Abstract
Myelofibrosis is a myeloproliferative neoplasm associated with progressive cytopenias and high symptom burden. MF patients with thrombocytopenia have poor prognosis but the presence of thrombocytopenia frequently precludes the use of JAK2 inhibitors. In this study, we assessed quality of life and symptom burden in 418 MF patients with (n = 89) and without (n = 329) thrombocytopenia using prospective data from the MPN-QOL study group database, including the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) and Total Symptom Score (MPN10). Thrombocytopenia, defined as platelet count < 100 × 109/L (moderate 51–100 × 109/L; severe ≤50 × 109/L), was associated with anemia (76% vs. 45%, p < 0.001), leukopenia (29% vs. 11%, p < 0.001), and need for red blood cell transfusion (35% vs. 19%, p = 0.002). Thrombocytopenic patients had more fatigue, early satiety, inactivity, dizziness, sad mood, cough, night sweats, itching, fever, and weight loss; total symptom scores were also higher (33 vs. 24, p < 0.001). Patients with severe thrombocytopenia were more likely to have anemia (86% vs. 67%, p = 0.04), leukopenia (40% vs. 20%, p = 0.04), and transfusion requirements (51% vs. 20%, p = 0.002) but few differences in symptoms when compared to patients with moderate thrombocytopenia. These results suggest that MF patients with thrombocytopenia experience greater symptomatic burden than MF patients without thrombocytopenia and may benefit from additional therapies.
Keywords: Myeloproliferative neoplasm, Myelofibrosis, Thrombocytopenia, Quality of life, Symptomatology
1. Introduction
Myelofibrosis (MF) is a clonally derived Philadelphia chromosome negative myeloproliferative neoplasm (MPN) associated with progressive cytopenias and potential to transform into acute myelogenous leukemia (AML). MPNs are often associated with dysregulated signaling of the Janus kinase-signal transducers and activators of transcription (JAK-STAT) pathway. The most common somatic mutation, JAK2 V617F, occurs in 50–60% of patients with MF [1–3].
MF patients exhibit a high degree of symptomatology with potentially dramatic impacts on quality of life (QOL). Common symptoms include abdominal pain, bone pain, fatigue, pruritus, night sweats, fever, and weight loss [4]. Cytopenic derangements are common and closely linked to disease progression. In particular, thrombocytopenia is a proven negative prognostic indicator and predictor of transformation to AML [5]. MF patients with platelets under 50 × 109/L have more frequent anemia and leukopenia and higher rates of both hemorrhagic and thrombotic complications compared to MF patients with higher platelet counts [6].
Treatment options are limited for patients with thrombocytopenia. Ruxolitinib, a JAK1/JAK2 inhibitor, was shown in the phase III COMFORT-I [7] and COMFORT-II [8] trials to have significant symptomatic benefit in intermediate-2 or high risk MF patients with platelets of at least 100 × 109/L. However, the drug may paradoxically contribute to thrombocytopenia and is presently only indicated in patients with platelets of at least 50 × 109/L.
To date, no investigation has evaluated the correlations between patient symptomatology and platelet count. In this study, we aimed to characterize the symptom burden in MF patients with and without thrombocytopenia using prospectively collected quality of life data. We also assessed differences in clinical characteristics and symptomatology among patients with varying degrees of thrombocytopenia.
2. Methods
2.1. Patient selection
This study was approved by the Institutional Review Board (IRB) of the Mayo Clinic. Data were prospectively collected from an international pool of MPN patients recruited from academic, private practice, and government-funded centers. A total of 418 patients were included in the study; all had a diagnosis of MF. Patients completed the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF), Brief Fatigue Inventory (BFI), and European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30) via self-reporting. All surveys were completed in the patients’ native language (English, German, Dutch, French, Spanish, Italian, Chinese, and Swedish) and translated to English using standard PRO translational methods. Clinical information was also gathered at the time of survey collection and included laboratory information, treatment history, prognostic scoring, and physical examination results as assessed by clinicians.
In this study, thrombocytopenia was defined as a platelet count less than 100 × 109/L, anemia was defined as hemoglobin less than 11 g/dL, and leukopenia was defined as a white blood cell (WBC) count less than 3.5 × 109/L. Moderate thrombocytopenia was defined as a platelet count of 51–100 × 109/L, and severe thrombocytopenia was defined as a platelet count less than or equal to 50 × 109/L. A broader categorization of patients with “lab abnormality” included any patient who met criteria for anemia, leukopenia, or thrombocytopenia.
