Abstract
BACKGROUND AND PURPOSE: Because of increased prevalence of macrocephaly in autism, head size must be controlled for in studies that examine volumetric findings of the temporal lobe in autistic subjects. We prospectively examined temporal lobe structures in individuals with autism who were normocephalic or macrocephalic (head circumference > 97th percentile) and in control subjects who were normocephalic or macrocephalic or who had a reading disorder (unselected for head size). The rationale for the reading disorder group was to have control subjects with potential temporal lobe anomalies, but who were not autistic.
METHODS: In individuals aged 7–31 years, autism was diagnosed on the basis of standardized interview and diagnostic criteria. Control subjects ranged in age from 7 to 22 years. All subjects were male. MR morphometrics of the major temporal lobe structures were based on ANALYZE segmentation routines, in which total brain volume and total intracranial volume (TICV) were calculated. Both group comparisons and developmental analyses were performed.
RESULTS: No distinct temporal lobe abnormalities of volume were observed once head size (TICV) was controlled for. In autistic and control subjects, robust growth patterns were observed in white and gray matter that differed little between the groups. Although subtle differences were observed in some structures (ie, less white matter volume in the region of the temporal stem and overall temporal lobe), none was statistically significant.
CONCLUSION: No major volumetric anomalies of the temporal lobe were found in cases of autism when IQ, TICV, and age were controlled. Temporal lobe abnormalities that may be associated with autism are likely to be more related to functional organization within the temporal lobe than to any gross volumetric difference.
Some form of temporal lobe abnormality has been suspect in autism since attention was first directed to neurobiologic explanations of this developmental disorder (1). Temporal lobe abnormality in autism is a likely candidate because core symptoms of the disorder center on deficits in language and social behavior, which are frequently accompanied by intellectual impairment—all functions thought to be subserved, at least in part, by the temporal lobes (2, 3). Several neuroimaging studies demonstrated temporal lobe abnormalities in autism, including volume differences in the hippocampus and amygdala when compared with age- and sex-matched control subjects (4–6), although not all found differences (7). Other studies demonstrated an association between autism and lesions of the temporal lobe in individuals with tuberous sclerosis (8, 9). Recently, Weidenheim et al (10) demonstrated the presence of dystrophic axons in hippocampus and other limbic structures in two cases of individuals with autistic behavior.
Normal brain growth has long been recognized as an index of healthy development, and therefore volume differences in the brain or its structures can be an index of abnormal development (11, 12). One of the most consistent morphologic findings in autism has been the increased prevalence of macrocephaly (12–22). Typically, since presence of macrocephaly is defined by head circumference greater than 97th percentile for age, by definition it would be expected that only approximately 3% of the population would have macrocephaly (23). For example, in the study by Petersson et al (24), in which they specifically examined for megalencephaly in a large sample of neonates, less than 1% was found in their sample. In contrast, prevalence studies of macrocephaly in autism have consistently reported levels to be between 10% and 20% (13–15, 17, 18). Several studies demonstrated that increased rates of macrocephaly are not present at birth in children who later receive a diagnosis of autism (5, 13, 25), but appear to develop by age 3 years (5, 25).
Accordingly, because of the increased prevalence rate of macrocephaly, the issue of macrocephaly in autism becomes central to studies that examine volumetric findings of the temporal lobe. Without proper control for presence of macrocephaly, size difference in comparison to neurologically normal control subjects could easily be a simple function of the over representation of macrocephaly in autism. What is needed is a control comparison group of subjects with benign macrocephaly who do not have autism. However, to our knowledge, no study has specifically addressed the issue of macrocephaly in autism and temporal lobe development. For example, are there differences in volume of temporal lobe structures between individuals with autism who are normocephalic versus those who are macrocephalic, that are not merely proportional to overall head size? We directly investigate such relationships by specifically comparing autistic subjects with head circumference greater than the 3rd but less than or equal to the 97th percentile versus those with macrocephaly (head circumference > 97th percentile) and similar control subjects.
