Abstract
Summary: An estimated two million Americans suffer from chemosensory disorders. We present the clinical and imaging findings in three hyposmic patients with bilateral olfactory bulb calcification detected by CT. To our knowledge, these are the first cases of olfactory bulb calcification reported in the literature. A review of the literature concerning calcification of cranial nerves, olfactory neuritis, and the potential etiology and clinical significance of olfactory bulb calcification in our patients is presented.
An estimated two million Americans suffer from taste and smell disorders, and as many as 25% of cases are classified as idiopathic (1). We present three patients with hyposmia and olfactory bulb calcification identified on coronal sinus CT scans. To our knowledge, these are the first cases of olfactory nerve calcification to be reported in the literature. Following the case presentations, the etiologies of olfactory dysfunction, clinical evaluation methods, and potential relationship of olfactory nerve calcification to hyposmia are discussed.
Case Reports
Patient 1
A 23-year-old white female patient presented with the complaint of diminished sense of smell. Her medical history was significant for basal cell nevus syndrome. She was taking no medications and denied any tobacco use. She denied any nasal obstruction, rhinorrhea, facial pain, or headaches. Findings on physical examination were unremarkable, as were those at nasal endoscopy. The University of Pennsylvania Smell Identification Test (UPSIT), a multiple-choice odor identification test, was administered, and the patient correctly identified 75% (30/40) of the odors on the right and 72.5% (29/40) on the left, which classified the patient’s condition as mildly hyposmic. Coronal sinonasal CT examination demonstrated calcification within the olfactory bulbs bilaterally (Fig 1).
Fig 1.
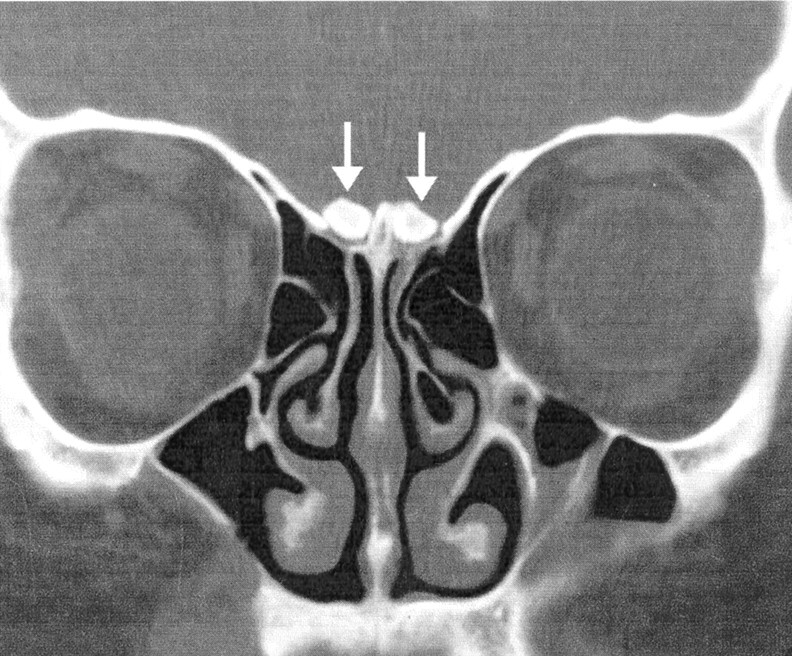
Coronal CT scan from patient 1 demonstrates attenuated, rounded calcifications above the cribriform plate (arrows) in the location of the olfactory bulbs.
Patient 2
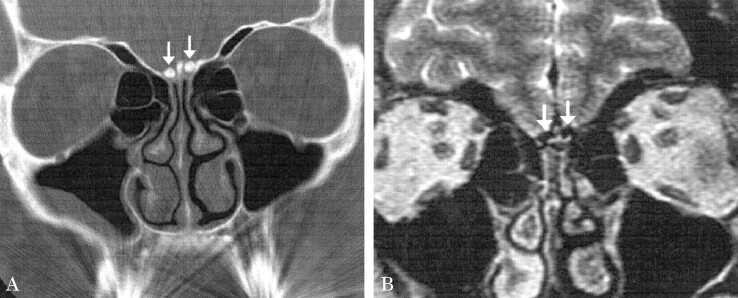
A 49-year-old African-American female patient presented with altered flavor perception and frontal headaches. She did not have specific olfactory complaints. Her medical history was significant for hypertension, diabetes, seizures, and migraines. She denied any tobacco use, nasal obstruction, rhinorrhea, or facial pain. Physical examination findings were unremarkable, as were those at nasal endoscopy. On UPSIT, the patient correctly identified 87.5% (35/40) of odors on each side, which classified her condition as mildly hyposmic when her score was adjusted for age. Taste testing results were normal. CT of the sinonasal cavities revealed bilateral olfactory bulb calcification (Fig 2A). MR images showed no abnormalities of the anterior skull base or cerebrum; however, hypointense signal on long-TR images was noted in the olfactory bulbs (Fig 2B), corresponding to the dense calcifications shown by CT.