2.2. Symptom evaluation
Symptom evaluation was performed using the previously validated MPN-SAF [9]. All participants were required to complete at least six of the ten MPN-SAF symptom questions to meet study inclusion criteria. Symptoms were scored on a scale from 0 (absent/as good as it can be) to 10 (worst imaginable/as bad as it can be), and items specifically assessed quality of life (QOL), early satiety, abdominal pain or discomfort, inactivity, headache, difficulty with concentration, dizziness, numbness, insomnia, sad mood, difficulty with sexual desire or function, cough, night sweats, itching, bone pain, fever, and weight loss. Total symptom score (TSS) was calculated by multiplying the average score across items by ten to achieve a 0–100 scaled score. The MD Anderson BFI was used to assess worst fatigue rating [10]. Splenomegaly was assessed by clinicians based on physical examination and reported as an estimated value in centimeters.
2.3. Prognostic scoring
The Dynamic International Prognostic Scoring System (DIPSS) was used to calculate MF prognostic scores [11]. Patients were stratified into low risk (0 points), intermediate-1 risk (1–2 points), intermediate-2 risk (3–4 points), and high risk (> 4 points) using the following scoring variables: circulating blasts greater than or equal to 1% (1 point), constitutional symptoms (1 point), age greater than 65 years (1 point), WBC count greater than or equal to 25 × 109/L (1 point), and hemoglobin less than 11 g/dL (2 points).
2.4. Statistical analysis
Demographics, clinical variables, and symptom scores were compared between subgroups of MF patients with and without thrombocytopenia using analysis of variance (ANOVA) F-tests for continuous variables or Chi-squared tests for discrete data. Comparisons between patients with severe and moderate thrombocytopenia were done similarly. Statistical significance was set at p < 0.05. All statistical analyses were conducted using SAS software version 9.4 (SAS Inc., Cary, NC).
3. Results
3.1. Demographic and clinical characteristics
A total of 418 MF patients with (n = 89) and without (n = 329) thrombocytopenia completed the MPN-SAF and BFI. Patients with thrombocytopenia were further classified as having moderate (n = 46) or severe (n = 43) thrombocytopenia. Demographic and clinical characteristics are shown in Tables 1 and 2. Mean age overall was 60.2 (SD = 12.3) years and 47.4% of patients were male. Most patients had a diagnosis of primary myelofibrosis (69.4%) and met intermediate-1 DIPSS risk criteria (54.9%). Anemia (51.6%) and laboratory abnormalities (62.4%) were common. However, most patients had no history of prior thrombosis (11.3%), prior hemorrhage (5.1%), or RBC transfusion requirements (22.5%).
Table 1.
Demographics and clinical characteristics of MF patients with and without thrombocytopenia.
| Normal (Plt > 100 × 109/L) | Thrombocytopenia (Plt < 100 × 109/L) | Total | p-value | |
|---|---|---|---|---|
| n = 329 | n = 89 | n = 418 | ||
| Age (mean, SD) | 61.0 (12.15) | 57.4 (12.67) | 60.2 (12.34) | 0.01 |
| Gender (male) | 156 (47.4%) | 42 (47.2%) | 198 (47.4%) | 0.97 |
| MF subtype | 0.01 | |||
| Primary MF | 217 (66%) | 73 (82%) | 290 (69.4%) | |
| ET-MF | 64 (19.5%) | 7 (7.9%) | 71 (17%) | |
| PV-MF | 48 (14.6%) | 9 (10.1%) | 57 (13.6%) | |
| DIPSS risk | 0.07 | |||
| Low | 38 (19.3%) | 5 (13.9%) | 43 (18.5%) | |
| Intermediate-1 | 113 (57.4%) | 15 (41.7%) | 128 (54.9%) | |
| Intermediate-2 | 41 (20.8%) | 14 (38.9%) | 55 (23.6%) | |
| High | 5 (2.5%) | 2 (5.6%) | 7 (3%) | |
| Anemia (Hgb < 11 g/dL) | 147 (44.8%) | 68 (76.4%) | 215 (51.6%) | < 0.001 |
| Leukopenia (WBC < 3.5 × 109/L) | 35 (10.6%) | 26 (29.2%) | 61 (14.6%) | < 0.001 |
| Lab abnormality | 172 (52.3%) | 89 (100%) | 261 (62.4%) | < 0.001 |
| Prior thrombosis | 42 (12.9%) | 5 (5.6%) | 47 (11.3%) | 0.06 |
| Prior hemorrhage | 14 (4.3%) | 7 (7.9%) | 21 (5.1%) | 0.17 |
| Required RBC transfusion | 62 (19.1%) | 31 (34.8%) | 93 (22.5%) | 0.002 |
| Mean MPN duration (years, SD) | 8.8 (8.33) | 11.9 (10.86) | 9.3 (8.81) | 0.049 |
DIPSS, Dynamic International Prognostic Scoring System; ET, essential thrombocythemia; Hgb, hemoglobin; MF, myelofibrosis; Plt, platelets; PV, polycythemia vera; RBC, red blood cell; SD, standard deviation; WBC, white blood cell.
Table 2.