Another confound in previous research has to do with the relationship between brain volume and intellectual status (26). At the extremes of brain size, there is greater likelihood of mental retardation (27). In fact, smaller brain volume in individuals who have moderate to severe levels of mental retardation has been established by several reports (28–31). Since mental retardation is often found in autism (32), not controlling for IQ likely represents an additional potential confound in studying volumetric differences between autistic and control subjects. However, since verbal skills are often diminished in autism (32), the match should be based on nonverbal abilities such as that obtained with the performance IQ (PIQ) from the Wechsler or other scales of intelligence (33, 34). In addition, to focus on the specificity of potential temporal lobe anomalies unique to autism, the control group should also contain subjects with a nonautistic but temporal lobe-based disorder like reading disability (35–39).
Accordingly, the purpose of this prospective investigation was to perform detailed temporal lobe volumetric analyses on normocephalic and macrocephalic individuals with and those without autism to test for differences in temporal lobe structures when head size is controlled for. Expanding the comparison sample to include subjects with a reading disorder ensures a broad sampling of individuals with potential brain anomalies of the temporal lobe but who do not have autism. Also, we examined autistic subjects with IQ scores of 62 or higher.
Detailed analysis of the five main temporal lobe gyri (superior, middle, inferior, fusiform, and parahippocampal) included total volume along with white and gray matter volumes. CSF volumes of the major temporal lobe structures were also calculated, including those of the sylvian fissure and superior, middle, inferior, and rhinal sulci along with the temporal horn of the lateral ventricle. Although the landmarks are distinct for the five gyri on the lateral and medial surface, at the very tip of the temporal lobe, some boundaries that separate the gyri become less distinct. Accordingly, we included a separate measure that involved just the tip or pole of the temporal lobe. Whereas the above measures cover most of the temporal lobe volume, there is a region of the temporal lobe, referred to as the temporal stem (40), that is not included in any gyral volume. This region of interest is particularly important in temporal lobe morphometrics because this structure represents the confluence of major temporal lobe pathways. Finally, we also determined the volume of the amygdala and hippocampus in these groups. Furthermore, we examined subregions of the hippocampus by calculating surface area (mm2) for the area dentata and hippocampal subregion of CA1-CA3 and subiculum (CAS) as outlined by Saitoh et al (4) who found smaller area dentata in their sample of autistic subjects.
Methods
Subjects
In this prospective, institutional review board-approved study, we actively recruited subjects for a 4-year period (June 1998 to July 2002) during which most autistic and comparison subjects were ascertained from community sources, including social skills training groups, parent support groups, youth groups, and schools. Some of the autistic subjects had participated in other research at the University of Utah. Five subject groups were studied: autism subjects unselected for head size, autistic subjects selected for macrocephaly (head circumference > 97th percentile for age and sex), healthy (typically developing) subjects unselected for head size, typically developing subjects with benign macrocephaly, and subjects with reading disorder unselected for head size. During the initial ascertainment phase of subject identification and classification, we recruited autistic subjects irrespective of head size; however, four of these subjects met criterion for macrocephaly. Later, we specifically selected for autistic subjects who met criteria for macrocephaly (n = 8), yielding a total of 12 autistic subjects with macrocephaly. This permitted creation of two autism groups, one characterized as normocephalic (n = 26), the other as macrocephalic (n = 12). Control subjects were selected as either normocephalic or macrocephalic; ascertainment of typically developing subjects was initially without selection for head size and later it was specific for subjects with benign macrocephaly. Control subjects with reading disorder were unselected for head size. When plotting growth curves, we used a single dichotomy of autism versus control subjects (including the subjects with reading disorder), irrespective of head size.
Autism associated with high versus low IQ may differ in etiology (41) and neuroanatomy (42). The same is true for autism in male versus female subjects and autism associated with causal medical conditions. Because we wanted our autism and comparison samples to be as homogeneous as possible with regard to IQ, sex, and etiology, all autistic subjects in this study were male subjects with idiopathic autism who had PIQs of 62 or higher based on either the Wechsler Intelligence Scale for Children-III (33) or the Wechsler Adult Intelligence Scale-III (34). Only three of the autistic subjects had PIQ scores equal to or less than 69, the mild range of mental retardation. The comparison group consisted of healthy (typically developing) subjects with or without macrocephaly and subjects with reading disorder, group-matched by age to the autistic subjects.