Fig 2.
Patient 2.
A, Coronal CT image shows attenuated calcification in the location of the olfactory bulbs (arrows) similar to that seen in patient 1.
B, Coronal T2-weighted MR image demonstrates markedly hypointense signal in the olfactory bulbs (arrows) corresponding to the areas of calcification noted on the corresponding CT scan (A). No other anterior cranial fossa lesions were identified.
Patient 3
A 61-year-old white female patient presented with the complaint of a “bad odor” as well as distortion of smell and flavor of most foods. Her medical history was significant for GERD (Gastroesophageal Reflux Disease), diverticulosis, and sensorineural hearing loss. She denied any tobacco use, nasal obstruction, rhinorrhea, facial pain, or headaches. Physical examination findings were unremarkable, as were those at nasal endoscopy. At smell identification testing, the patient correctly identified 62.5% (25/40) of odors, classifying her as moderately hyposmic. A CT scan demonstrated punctate bilateral olfactory bulb calcification (Fig 3A). MR imaging revealed mild enhancement of both olfactory bulbs, but no other anterior cranial fossa abnormalities (Fig 3B and C). Follow-up CT 1 year later demonstrated no progression of the amount of calcification within the olfactory bulbs.
Fig 3.
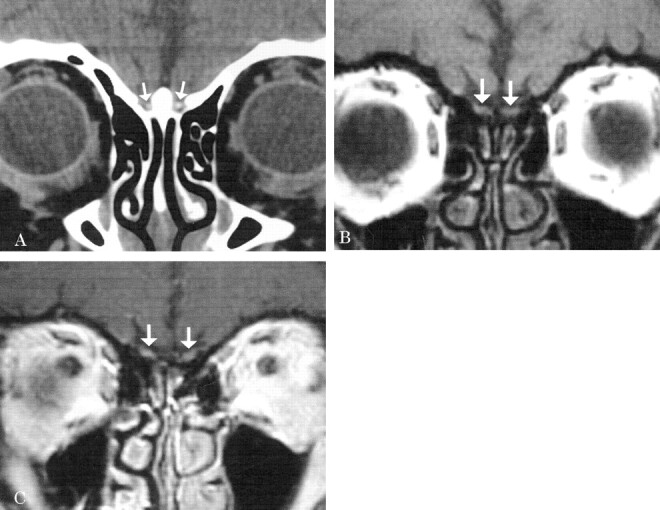
Patient 3.
A, Coronal CT scan shows punctate calcifications in the expected location of the olfactory bulbs (arrows).
B, Coronal T1-weighted MR image demonstrates the olfactory bulbs (arrows) before contrast agent administration.
C, Postcontrast coronal T1-weighted MR image demonstrates mild enhancement of both olfactory bulbs (arrows).
Discussion
The olfactory neuroepithelium occupies a 1-cm2 area in the superior nasal vault. Smell is perceived when an odorant molecule reaches the olfactory epithelium and interacts with a specific complementary receptor. This results in the generation of afferent impulses, which are transmitted across the cribriform plate to the central olfactory centers. Smell disorders are classified as anosmia (a complete loss of smell), hyperosmia (an enhanced sense of smell), and hyposmia (a decreased sense of smell). Dysosmia refers to distorted odor perception. Dysosmias can be further classified as either parosmias, a distortion of an odorant stimulus, or phantosmias, an intermittent or continuous perception of an odor when no odorant stimulus is present. It is important to note that most patients with a chemosensory dysfunction will complain of distorted taste, although this perception usually results from olfactory dysfunction.