Demographics and clinical characteristics of MF patients with moderate and severe thrombocytopenia.
| Moderate Thrombocytopenia (Plt 51–100 × 109/L) | Severe Thrombocytopenia (Plt < 50 × 109/L) | Total | p-value | |
|---|---|---|---|---|
| n = 46 | n = 43 | n = 89 | ||
| Age (mean, SD) | 57.9 (11.29) | 56.9 (14.11) | 57.4 (12.67) | 0.72 |
| Gender (male) | 21 (45.7%) | 21 (48.8%) | 42 (47.2%) | 0.76 |
| MF subtype | 0.87 | |||
| Primary MF | 38 (82.6%) | 35 (81.4%) | 73 (82%) | |
| ET-MF | 4 (8.7%) | 3 (7%) | 7 (7.9%) | |
| PV-MF | 4 (8.7%) | 5 (11.6%) | 9 (10.1%) | |
| DIPSS risk | 0.09 | |||
| Low | 4 (21.1%) | 1 (5.9%) | 5 (13.9%) | |
| Intermediate-1 | 10 (52.6%) | 5 (29.4%) | 15 (41.7%) | |
| Intermediate-2 | 5 (26.3%) | 9 (52.9%) | 14 (38.9%) | |
| High | 0 (0%) | 2 (11.8%) | 2 (5.6%) | |
| Anemia (Hgb < 11 g/dL) | 31 (67.4%) | 37 (86.0%) | 68 (76.4%) | 0.04 |
| Leukopenia (WBC < 3.5 × 109/L) | 9 (19.6%) | 17 (39.5%) | 26 (29.2%) | 0.04 |
| Lab abnormality | 46 (100%) | 43 (100%) | 89 (100%) | |
| Prior thrombosis | 2 (4.3%) | 3 (7%) | 5 (5.6%) | 0.59 |
| Prior hemorrhage | 2 (4.3%) | 5 (11.6%) | 7 (7.9%) | 0.20 |
| Required RBC transfusion | 9 (19.6%) | 22 (51.2%) | 31 (34.8%) | 0.002 |
| Mean MPN duration (years, SD) | 12.3 (12.52) | 11.5 (8.58) | 11.9 (10.86) | 0.83 |
DIPSS, Dynamic International Prognostic Scoring System; ET, essential thrombocythemia; Hgb, hemoglobin; MF, myelofibrosis; Plt, platelet; PV, polycythemia vera; RBC, red blood cell; SD, standard deviation; WBC, white blood cell.
Total Symptom Scores (TSS) stratified by presence or absence of clinical and lab abnormalities are shown in Table 3. Patients with anemia (p < 0.001), any lab abnormality (p < 0.001), those requiring RBC transfusion (p = 0.003), and those with higher DIPSS risk scores (p < 0.001) reported significantly higher TSS. There was no significant association between leukopenia and overall symptom burden as reported by TSS, though a trend toward high TSS in patients with leukopenia was observed.
Table 3.
Association between clinical characterstics and MPN-SAF Total Symptom Score in MF patients
| Total Symptom Score (TSS) (mean, SD) | p-value | |
|---|---|---|
| n = 418 | ||
| Anemia (Hgb < 11 g/dL) | < 0.001 | |
| Yes | 29.6 (18.4) | |
| No | 22.3 (15.6) | |
| Leukopenia (WBC < 3.5 × 109/L) | 0.06 | |
| Yes | 29.9 (19.3) | |
| No | 25.3 (17.1) | |
| Lab abnormality | < 0.001 | |
| Yes | 28.7 (18.1) | |
| No | 21.5 (15.6) | |
| Required RBC transfusion | 0.003 | |
| Yes | 30.8 (19.0) | |
| No | 24.6 (16.8) | |
| DIPSS risk | < 0.001 | |
| Low | 15.2 (11.1) | |
| Intermediate-1 | 23.3 (15.4) | |
| Intermediate-2/High | 31.5 (17.1) |
DIPSS, Dynamic International Prognostic Scoring System; Hgb, hemoglobin; MF, myelofibrosis; RBC, red blood cell; SD, standard deviation; WBC, white blood cell.
Overall, the highest symptom scores in this cohort were reported for worst fatigue (4.8/10), sexuality (4.3/10), overall compromise in QOL (3.4/10), and inactivity (3.2/10). In contrast, lowest scores were reported for fever (0.6/10), cough (1.6/10), abdominal pain (1.7/10), and headache (1.7/10).
3.2. Impact on symptoms and phenotype: thrombocytopenia vs. no thrombocytopenia
Patient demographics, clinical features, prognostic scores, and MPN-SAF and BFI results including symptom scores (Fig. 1) and prevalence (Fig. 2) were compared among MF patients with and without thrombocytopenia. Overall, patients with thrombocytopenia were slightly younger (57.4 vs. 61.0 years, p = 0.01), had longer disease duration (11.9 vs. 8.8 years, p = 0.05), had more RBC transfusion requirements (34.8% vs. 19.1%, p = 0.002), were more likely to have PMF (82% vs. 66%, p = 0.01), and had more laboratory abnormalities including anemia (76.4% vs. 44.8%, p < 0.001) and leukopenia (29.2% vs. 10.6%, p < 0.001). Patients with thrombocytopenia did not differ from patients without thrombocytopenia by DIPSS risk score, gender, history of prior thrombosis, or prior hemorrhage (Table 1).