Autistic Subjects.
Autism was rigorously diagnosed. The subject’s mother was interviewed by using the Autism Diagnostic Interview-Revised (ADI-R), a semistructured, investigator-based interview with good reliability and validity (43). In addition, autistic subjects were directly assessed by using the Autism Diagnostic Observation Schedule-Generic (ADOS-G), a semistructured play and interview session designed to elicit social, communication, and stereotyped repetitive behaviors characteristic of autism (44). All autistic subjects met ADI-R, ADOS-G, and Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (45) criteria for autism. History, physical examination, Fragile-X gene testing, and karyotype excluded medical causes of autism and were performed on all subjects.
Comparison Subjects.
All subjects with reading disorder had a documented history of a reading disorder and on all of the following reading tests performed below their full scale IQ (FSIQ) scores: Woodcock-Johnson Psycho-Educational Battery-Revised (46) word attack and letter word identification (tests of phonologic processing); Wide Range Achievement Test (47) of spelling; and the Gray Oral Reading Tests, Fourth Edition (48) fluency score (reading speed and accuracy). All subjects with reading disorder scored 1.5 standard deviations (SDs) or more below FSIQ on at least two of the tests or 2 SDs below FSIQ on one of the tests. Typically developing subjects, both healthy controls unselected for head size and those with benign macrocephaly, had no developmental, neurologic, or severe psychiatric disorders based on history, IQ, reading and language tests, physical examination, and structured psychiatric assessment. Pervasive developmental disorders were excluded in all comparison subjects by history, direct observation, and an interview of the mother by using the Family History Interview for Disorders of Social Development and Cognition (FHI [49, 50]). The FHI was specifically designed to inquire about signs of autism-spectrum disorders and milder isolated autismlike features.
Head Circumference and Height.
Head circumference and height were measured in all subjects by using the standardized methods and reference data described by Farkas et al (51). Head circumference and height data were converted into standardized z scores (zHC and zHgt), which controls for age and sex: zHC, zHgt = (subject value − mean for age and sex)/SD for age and sex.
Neuroimaging
MR images were acquired with a Eclipse 1.5-T unit (Philips Medical Systems, Highland Heights, Ohio). Axial 3D T1-weighted (13/4.47 [TR/TE], 20° flip angle, 1.2-mm section thickness, 25.6-cm FOV) and coronal 3D T2-weighted fast spin-echo (3500/114, 1.5-mm section thickness, 25.6-cm FOV) images were used for quantitative image analysis. In some cases, sedation was necessary and followed a strict clinical protocol approved by the institutional review board of the university and was performed by an onsite, approved anesthesiologist. The procedure was clearly explained, as best as possible, to the subject and parent or guardian. In several situations, rehearsal was used to ‘practice’ lying in the MR unit. In all cases, written informed consent was obtained before any imaging. No complications or untoward effects were encountered. All images were reviewed by a clinical radiologist with special competence in neuroradiology. Sixteen subjects were voluntarily reimaged because of movement artifact. Four of the subjects recruited for reading disorder and who were imaged were not included as control subjects, because they did not meet all criteria of reading disorder classification.
Volumetric Image Analysis.
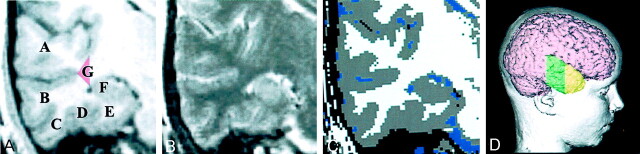
Quantitative analyses followed well-established, published protocols (52,53). Briefly, the coregistered T1- and T2-weighted images were segmented into white, gray, and CSF pixels by using the ANALYZE (54, 55) multispectral tool (Fig 1). Total brain volume (TBV) was the combination of white and gray matter summed. Total CSF was the sum of subarachnoid and ventricular CSF. By using the inner table of the skull as a landmark, total intracranial volume (TICV) was determined by the total sum of whole-brain parenchyma and CSF. Temporal lobe structures including the hippocampus were identified according to the methods outlined by Bigler et al (52) and included total white and gray matter volumes of the five gyri and CSF volumes of the major temporal lobe sulci and the temporal horn along with the temporal stem (see Fig 1 for definition). Cross-sectional surface areas (mm2) of the area dentata and CAS subregions of the hippocampus were measured according to the methods of Saitoh et al (4) and the amygdala volume by the method of Aylward et al (6). The landmarks that define gyri at the anterior most extension of the temporal lobe, the temporal pole, are sometimes difficult to identify, and so a separate measure of gray, white, and total volume of the temporal pole was included (Fig 1). The temporal pole was defined as the region anterior to the temporal horn. The sum of all gyral volumes, hippocampal and amygdala volumes, temporal stem, and the temporal pole was used to calculate total temporal lobe volume.