Etiology of Olfactory Dysfunction
Potential etiologies of hyposmia include idiopathic origin (26%), head injury (19%), prior upper respiratory tract infection (URI) (17%), sinonasal disease (16%), toxic exposure (6%), multiple causes (5%), congenital anosmia (2%), age-related olfactory loss (1%), and miscellaneous causes (8%) (2). An estimated 5–30% of head injury patients will have olfactory dysfunction, most often anosmia (3). The mechanism is presumed to be tearing of the olfactory epithelium, shearing of axons as they pass through the cribriform plate, or both. Prognosis for these patients is higher than other categories of olfactory loss, with 40% of trauma patients regaining some or all of their sense of smell (4).
Postinfectious olfactory loss, usually secondary to viral URI, is diagnosed on the basis of the temporal relationship of the olfactory loss to a URI. These patients most commonly experience hyposmia and dysosmia (2). The mechanism of olfactory dysfunction in these cases is not clearly understood, but studies have shown that the olfactory neuroepithelium undergoes a direct insult from the virus (5). Spontaneous recovery may occur slowly over years but is uncommon (6).
Olfactory loss due to sinonasal inflammatory and neoplastic disorders is usually caused by direct obstruction of the olfactory clefts. Chronic rhinosinusitis, allergic rhinitis, and polyposis are the most common etiologies of mucosal congestion. These patients describe fluctuating levels of olfactory function with intermittent hyposmia or anosmia. Phantosmias are often indicative of sinus infection although, in rare cases, they may be caused by metabolic disorders (2). Obstructive olfactory loss can sometimes be reversed with treatment of the underlying nasal or sinus disease. Thus, the prognosis for recovery is favorable.
Olfactory dysfunction from toxic exposure is related to either an acute, large, inadvertent exposure to a toxin or a long-term, low-grade exposure (2). Phosphorus fire, chlorine gas, metal dusts, solvents, acids, oil vapors, and household cleaners have all been associated with olfactory loss (2, 7). These substances directly injure the olfactory neuroepithelium, the olfactory nerves, or both (7). Conflicting data about the effects of chronic cigarette smoking have been presented, but these likely represent other causes of olfactory loss (3). The prognosis for recovery depends on the substance and intensity of exposure, but it is often poor.
Congenital olfactory dysfunction with resulting anosmia have been noted in 2% of patients (2). Several syndromes have been associated with olfactory loss, including Turner and Kallman syndromes. Biopsy results from these patients have shown the olfactory neuroepithelium to be underdeveloped or immature (8).
Smell disorders related to age have been cited most commonly after the 7th decade of life. Both men and women experience a marked decrease in olfaction after the age of 69 years and are usually classified as hyposmic (9). Age-related olfactory loss is attributed to degenerative changes in nasal epithelial receptors and the replacement of the olfactory neuroepithelium by respiratory epithelium in a random pattern (10).
Evaluation of Patients with Olfactory Dysfunction
The evaluation of patients with disorders of smell and taste begins with a thorough history and physical examination. The history should include present medications, history of prior head trauma, toxic exposure, and recent URI. Physical examination should include a complete ear, nose, and throat examination as well as a cranial nerve examination. Nasal endoscopy is helpful to exclude sinonasal inflammatory or neoplastic disease. Chemosensory testing should be conducted because of the frequent confusion between smell, taste, and flavor perception. Olfactory testing involves identification of specific odors, threshold testing, or both. The UPSIT is a standardized, easily administered odor identification test that uses a multiple-choice format (11).
Imaging studies are indicated in the workup of chemosensory disorders if the history and physical examination do not provide a clear diagnosis. CT is the initial study in evaluating patients with hyposmia, particularly when the etiology is thought to be sinonasal obstructive disease. CT scanning demonstrates the bony anatomy and air passages of the sinonasal cavities and detects mucosal thickening, polyps, and masses. CT scanning is more sensitive for detecting calcification than MR imaging, and the olfactory bulb calcifications in our patients were well demonstrated with standard thin-section coronal sinonasal CT.
MR imaging is a valuable tool for evaluating the hyposmic patient, particularly those in whom a causative disease process is not detected within the nasal cavity at CT. MR imaging is superior to CT scanning for excluding intracranial disease and for evaluating the extent of known anterior cranial fossa pathologic process. The multiplanar capability of MR imaging allows optimal visualization of the olfactory bulbs, tracts, and anterior cranial fossa structures responsible for olfactory function (12). Although MR imaging is somewhat less sensitive for evaluating bony anatomy and detecting calcification, it is clearly superior for delineating the extent of soft-tissue masses traversing the skull base, evaluating parenchymal lesions within the frontal lobes and hypothalamus, and confirming the precise anatomic location of an abnormality seen on CT scans, such as calcification of the olfactory bulbs as noted in patient 2 in our series.