Fig. 1.
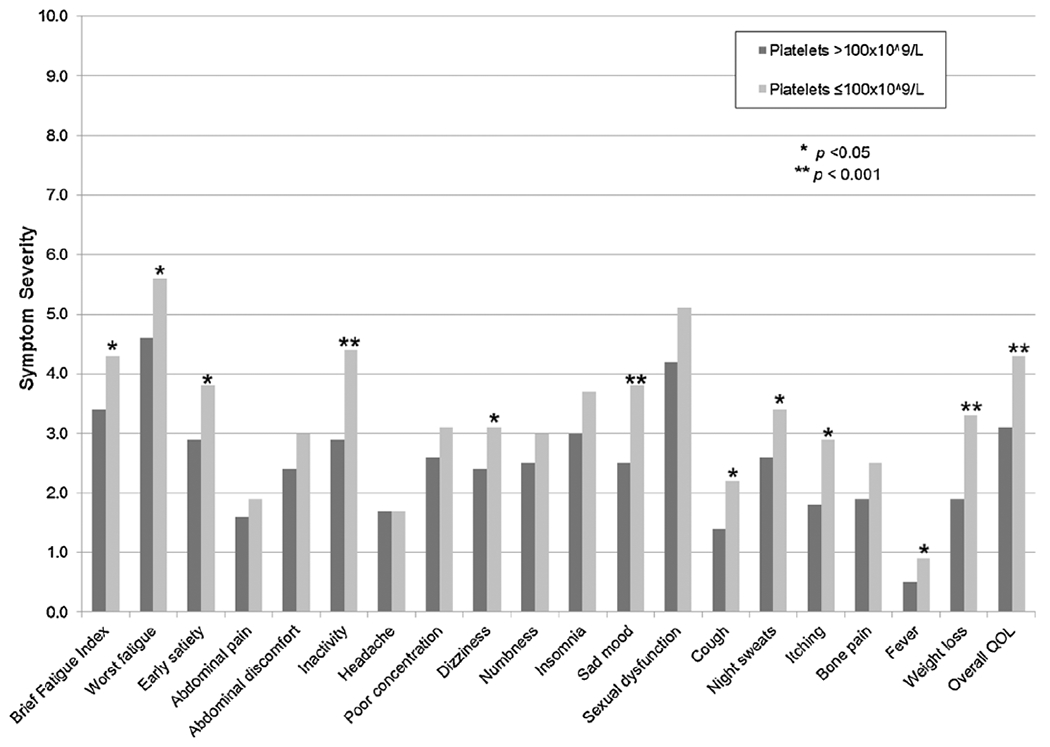
Thrombocytopenia versus no thrombocytopenia by individual MPN-SAF score.
Fig. 2.
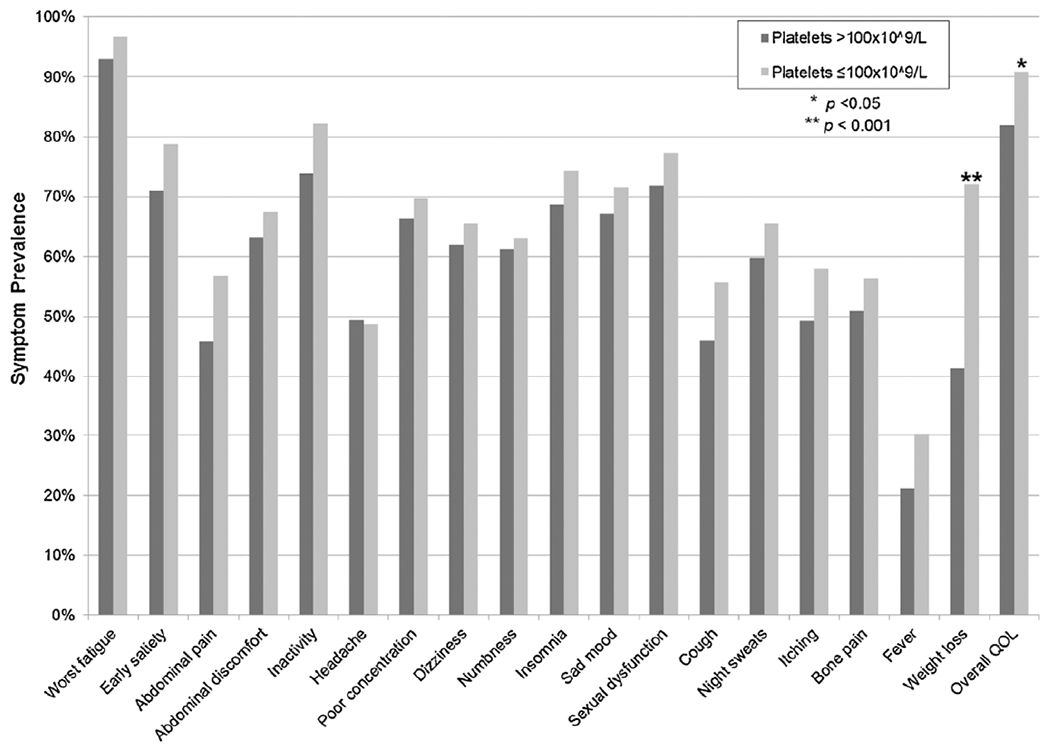
Thrombocytopenia versus no thrombocytopenia by individual MPN-SAF symptom prevalence.
