOI] [PubMed] [Google Scholar]
- 8.Passamonti F, Rumi E, Pietra D, et al. A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia. 2010:24:1574–9. [DOI] [PubMed] [Google Scholar]
- 9.Rumi E, Pietra D, Ferretti V, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood. 2014;123:1544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–98. [DOI] [PubMed] [Google Scholar]
- 12.Verstovsek S, Vannucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica. 2016;101:821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29:761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez-Larran A, Pereira A, Cervantes F, et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood. 2012;119:1363–9. [DOI] [PubMed] [Google Scholar]
- 15.Silver RT Long-term effects of the treatment of polycythemia vera with recombinant interferon-alpha. Cancer. 2006;107:451–8. [DOI] [PubMed] [Google Scholar]
- 16.Stauffer Larsen T, Iversen KF, Hansen E, et al. Long term molecular responses in a cohort of Danish patients with essential thrombocythemia, polycythemia vera and myelofibrosis treated with recombinant interferon alpha. Leuk Res. 2013;37:1041–5. [DOI] [PubMed] [Google Scholar]
- 17.Kovacsovics-Bankowski M, Kelley TW, Efimova O, et al. Changes in peripheral blood lymphocytes in polycythemia vera and essential thrombocythemia patients treated with pegylated-interferon alpha and correlation with JAK2V617F allelic burden. Exp Hematol Oncol. 2016;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang BT, Zeng QC, Zhao WH, et al. Interferon alpha-2b gains high sustained response therapy for advanced essential thrombocythemia and polycythemia vera with JAK2V617F positive mutation. Leuk Res. 2014;38:1177–83. [DOI] [PubMed] [Google Scholar]
- 19.Hansen IO, Sorensen AL, Hasselbalch HC. Second malignancies in hydroxyurea and interferon-treated Philadelphia-negative myeloproliferative neoplasms. Eur J Haematol. 2017;98:75–84. [DOI] [PubMed] [Google Scholar]
- 20.Hasselbalch HC, Silver RT. Interferon in polycythemia vera and related neoplasms. Can it become the treatment of choice without a randomized trial? Expert Rev Hematol. 2015;8:439–45. [DOI] [PubMed] [Google Scholar]
- 21.Gisslinger H, Klade C, Georgiev P, et al. Final results from PROUD-PV: a randomized controlled phase 3 trial comparing ropeginterferon alfa-2b to hydroxyurea in polycythemia patients. Presented at the 58th Annual Meeting of the American Society of Hematology; Dec 3–6, 2016; San Diego, CA. Abstr 475. [Google Scholar]
- 22.Mascarenhas J, Prchal JT, Rambaldi A, et al. Interim analysis of the Myeloproliferative Disorders Research Consortium (MPD-RC) 112 global phase III trial of front line pegylated interferon alpha-2a vs. hydroxyurea in high risk polycythemia vera and essential thrombocythemia. Presented at the 58th Annual Meeting of the American Society of Hematology; Dec 3–6, 2016; San Diego, CA. Abstr 479. [Google Scholar]
- 23.Mesa RA, Cortes J. Optimizing management of ruxolitinib in patients with myelofibrosis: the need for individualized dosing. J Hematol Oncol. 2013;6:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jakafi (ruxolitinib) tablets [prescribing information]. http://www.jakafi.com/pdf/prescribing-information.pdf. Accessed June 8, 2017.
- 25.Bryan JC, Verstovsek S. Overcoming treatment challenges in myelofibrosis and polycythemia vera: the role of ruxolitinib. Cancer Chemother Pharmacol. 2016;77:1125–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verstovsek S, Odenike O, Singer JW, et al. Phase 1/2 study of pacritinib, a next generation JAK2/FLT3 inhibitor, in myelofibrosis or other myeloid malignancies. J Hematol Oncol. 2016;9:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mesa R, Egyed M, Szoke A, et al. Results of the PERSIST-1 phase III study of pacritinib (PAC) versus best available therapy (BAT) in primary myelofibrosis (PMF), post-polycythemia vera myelofibrosis (PPV-MF), or post-essential thrombocythemia-myelofibrosis (PET-MF). J Clin Oncol. 2015;33(suppl):abstr LBA7006. [Google Scholar]
- 28.Mascarenhas J, Hoffman R, Talpaz M, et al. Results of the Persist-2 phase 3 study of pacritinib (PAC) versus best available therapy (BAT), including ruxolitinib (RUX), in patients (pts) with myelofibrosis (MF) and platelet counts <100,000/μl. Presented at the 58th Annual Meeting of the American Society of Hematology; Dec 3–6, 2016; San Diego, CA. Abstr LBA-5. [Google Scholar]
- 29.Gupta V, Mesa RA, Deininger MW, et al. A phase 1/2, open-label study evaluating twice-daily administration of momelotinib in myelofibrosis. Haematologica. 2017;102:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mesa RA, Li CY, Ketterling RP, et al. Leukemic transformation in myelofibrosis with myeloid metaplasia: a single-institution experience with 91 cases. Blood. 2005;105:973–7. [DOI] [PubMed] [Google Scholar]