Fig 1.
A, T1-weighted coronal image shows the five temporal gyri: superior temporal gyrus (A), middle temporal gyrus (B), inferior temporal gyrus (C), fusiform gyrus (D), parahippocampal gyrus (E), and hippocampus (F). The red triangle (G) defines the region of classification for determining the volume of the temporal stem, which represents the trunk of the white matter projections of the temporal lobe in the coronal plane.
B, Coregistered T2-weighted image.
C, Edited segmented image from the T1- and T2-weighted images depicts gray matter in gray, white matter in white, and CSF in blue. The Volume Render Module and region-of-interest feature in ANALYZE (54, 55) were used to identify gyral boundaries, defined by each sulcus.
D, Three-dimensional reconstruction of this subject’s brain depicts a lateral view showing the region where gyral quantification occurred (green) and the temporal pole (yellow). Anterior to the coronal sections in A and B, where the temporal horn is no longer definable, was the landmark to begin measuring the temporal pole. The red triangle (G) in A defines the region of classification for determining the volume of the temporal stem. The mesial vertical boundary was defined by connecting the inner extension of the sylvian fissure to the most lateral aspect of the temporal horn, triangulated with the gray matter extension at the innermost point of the superior temporal sulcus. CSF boundaries were defined by CSF segmented pixels in the region bounded by the inferior frontal lobe and the superior temporal gyrus for the sylvian fissure; between the superior and inferior temporal gyri for the middle temporal sulcus; between the inferior and fusiform for the inferior temporal sulcus; and the rhinal sulcus between the fusiform and parahippocampal gyrus.
Statistical Analysis
Within groups, right and left volumetric differences were first computed; although some differences were noted, particularly with sulcal CSF, none remained significant after Bonferroni corrections. Accordingly, right and left values were collapsed into a single measure and the average taken. Individual scores for each region were then plotted and mean differences ascertained across the five groups (autism with normocephaly, autism with macrocephaly, controls with normocephaly, controls with benign macrocephaly, and controls with reading disorders). Gray matter and white matter volumes for each temporal lobe gyrus, along with total volume of the amygdala, hippocampus, and hippocampal subregions, were determined individually and plotted by age for the total autism sample (normocephalic and macrocephalic) and total control sample. As above, for each structure, right and left comparisons were first made and the best fit (linear or quadratic) determined. Again, there were no distinct or significant findings between right and left structures that uniquely differentiated the groups; therefore, each structure was combined into a single measure. Growth curves were then plotted by age and the best fit (linear versus quadratic) determined. Because when plotted by age the sample size was limited for the macrocephaly groups in particular, growth plots collapsed all autistic subjects into a single category of ‘autism’ plotted against all control subjects collapsed into a single category of ‘controls.’ Accordingly, the control sample for the growth plots included healthy controls, healthy controls with macrocephaly, and the controls with reading disorders. For autism, growth plots included, in a single group, normocephalic and macrocephalic subjects with autism. The correlation for each model was compared to determine if the quadratic information significantly contributed to the linear information with a “full versus reduced” F test. If it did, then the quadratic model was determined to be a better fit; if not, then the linear was accepted. Because of the multiple comparisons undertaken, Bonferroni correction procedures were made where appropriate, with the level of significance at .05. Raw data points uncorrected for head size were the basis for the frequency plots and growth curves. For statistical comparisons, age, height, and TICV were control variables.