Patients with thrombocytopenia experienced significantly worse symptom burden in comparison to patients without thrombocytopenia with higher symptoms scores for worst fatigue (5.6 vs. 4.6, p = 0.002), inactivity (4.4 vs. 2.9, p < 0.001), impaired overall QOL (4.3 vs. 3.1, p < 0.001), dizziness (3.2 vs 2.4, p = 0.046), sad mood (3.8 vs. 2.5, p < 0.001), cough (2.2 vs. 1.4, p = 0.003), night sweats (3.4 vs. 2.6, p = 0.02), itching (2.9 vs. 1.8, p = 0.001), fever (0.9 vs. 0.5, p = 0.04), weight loss (3.3 vs. 1.9, p < 0.001), and early satiety (3.8 vs. 2.9, p = 0.01); see Fig. 1. Overall TSS was also significantly higher (32.8 vs. 24.1, p < 0.001) in patients with thrombocytopenia. There were no differences in abdominal discomfort, abdominal pain, bone pain, headache, poor concentration, sexual dysfunction, numbness, or insomnia. With regard to prevalence of symptoms, weight loss (72.1% vs. 41.3%, p < 0.001) and decline in overall QOL (90.8% vs. 81.9%, p = 0.045) were seen with significantly higher frequency in the thrombocytopenic group compared to MF patients without thrombocytopenia; see Fig. 2.
3.3. Impact on symptoms and phenotype: moderate vs. severe thrombocytopenia
Patient demographics, clinical features, prognostic scores, and MPN-SAF and BFI results were compared between MF patients with moderate (platelets 51–100 × 109/L) and severe (platelets < 50 × 109/L) thrombocytopenia. As seen in Table 2, patients with severe thrombocytopenia were more likely to have anemia (86.0% vs. 67.4%, p = 0.04), leukopenia (39.5% vs. 19.6%, p = 0.04), and require RBC transfusions (51.2% vs. 19.6%, p = 0.002). Patients did not differ by MF subtype, age, gender, history of prior thrombosis, prior hemorrhage, DIPSS risk scores, or mean MPN duration. No differences were noted in individual or total symptom scores between groups (Figs. 3 and 4) with one exception; patients with severe thrombocytopenia experienced less frequent sad mood (60.5% vs. 82.2%, p = 0.02).
Fig. 3.
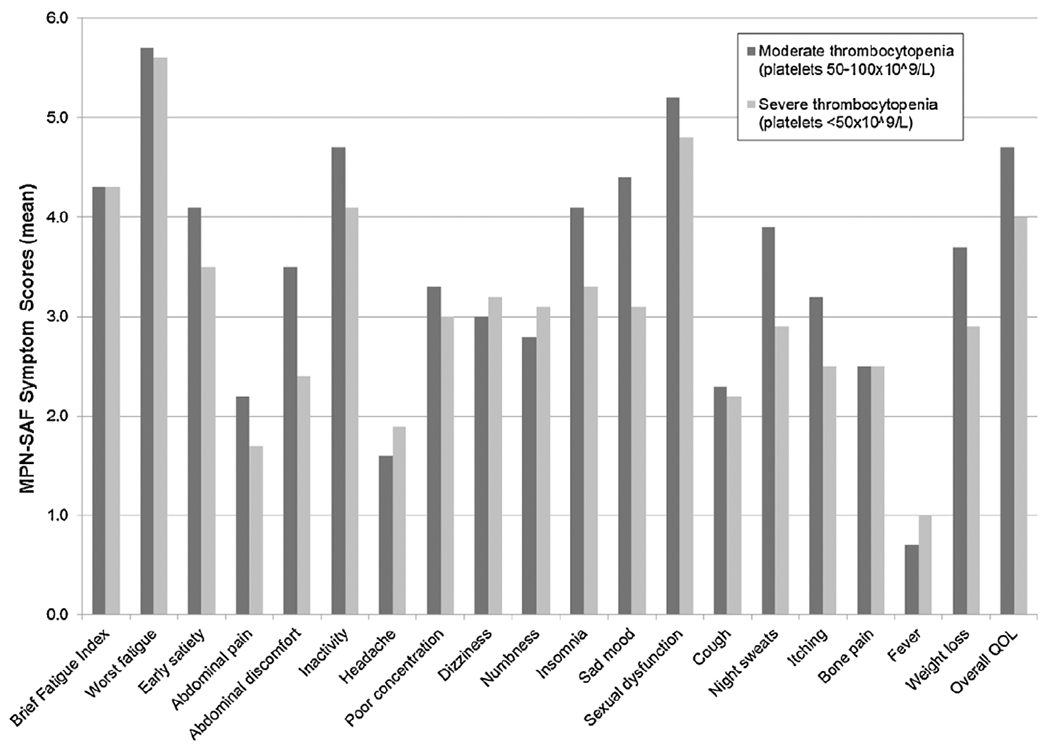
Moderate versus severe thrombocytopenia by individual MPN-SAF Score.