Relationship of Calcification to Nerve Function
The literature was reviewed with respect to olfactory neuritis as a cause of hyposmia, olfactory (and other cranial nerve) calcification, and the relationship of calcification to olfactory dysfunction. No previous cases of olfactory bulb calcification in hyposmic patients have been reported, and little literature about olfactory neuritis was discovered. Olfactory neuritis, as a distinct entity, was described in a 1972 article that studied 17 patients (13). In this article, Kitsera hypothesized that anosmia or hyposmia may result from olfactory neuritis, but no convincing scientific evidence was given to support this claim. Imaging studies were not obtained in these patients. Little literature is available describing the relationship between inflammatory cranial neuritis and nerve calcification. A study by Bajandas and Smith (14) found that calcification within nutrient vessels supplying the optic nerve may play a role in the development of optic neuropathy. The authors hypothesized that the vascular calcification led to ischemia of the nerve, producing neuritis.
Several studies have discussed the role of imaging in the evaluation of patients with chemosensory dysfunction. A study by Schellinger et al (15) described 354 patients with taste, smell, or both taste and smell disorders evaluated with CT. CT abnormalities including frontal encephalomalacia, subfrontal atrophy in the area of the olfactory bulbs, and anterior temporal lobe atrophy, all related to prior trauma were found in 31% of patients. Schellinger et al described no olfactory nerve calcification. Calcification around, but not within, the nerves has been reported infrequently in the literature. Doty (9) investigated the etiology of age-related olfactory dysfunction and found that calcification of the cribriform plate was potentially related to a decline in sense of smell. The hypothesis in the study was that calcification led to degenerative changes of the olfactory neurons. MR imaging has also been used to evaluate patients with smell disorders. As previously mentioned, MR imaging is the method of choice for visualization of the olfactory bulbs and olfactory tracts and for detecting intracranial lesions affecting olfactory function (12). MR imaging evaluation studies of congenital and posttraumatic olfactory dysfunction have shown volume loss of the olfactory bulbs and tracts but make no note of neural calcification (16, 17).
Other authors have noted calcification of degenerating myelinated axons elsewhere within the nervous system that may suggest a causal relationship between calcification and neurodegenerative changes (18). Axonal calcification has been seen in traumatized spinal cord tissue in humans and animals (19). Using an animal model, these authors demonstrated calcium-induced paraplegia. Histologic analysis supported the authors supposition that calcium-mediated necrosis led to nerve dysfunction. Several metabolic states and syndromes have been associated with cerebral and meningeal calcification, and these patients may develop cranial nerve dysfunction. Some of these conditions include idiopathic hypoparathyroidism, pseudohypoparathyroidism, Paget disease, and carbonic anhydrase II deficiency (20). Cranial nerve dysfunction in these cases may be related to nerve compression from foraminal narrowing in the skull base. Peripheral neuropathy, commonly seen in diabetic patients, has also been linked to perineural calcification (21). Peripheral and cranial nerve neuropathies due to neural calcification have been reported in association with scleroderma and its CREST variant (22).
The etiology of olfactory dysfunction in the three patients we present is presumed to be idiopathic, and the relationship of olfactory bulb calcification to their hyposmia is uncertain. The calcification may represent the sequela of previous olfactory neuritis or unrecognized toxic exposure. Alternatively, the calcification of the nerve may be the primary disease causing olfactory dysfunction. In no cases were images of our patients available from prior to their presentation with hyposmia; thus, the temporal relationship between the onset of hyposmia and the development of the olfactory bulb calcification cannot be determined.
Conclusion
Disorders of olfactory function are frequently attributed to prior head injury, previous URIs, sinonasal disease, toxic exposures, or are considered idiopathic. The origin of olfactory bulb calcification in the three patients presented and relationship to their olfactory dysfunction remain uncertain. Although evidence exists to support that calcium can have a negative effect on nerve function in the experimental setting, no direct evidence that calcification of the olfactory nerve is a cause of olfactory dysfunction has been confirmed. Further study is necessary to clarify the role of olfactory bulb calcification in patients with chemosensory symptoms.
Footnotes
Presented at the 40th annual meeting of the American Society of Neuroradiology, Vancouver, B.C., May, 11–17, 2002.