Results
Clinical Neuroimaging Findings
Characteristics of Autism and Comparison Subjects.
Table 1 summarizes the demographic variables for the autistic and comparison subjects. Despite the attempts at matching for PIQ, a significant difference was noted in which the control subjects with benign macrocephaly had higher PIQ. As expected, size differences in head circumference were noted among the groups, since group composition was based on head size. Accordingly, as previously mentioned, significant demographic differences were controlled statistically where appropriate. Table 2 summarizes the blind and independent clinical reading of the MR images before any quantitative analysis. Most images were interpreted as normal; where abnormalities were identified, none involved any neoplastic process or vascular abnormality requiring any form of treatment. No heterotopias were identified, but, as can be seen, the autistic subjects with normocephaly had the highest frequencies of clinical abnormalities noted.
TABLE 1:
Age, PIQ, head circumference, and height data for autistic and control subjects
| Characteristic | Autistic Subjects |
Control Subjects |
F Values | |||
|---|---|---|---|---|---|---|
| Normocephalic | Macrocephalic | Normocephalic | Macrocephalic | Reading Disorder | ||
| No. of subjects* | 26 | 12 | 19 | 8 | 17 | |
| Age (y) | ||||||
| Mean (SD) | 14.15 (6.05) | 12.75 (4.43) | 12.53 (4.48) | 12.50 (3.46) | 12.65 (3.10) | .48, P = .75 |
| Range | 8–31 | 7–19 | 7–22 | 8–16 | 7–18 | |
| No. of subjects older than 19 y | 4 | 0 | 2 | 0 | 0 | |
| PIQ | ||||||
| Mean (SD) | 97.50 (19.29) | 108.58 (18.22) | 101.25 (15.55) | 117.88 (11.69) | 100.18 (10.88) | 2.82, P = .03 |
| Range | 62–141 | 69–125 | 74–126 | 103–136 | 82–129 | |
| zHC | ||||||
| Mean (SD) | 0.45 (0.98) | 2.78 (0.77) | 0.20 (1.18) | 3.02 (1.01) | −0.02 (1.36) | 21.86, P < .001 |
| Range | −1.81–1.46 | 1.93–4.12 | −1.75–1.81 | 2.14–5.20 | −2.30–2.04 | |
| zHgt | ||||||
| Mean (SD) | −0.23 (1.12) | 0.72 (1.26) | 0.56 (1.05) | 0.83 (.67) | 0.50 (.98) | 2.97, P = .02 |
| Range | −2.51–1.98 | −1.38–3.17 | −0.69–2.86 | 0.24–1.98 | −1.20–2.86 | |
| Grade level mean | 6 | 7 | 5 | 9 | 6 | |
Note.—zHC indicates z score for head circumference; zHgt, z score for height. The z score is the number of SDs a value is away from the reference data mean for age and sex. In reference data mean, zHC + zHgt = 0.
All subjects are male.
TABLE 2:
Clinical MR imaging findings
| Finding | Autistic Subjects |
Control Subjects |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normocephaly (n = 26) | Macrocephaly (n = 12) | Normocephaly (n = 19) | Benign Macrocephaly (n = 8) | Reading Disorder (n = 17) | ||||||
| Signal hyperintensity | 4 | (15.4) | 1 | (8.3) | 2 | (10.5) | 2* | (25.0) | 1 | (5.9) |
| Mild atrophy | 2 | (7.7) | - | - | - | - | - | - | - | - |
| Prominent perivascular spaces | 2 | (7.7) | 1 | (8.3) | 1 | (5.3) | 2* | (25.0) | - | - |
| Temporal horn prominence | 1 | (3.8) | 1 | (8.3) | - | - | - | - | 1 | (5.9) |
| Hypothalamic signal abnormality | 1 | (3.8) | - | - | - | - | - | - | ||
| Ventricular asymmetry | - | - | - | - | - | - | - | - | 1 | (5.9) |
| Arachnoid cyst | - | - | - | - | 1 | (5.3) | - | - | 3 | (17.6) |
| Left choroidal fissure cyst | 1 | (3.8) | - | - | - | - | - | - | 1 | (5.9) |
| Possible pineal cyst | 1 | (3.8) | - | - | - | - | - | - | - | - |
Note.—Data are the number of subjects. Numbers in parentheses are percentages.