Fig. 4.
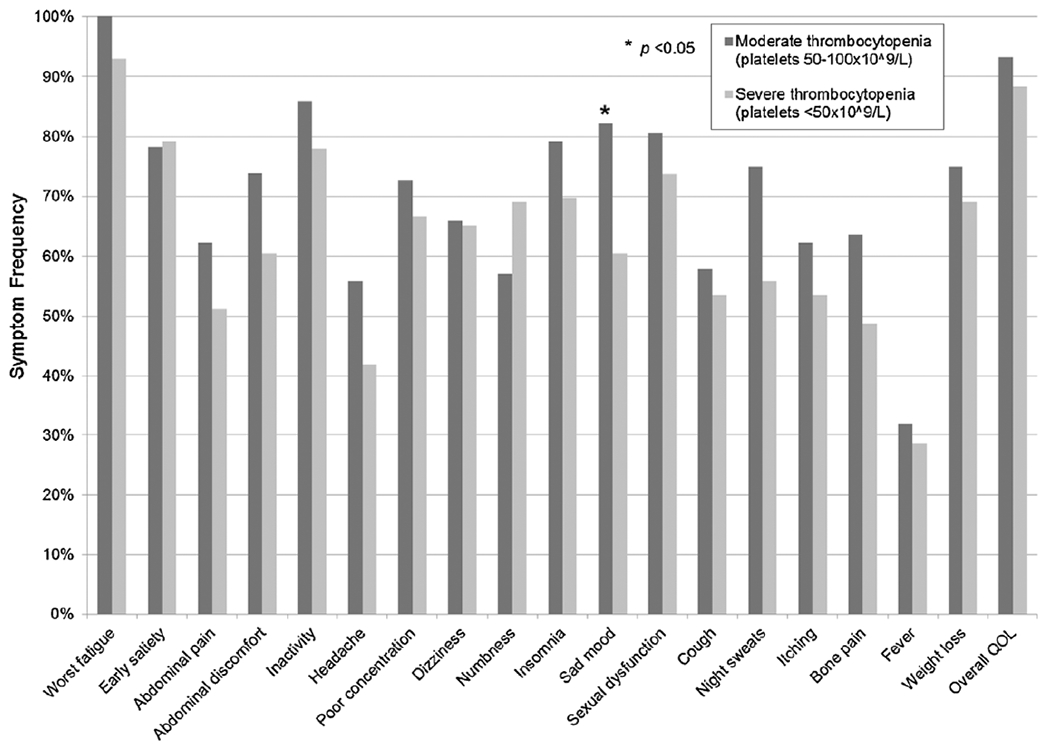
Moderate versus severe thrombocytopenia by individual MPN-SAF symptom frequency.
4. Discussion
As a group, myelofibrosis patients experience a high symptom burden affecting quality of life. In this analysis, we demonstrate that MF patients with thrombocytopenia exhibit an even greater symptom burden, as well as distinct clinical characteristics, when compared to MF patients without thrombocytopenia. Several important symptoms were both more prevalent and more severe in MF patients with thrombocytopenia including fatigue, inactivity, early satiety, and impaired quality of life. In accordance with this, the total symptom score was also significantly higher. Notably, there do appear to be some symptom clusters for which there are no major differences between MF patients with and without thrombocytopenia. These include symptoms related to abdominal complaints (pain, discomfort), neurological issues (headache, poor concentration, numbness, insomnia), or extramedullary hematopoiesis (bone pain, abdominal pain).
