H&O What is polycythemia vera?
RM Polycythemia vera is a myeloproliferative neoplasm. There are approximately 150,000 patients with polycythemia vera in the United States. The disease is characterized by bone marrow that produces too many red blood cells. Patients may also have elevated levels of white blood cells and platelets. The key clinical consequence of polycythemia vera is the risk of blood clots or bleeding. Other significant disease-related symptoms include headaches, microvascular complications, and pruritus. Patients with polycythemia vera have the potential to develop more advanced chronic blood diseases, such as myelofibrosis or even acute leukemia. Polycythemia vera can lead to fatal complications in some cases.
The diagnosis of polycythemia vera is based on results from tests assessing elevations in blood counts and the presence of molecular mutations associated with the disease. Most of these patients have mutations in the Janus kinase 2 (JAK2) gene. There may also be abnormalities in bone marrow biopsies.
H&O When was polycythemia vera added to the NCCN guidelines?
RM The National Comprehensive Cancer Network (NCCN) added myeloproliferative neoplasms as a whole to the guidelines in the fall of 2016. Treatment guidelines for polycythemia vera were added in the summer of 2017. These guidelines were added to introduce greater uniformity in the diagnosis and treatment of polycythemia vera throughout the United States.
H&O What are the general principles of treatment?
RM There are several principles of treatment. The first is to prevent thrombosis and bleeding through the use of either phlebotomy or medication to reduce elevations in blood counts. The second principle of treatment is to reverse disease-associated symptoms and toxicities, such as headache, pruritus, and spleen enlargement. The third principle, which may not be possible in most cases, is to avoid disease progression.
H&O What risk categories are used in the guidelines?
RM In polycythemia vera, the predominant risks are thrombosis and/or bleeding. The NCCN guidelines use high- and low-risk categories that have been previously validated in the field of myeloproliferative neoplasms. Low-risk patients are younger than 60 years and have no history of thrombotic events. To further refine treatment selection, the guidelines incorporate factors such as features of disease progression (eg, pruritus, night sweats, fatigue), progressive splenomegaly, thrombocytosis, leukocytosis, and poor tolerance of phlebotomy.
H&O What is the initial treatment for low-risk vs high-risk patients?
RM The cornerstone of treatment for polycythemia vera has been low-dose aspirin and phlebotomy to maintain a hematocrit value of less than 45%. This treatment approach is now reserved for asymptomatic patients at low risk for progression. Low-risk patients with persistent symptoms or other problematic features are candidates for cytoreductive therapy. For patients who are at high risk, the recommendation is for cytoreductive therapy—even as initial treatment—along with phlebotomy and aspirin. Hydroxyurea and interferon are also recommended as frontline therapy for these patients.
H&O How does cytoreductive therapy work?
RM Cytoreductive therapy, such as hydroxyurea or interferon, can directly reduce counts of red cells, white cells, and platelets. (In contrast, phlebotomy is a mechanical reduction in the number of red cells.) Cytoreductive therapy is used as frontline treatment in patients at high risk and in low-risk patients who have rising blood counts, who are intolerant of phlebotomy, or who have progressive splenomegaly. Hydroxyurea and interferon are currently being compared in randomized phase 3 studies. There are subtleties in terms of the selection between these therapies, with interferon being clearly preferred in women of childbearing age and, most likely, in younger patients.
H&O How effective is cytoreductive therapy?
RM Cytoreductive therapy effectively reduces elevations in red cell counts. It tends to be inadequate, however, in the control of white cell counts and platelet counts. Cytoreductive therapy may also fail to control symptoms of arthritis, fatigue, and splenomegaly. In some cases, cytoreductive therapy will control the red cell count but cause leukopenia and other toxicities that lead patients to discontinue treatment. For example, hydroxyurea is associated with skin changes, leg ulcerations, mucosal ulcers, and excessive myelosuppression. Interferon can lead to elevations in liver function tests, fatigue, flulike symptoms, fever, and autoimmune complications. Approximately 30% to 40% of patients with polycythemia vera who receive cytoreductive therapy have a suboptimal response.
H&O How are patients monitored once treatment begins?
RM Monitoring consists of several components. Patients undergo continual assessment of the hematocrit level, to ensure it remains under 45%. Patients are monitored for any signs of the disease, such as thrombosis or bleeding, as well as for complications from therapy. Monitoring also includes assessment of symptoms, such as pruritus, headache, and microvascular complications. Symptoms that were present at baseline might resolve, and new symptoms can arise. Additionally, patients are followed for changes in their white blood cell count or platelet count, as well as for enlargement of the spleen.
H&O What are the criteria indicating that cytoreductive therapy is not effective or has stopped being effective?
RM Currently, there are criteria for resistance and intolerance to hydroxyurea. These criteria are provided by the European LeukemiaNet research network and have been used in clinical trials. An effective response is defined by control of the platelet count, a white cell count that normalizes, minimal use of phlebotomy, and, if present, resolution of splenomegaly after treatment with a maximum tolerated dose given up to 3 months. Failure to achieve these benchmarks is a sign of resistance to treatment. The criteria for intolerance include leg ulcerations, gastrointestinal disturbances, mucosal ulcerations, and secondary skin cancers.
H&O What are the options for patients who require treatment after cytoreductive therapy?
RM Ruxolitinib (Jakafi, Incyte) is the cornerstone of second-line therapy for patients who are resistant or intolerant to hydroxyurea. Ruxolitinib was approved for the treatment of myelofibrosis in 2011 and for polycythemia vera in 2014. This agent is an inhibitor of the native JAK2. The JAK2 mutation is seen in nearly all patients with polycythemia vera. Mutations can occur in the JAK2 V617F allele and the JAK2 exon 12. The JAK signal transducer and activator of transcription (STAT) pathway and the JAK2 protein are overly active in all patients with polycythemia vera.