One subject with benign macrocephaly had both a signal abnormality and prominent perivascular space noted. No other dual clinical abnormalities were identified in other subjects.
Quantitative Neuroimaging Findings
Volumetric Comparisons of Temporal Lobe Parenchyma.
Figure 1 shows the temporal lobe regions measured. Figures 2 and 3 compare each temporal lobe structure by the five group classifications: autism with normocephaly, autism with macrocephaly, controls with normocephaly, controls with benign macrocephaly, and controls with reading disorder. Within each group, right and left differences were examined, and although several asymmetries were present, none was found to be prominent or significant after Bonferroni correction nor did one group have a distinct or unique pattern of asymmetry. Accordingly, right and left volumes for each structure were combined into a single measure as depicted in Figures 2 and 3. Although some significant volume differences were found, they were always between macrocephalic subjects (autism or control) and either the normocephalic autism group or one of the nonmacrocephalic control groups. There was no circumstance in which the macrocephalic autistic and the macrocephalic control subjects differed on any measure or in which the normocephalic autistic and normocephalic control subjects differed.
Fig 2.
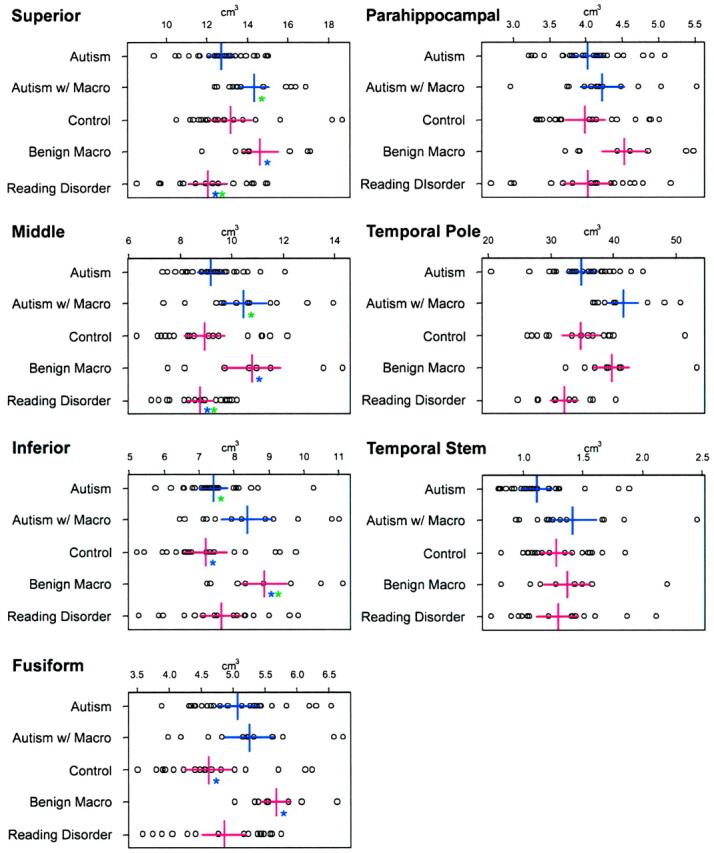
Parenchymal gyral volumes of the temporal lobe, volume of the temporal lobe tip, and white matter stem. Each box contains the actual data point representing the uncorrected volume for each structure. The five groups are represented on the y axis. The x axis is in cm3. Colored asterisks indicate where significance (P < .05) is present and between which groups the significance resides (ie, same color). For example, in the superior temporal gyrus panel in the upper left, the two blue asterisks indicate that subjects with reading disorder had smaller volume than that of the control subjects with benign macrocephaly (Macro). Note that there are basically no significant differences between autistic and control subjects with normal head size or between autistic and control subjects with large head size. The only comparisons that were significant were between one of the macrocephalic groups and the normocephalic groups.
Fig 3.