Interestingly, data from this study do not demonstrate a significant association between DIPSS risk category and either presence or degree of thrombocytopenia, despite the fact that thrombocytopenia is a known risk factor for disease advancement. In fact, the DIPSS Plus score, an updated version of the DIPSS scoring system, includes platelet count in its calculation [5]. While we did observe a trend toward patients with lower platelet counts having higher DIPSS scores, this did not reach statistical significance. This may be because other clinical factors included in the DIPSS score such as age did not differ significantly between study groups, thus diluting the effect of thrombocytopenia or other laboratory abnormalities. The fact that thrombocytopenic patients experience a high degree of symptoms despite them not being in a higher risk category suggests that clinical risk score is not necessarily an accurate surrogate for symptom burden or quality of life for MF patients. This aligns with previously published data suggesting that high symptom burden can be seen even in patients with lower clinical risk scores due to significant heterogeneity of symptomatology within MF and other MPN subtypes [12].
In this study, patients with thrombocytopenia were no more likely to have a history of thrombosis or hemorrhage than patients without thrombocytopenia. This is supported by other literature suggesting that low platelet counts in this population do not necessarily correlate with risk for bleeding complications [13]. Additionally, some data have shown that MF patients with lower platelet counts at diagnosis actually have a higher risk for arterial, venous, and total thrombotic events [14,15]. This likely because thrombocytopenia is associated with more advanced disease and higher degrees of inflammation and is therefore seen in association with thrombosis. In addition, cytoreductive therapy to control thrombocytosis does not necessarily result in decreased risk for thrombosis [16].
Of note, in this study abdominal pain and discomfort were two of the few symptoms that were not rated as more severe in thrombocytopenic patients with MF as compared to patients with normal platelet counts. This is somewhat surprising, as thrombocytopenic MF patients frequently experience splenomegaly due to both extramedullary hematopoiesis and splenic platelet sequestration. This in turn can cause abdominal symptomatology that negatively impacts QOL. The fact that these symptoms were not reported to a high degree in this study population suggests that bone marrow failure more than splenic sequestration may be the underlying cause of thrombocytopenia in this patient group. In addition, the lack of symptom differences between the moderate and severe thrombocytopenia groups indicates that symptom burden may not be related directly to low platelet counts, but rather to the underlying bone marrow failure and its sequelae that are seen in the more advanced disease states characteristic of these patients. In short, the utility of platelet count as a clinical indicator of disease status and burden is at least in part related to its role as a marker of adequate bone marrow function for MF patients.
While treatment options for MF patients with thrombocytopenia have been limited due to risk of exacerbating cytopenias, more recent data indicate that judicious use of ruxolitinib in this group can be both safe and clinically effective. An interim analysis of the phase III COMFORT-I and -II trials evaluating the subset of MF patients with platelet counts of 50–100 × 109/L found that more than half of patients achieved stable dosing and demonstrated an average 24% reduction in spleen volume and 44% reduction in TSS [17]. Similarly, the first post-market analysis of ruxolitinib showed that patients with platelets under 100 × 109/L experienced improvement in symptom burden and a decrease in splenomegaly with only minimal decline in platelet count and no serious bleeding complications [18]. Successful use of ruxolitinib, even in patients with severe thrombocytopenia, has also been reported with minimal adverse effects [19].
Pacritinib, an inhibitor of both JAK2 and Fms-related tyrosine kinase 3 (FLT3), has also been tested in phase III trials that included patients with baseline cytopenias. The PERSIST-1 trial demonstrated that pacritinib reduces MF symptom burden even amongst patients with baseline thrombocytopenia [20]. The PERSIST-2 trial specifically evaluated pacritinib in MF patients with platelets less than 100 × 109/L, including those who previously received ruxolitinib, and found it be more effective than best available therapy at inducing spleen volume reduction and decreasing TSS [21].
Given the high symptomatic burden in this population, these data support a paradigm where MF patients with thrombocytopenia can be reasonably considered for targeted therapies. While there is some risk of lowering platelet count further or exacerbating concomitant cytopenias in this setting, there is now evidence that with close monitoring these patients can be safely treated in many cases. In conclusion, the results of this study demonstrate that MF patients with thrombocytopenia face a significant symptom burden and are likely to benefit from close surveillance of their symptomatology while receiving consideration for additional therapies.
Acknowledgements
The authors wish to acknowledge the patients, families, and clinicians whose participation made this research possible. All research was funded by internal grant funding.