References
- 1.Schiffman SS. Taste and smell in disease. N Engl J Med 1983;308:1337–1343 [DOI] [PubMed] [Google Scholar]
- 2.Seiden AM. The initial assessment of patients with taste and smell disorders. In: Seiden AM, ed. Taste and Smell Disorders. 1st ed. New York: Thieme;1997. :4–19
- 3.Duncan HJ, Smith DV. Clinical disorders of olfaction: a review. In: Doty RL, ed. Handbook of Olfaction and Gustation. 1st ed. New York: Marcel Dekker;1995. :345–365
- 4.Truwit CL, Kelly WM. The olfactory system. Neuroimaging Clin North Am 1993;3:47–70 [Google Scholar]
- 5.Jafek BW, Hartman D, Eller PM, et al. Postviral olfactory dysfunction. Am J Rhinol 1990;4:91–100 [Google Scholar]
- 6.Mott AE, Leopold DA. Disorders in taste and smell. Med Clin North Am 1991;75:1321–1353 [DOI] [PubMed] [Google Scholar]
- 7.Amoore JE. Effects of chemical exposure on olfaction in humans. In: Barrows CS, ed. Toxicology of the Nasal Passages. 1st ed. Washington, DC: Hemisphere;1986. :155–190
- 8.Leopola DA, Hornung DE, Schwob JE. Congenital lack of olfactory ability. Ann Otol Rhinol Laryngol 1992;101:229–36 [DOI] [PubMed] [Google Scholar]
- 9.Doty RL. Influence of age and age-related diseases on olfactory function. Ann N Y Acad Sci 1989;561:76–86 [DOI] [PubMed] [Google Scholar]
- 10.Paik S, Lehman MN, Seiden AM, et al. Human olfactory biopsy. Arch Otolaryngol Head Neck Surg 1992;118:731–738 [DOI] [PubMed] [Google Scholar]
- 11.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania smell identification test: a standardized microencapsulated test of olfactory function. Physiol Behavior 1984;32:489–502 [DOI] [PubMed] [Google Scholar]
- 12.Li C, Yousem DM, Doty RL, Kennedy DW. Evaluation of olfactory deficits by medical imaging. In: Doty RL, ed. Handbook of Olfaction and Gustation. 1st ed. New York: Marcel Dekker;1995. :395–419
- 13.Kitsera AE. Diagnosis and treatment of olfactory neuritis. Zhurnal Ushnykh Nosovykh i Gorlovykh Boleznei (Journal of Otology, Rhinology, and Laryngology) 1972;32:26–30 [PubMed] [Google Scholar]
- 14.Bajandas FJ, Smith JL. Optic neuritis in hypoparathyroidism. Neurology 1976;26:451–454 [DOI] [PubMed] [Google Scholar]
- 15.Schellinger D, Henkin RT, Smirniotopoulos JG. CT of the brain in taste and smell dysfunction. AJNR Am J Neuroradiol 1983;4:752–754 [PMC free article] [PubMed] [Google Scholar]
- 16.Yousem DM, Geckle RJ, Bilker WB, et al. Posttraumatic olfactory dysfunction: MR and clinical evaluation. AJNR Am J Neuroradiol 1996;17:1171–1179 [PMC free article] [PubMed] [Google Scholar]
- 17.Yousem DM, Geckle RJ, Bilker WB, et al. MR evaluation of patients with congenital hyposmia or anosmia. AJR Am J Roentgenol 1996;166:439–443 [DOI] [PubMed] [Google Scholar]
- 18.Korf J, Postema F. Regional calcium accumulation and cation shifts in rat brain kainate. J Neurochem 1984;43:1052–1060 [DOI] [PubMed] [Google Scholar]
- 19.Balentine JD, Dean DL Jr. Calcium-induced spongiform and necrotizing myelopathy. Lab Invest 1982;47:286–295 [PubMed] [Google Scholar]
- 20.Whyte MP. Carbonic anhydrase II deficiency. Clin Orthop 1993;294:52–63 [PubMed] [Google Scholar]
- 21.Kalimo H, Maki J, Paetau A, Haltia M. Microanalysis of perineural calcification in diabetic nephropathy. Muscle Nerve 1981;4:228–233 [DOI] [PubMed] [Google Scholar]
- 22.Polio JL, Stern PJ. Digital nerve calcification in CREST syndrome. J Hand Surg 1989;14:201–203 [DOI] [PubMed] [Google Scholar]