In randomized studies of patients with polycythemia vera, ruxolitinib was superior to the best alternative therapy in controlling red cell counts, decreasing vascular events, improving symptoms, and reducing splenomegaly. The RESPONSE trial (Study of Efficacy and Safety in Polycythemia Vera Subjects Who Are Resistant to or Intolerant of Hydroxyurea: JAK Inhibitor INC424 [INCB018424] Tablets Versus Best Available Care) evaluated ruxolitinib in patients with an inadequate response to hydroxyurea and who had splenomegaly and inadequately controlled disease. The primary endpoint was the proportion of patients with both control of hematocrit levels and a reduction in spleen volume of at least 35% from baseline to week 32. This endpoint was achieved in 21% of patients treated with ruxolitinib vs 1% of those treated with standard therapy (P<.001). Ruxolitinib was also superior to best available therapy in improving symptom burden and decreasing the need for phlebotomy. Assessment after 80 weeks of follow-up showed that the primary response was durable (Figure). There was a trend toward improved control of thrombotic events.
Figure.
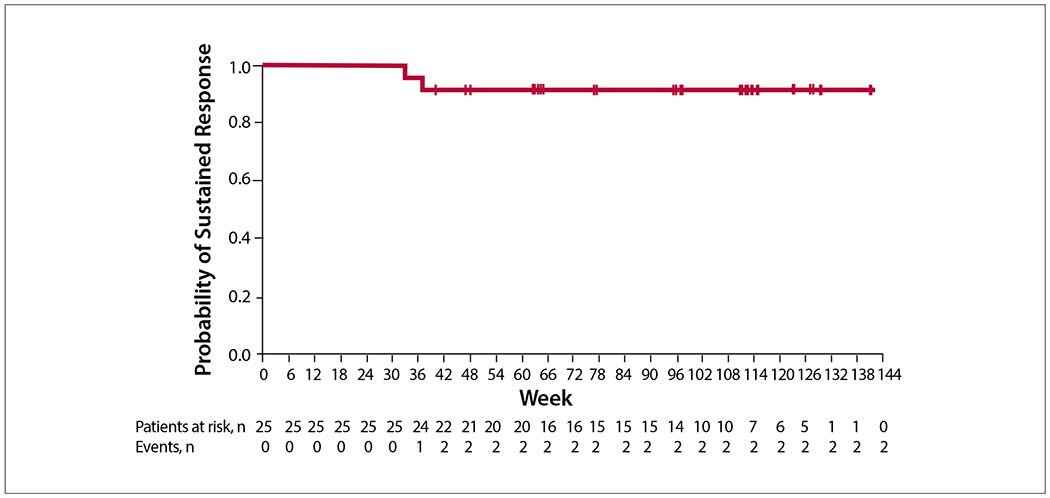
Durability of the primary response at 80-week follow-up among patients treated with ruxolitinib in the RESPONSE trial. RESPONSE, Study of Efficacy and Safety in Polycythemia Vera Subjects Who Are Resistant to or Intolerant of Hydroxyurea: JAK Inhibitor INC424 (INCB018424) Tablets Versus Best Available Care. Adapted from Verstovsek S et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica. 2016;101(7):821-829. © 2016 Ferrata Storti Foundation.
The more recent RESPONSE-2 trial (Ruxolitinib Efficacy and Safety in Patients With HU Resistant or Intolerant Polycythemia Vera vs Best Available Therapy) evaluated ruxolitinib in patients with an inadequate response to hydroxyurea and inadequately controlled disease, but who did not have splenomegaly. Ruxolitinib was similarly effective in this population.
The toxicities associated with ruxolitinib tend to be manageable. The main toxicity is a slight increase in herpes zoster infections or reactivation. Clinicians should be mindful of the risk for shingles.
There are upcoming studies in the United States and some ongoing studies in other parts of the world evaluating the use of ruxolitinib as frontline therapy in certain groups of patients.
H&O Do you have any other suggestions for the management of polycythemia vera?
RM The key to successful treatment in polycythemia vera is serial assessment of the patient. Frequently, patients continue to experience inadequately controlled symptoms or other difficulties during treatment with phlebotomy and aspirin alone, or even with hydroxyurea. Control of hematocrit is usually the primary goal, but management should encompass all aspects of the disease.
Acknowledgments
Disclosure
Dr Mesa is a consultant for Novartis, Shire, and AOP Orphan. He has received research support from Incyte, Gilead, CTI BioPharma, and Promedior.
Biography

Suggested Readings
- ClinicalTrials.gov. Randomized trial of pegylated interferon alfa-2a versus hydroxyurea in polycythemia vera (PV) and essential thrombocythemia (ET). https://clinicaltrials.gov/ct2/show/NCT01259856. Identifier: NCT01259856. Accessed September 27, 2017.
- Gisslinger H, Klade C, Georgiev P, et al. Final results from PROUD-PV a randomized controlled phase 3 trial comparing ropeginterferon alfa-2b to hydroxyurea in polycythemia vera patients. [ASH abstract 475]. Blood. 2016;128(suppl 22). [Google Scholar]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: myeloproliferative neoplasms. Version 2.2018. https://www.nccn.org/professionals/physician_gls/pdf/mpn.pdf. Updated September 7, 2017. Accessed September 26, 2017.
- Passamonti F, Griesshammer M, Palandri F, et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. Lancet Oncol. 2017;18(1):88–99. [DOI] [PubMed] [Google Scholar]
- Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372(5): 426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstovsek S, Vannucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica. 2016;101(7):821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]