References
- [1].Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. , Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders, Lancet (London, England) 365 (9464) (2005) 1054–1061. [DOI] [PubMed] [Google Scholar]
- [2].Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. , A gain-of-function mutation of JAK2 in myeloproliferative disorders, N. Engl. J. Med 352 (17) (2005) 1779–1790. [DOI] [PubMed] [Google Scholar]
- [3].Zhao ZJ, Vainchenker W, Krantz SB, Casadevall N, Constantinescu SN, Role of tyrosine kinases and phosphatases in polycythemia vera, Semin. Hematol 42 (4) (2005) 221–229. [DOI] [PubMed] [Google Scholar]
- [4].Mesa RA, Niblack J, Wadleigh M, Verstovsek S, Camoriano J, Barnes S, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international internet-based survey of 1179 MPD patients, Cancer 109 (1) (2007) 68–76. [DOI] [PubMed] [Google Scholar]
- [5].Gangat N, Caramazza D, Vaidya R, George G, Begna K, Schwager S, et al. , DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status, J. Clin. Oncol 29 (4) (2011) 392–397. [DOI] [PubMed] [Google Scholar]
- [6].Hernandez-Boluda JC, Correa JG, Alvarez-Larran A, Ferrer-Marin F, Raya JM, Martinez-Lopez J, et al. , Clinical characteristics, prognosis and treatment of myelofibrosis patients with severe thrombocytopenia, Br. J. Haematol (2017). [DOI] [PubMed] [Google Scholar]
- [7].Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. , JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis, N. Engl. J. Med 366 (9) (2012) 787–798. [DOI] [PubMed] [Google Scholar]
- [8].Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. , A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis, N. Engl. J. Med 366 (9) (2012) 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Scherber R, Dueck AC, Johansson P, Barbui T, Barosi G, Vannucchi AM, et al. , The myeloproliferative neoplasm symptom assessment form (MPN-SAF): international prospective validation and reliability trial in 402 patients, Blood 118 (2) (2011) 401–408. [DOI] [PubMed] [Google Scholar]
- [10].Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, et al. , The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory, Cancer 85 (5) (1999) 1186–1196. [DOI] [PubMed] [Google Scholar]
- [11].Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A, et al. , A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment), Blood 115 (9) (2010) 1703–1708. [DOI] [PubMed] [Google Scholar]
- [12].Geyer HL, Scherber RM, Dueck AC, Kiladjian JJ, Xiao Z, Slot S, et al. , Distinct clustering of symptomatic burden among myeloproliferative neoplasm patients: retrospective assessment in 1470 patients, Blood 123 (24) (2014) 3803–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kander EM, Raza S, Zhou Z, Gao J, Zakarija A, McMahon BJ, et al. , Bleeding complications in BCR-ABL negative myeloproliferative neoplasms: prevalence, type, and risk factors in a single-center cohort, Int. J. Hematol 102 (5) (2015) 587–593. [DOI] [PubMed] [Google Scholar]
- [14].Buxhofer-Ausch V, Gisslinger H, Thiele J, Gisslinger B, Kvasnicka HM, Mullauer L, et al. , Leukocytosis as an important risk factor for arterial thrombosis in WHO-defined early/prefibrotic myelofibrosis: an international study of 264 patients, Am. J. Hematol 87 (7) (2012) 669–672. [DOI] [PubMed] [Google Scholar]
- [15].Schwarz J, Penka M, Ovesna P, Cerna O, Brychtova Y, Dulicek P, Thrombosis in MPN with thrombocythemia is associated with higher platelet count at the time of the event: data from the czech registry of patients treated with anagrelide, Blood 118 (21) (2011) 3857. [Google Scholar]
- [16].Palandri F, Catani L, Testoni N, Ottaviani E, Polverelli N, Fiacchini M, et al. , Long-term follow-up of 386 consecutive patients with essential thrombocythemia: safety of cytoreductive therapy, Am. J. Hematol 84 (4) (2009) 215–220. [DOI] [PubMed] [Google Scholar]
- [17].Talpaz M, Paquette R, Afrin L, Hamburg SI, Prchal JT, Jamieson K, et al. , Interim analysis of safety and efficacy of ruxolitinib in patients with myelofibrosis and low platelet counts, J. Hematol. Oncol 6 (1) (2013) 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Geyer H, Cannon K, Knight E, Fauble V, Camoriano J, Emanuel R, et al. , Ruxolitinib in clinical practice for therapy of myelofibrosis: single USA center experience following Food and Drug Administration approval, Leuk. Lymphoma 55 (1) (2014) 195–197. [DOI] [PubMed] [Google Scholar]
- [19].Bjorn ME, Holmstrom MO, Hasselbalch HC, Ruxolitinib is manageable in patients with myelofibrosis and severe thrombocytopenia: a report on 12 Danish patients, Leuk. Lymphoma 57 (1) (2016) 125–128. [DOI] [PubMed] [Google Scholar]
- [20].Mesa RA, Vannucchi AM, Mead A, Egyed M, Szoke A, Suvorov A, et al. , Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): an international, randomised, phase 3 trial, Lancet Haematol. 4 (5) (2017) e225–e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mascarenhas J, Hoffman R, Talpaz M, Gerds AT, Stein B, Gupta V, et al. , Results of the persist-2 phase 3 study of pacritinib (PAC) versus best available therapy (BAT), including ruxolitinib (RUX), in patients (pts) with myelofibrosis (MF) and platelet counts < 100,000/μl, Blood 128 (22) (2016) LBA–5. [Google Scholar]