- 31.Tam CS, Nussenzveig RM, Popat U, et al. The natural history and treatment outcome of blast phase BCR-ABL- myeloproliferative neoplasms. Blood. 2008;112:1628–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devillier R, Raffoux E, Rey J, et al. Combination therapy with ruxolitinib plus intensive treatment strategy is feasible in patients with blast-phase myeloproliferative neoplasms. Br J Haematol. 2016;172:628–30. [DOI] [PubMed] [Google Scholar]
- 33.Mwirigi A, Galli S, Keohane C, et al. Combination therapy with ruxolitinib plus 5-azacytidine or continuous infusion of low dose cytarabine is feasible in patients with blast-phase myeloproliferative neoplasms. Br J Haematol. 2014;167:714–6. [DOI] [PubMed] [Google Scholar]
- 34.Daver N, Cortes JE, Pemmaraju N, et al. Ruxolitinib (RUX) in combination with 5-azacytidine (AZA) as therapy for patients (pts) with myelofibrosis (MF). Blood. 2016;128:abstr 1127. [Google Scholar]
- 35.Rampal R, Mascarenhas JO, Kosiorek HE, et al. Safety and efficacy of combined ruxolitinib and decitabine in patients with blast-phase MPN and post-MPN AML: results of a phase I study (Myeloproliferative Disorders Research Consortium 109 trial). Presented at the 58th Annual Meeting of the American Society of Hematology; Dec 3–6, 2016; San Diego, CA. Abstr 1124. [Google Scholar]
- 36.Bose P, Verstovsek S, Gasior Y, et al. Phase I/II study of ruxolitinib (RUX) with decitabine (DAC) in patients with post-myeloproliferative neoplasm acute myeloid leukemia (post-MPN AML): phase I results. Presented at the 58th Annual Meeting of the American Society of Hematology; Dec 3–6, 2016; San Diego, CA. Abstr 4262. [Google Scholar]
- 37.Tefferi A, Lasho TL, Begna KH, et al. A pilot study of the telomerase inhibitor imetelstat for myelofibrosis. N Engl J Med. 2015;373:908–19. [DOI] [PubMed] [Google Scholar]
- 38.Bartalucci N, Guglielmelli P, Vannucchi AM. Rationale for targeting the PI3K/Akt/mTOR pathway in myeloproliferative neoplasms. Clin Lymphoma Myeloma Leuk. 2013;13(suppl 2):S307–S309. [DOI] [PubMed] [Google Scholar]
- 39.Guglielmelli P, Barosi G, Rambaldi A, et al. Safety and efficacy of everolimus, a mTOR inhibitor, as single agent in a phase 1/2 study in patients with myelofibrosis. Blood. 2011;118:2069–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andersen CL, McMullin MF, Ejerblad E, et al. A phase II study of vorinostat (MK-0683) in patients with polycythaemia vera and essential thrombocythaemia. Br J Haematol. 2013;162:498–508. [DOI] [PubMed] [Google Scholar]
- 41.Mascarenhas J, Sandy L, Lu M, et al. A phase II study of panobinostat in patients with primary myelofibrosis (PMF) and post-polycythemia vera/essential thrombocythemia myelofibrosis (post-PV/ET MF). Leuk Res. 2017;53:13–9. [DOI] [PubMed] [Google Scholar]
- 42.Sasaki K, Gotlib JR, Mesa RA, et al. Phase II evaluation of IPI-926, an oral Hedgehog inhibitor, in patients with myelofibrosis. Leuk Lymphoma. 2015;56:2092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zingariello M, Martelli F, Ciaffoni F, et al. Characterization of the TGF-beta1 signaling abnormalities in the Gata1low mouse model of myelofibrosis. Blood. 2013;121:3345–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29:392–7. [DOI] [PubMed] [Google Scholar]
- 45.Mesa RA, Scherber RM, Geyer HL. Reducing symptom burden in patients with myeloproliferative neoplasms in the era of Janus kinase inhibitors. Leuk Lymphoma. 2015;56:1989–99. [DOI] [PubMed] [Google Scholar]
- 46.Mesa R, Vannucchi AM, Yacoub A, et al. The efficacy and safety of continued hydroxycarbamide therapy versus switching to ruxolitinib in patients with polycythaemia vera: a randomized, double-blind, double-dummy, symptom study (RELIEF). Br J Haematol. 2017;176:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marchioli R, Finazzi G, Specchia G, et al. The CYTO-PV: a large-scale trial testing the intensity of CYTOreductive therapy to prevent cardiovascular events in patients with polycythemia vera. Thrombosis. 2011;2011:794240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scherber RM, Geyer HL, Dueck AC, et al. The potential role of hematocrit control on symptom burden among polycythemia vera patients: insights from the CYTO-PV and MPN-SAF patient cohorts. Leuk Lymphoma. 2016;58:1481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scherber RM, Geyer H, Dueck AC, et al. Symptom burden as primary driver for therapy in patients with myelofibrosis: an analysis by MPN International Quality of Life Study Group. Presented at the 58th Annual Meeting of the American Society of Hematology; Dec 3–6, 2016; San Diego, CA. Abstr 3117. [Google Scholar]
- 50.McFarland DC, Shen MJ, Polizzi H, et al. Preferences of patients with myeloproliferative neoplasms for accepting anxiety or depression treatment. Psychosomatics. 2017;58:56–63. [DOI] [PubMed] [Google Scholar]