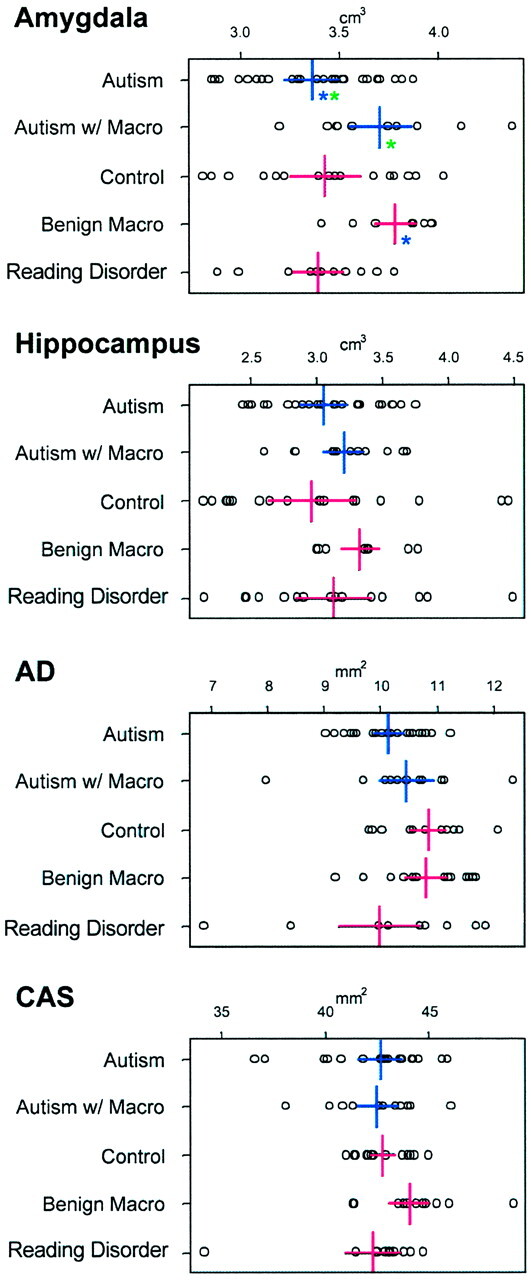
Volumetric comparisons of subcortical regions of the temporal lobe including the amygdala, total hippocampal volume, area dentata (AD), and the CA1-CA3 region plus subiculum (CAS) of the hippocampus. Legend is otherwise the same as that in Figure 2. Note that AD and CAS values are in mm2 as they represent the surface area at that level.
Volumetric Comparisons of Temporal Lobe CSF.
Table 3 depicts temporal lobe CSF volumes for the different regions of interest. As with the parenchymal measures, asymmetries were present, but none was significant after Bonferroni correction; therefore, left and right were combined into a single measure. No significant differences were noted across the groups in terms of CSF volumes. The temporal horns approached significance (P = .09), with the normocephalic autistic subjects having smaller volumes than all other comparisons.
TABLE 3:
Temporal lobe CSF volumes by sulcus and horn of the lateral ventricle
| Region | Volumes (cm3) by Diagnostic Group |
||||
|---|---|---|---|---|---|
| Autism (n = 26) | Autism with Macrocephaly (n = 12) | Normal (n = 19) | Benign (n = 8) | Reading Disorder (n = 17) | |
| Sylvian | 0.89 (0.60) | 1.02 (0.50) | 0.71 (0.45) | 0.95 (0.39) | 0.69 (0.36) |
| Superior | 0.22 (0.17) | 0.26 (0.14) | 0.18 (0.13) | 0.18 (0.08) | 0.15 (0.09) |
| Middle | 0.09 (0.07) | 0.08 (0.04) | 0.06 (0.05) | 0.10 (0.06) | 0.09 (0.08) |
| Inferior | 0.04 (0.02) | 0.04 (0.03) | 0.04 (0.02) | 0.05 (0.04) | 0.04 (0.04) |
| Rhinal | 0.06 (0.03) | 0.06 (0.03) | 0.06 (0.05) | 0.08 (0.05) | 0.05 (0.04) |
| Horn | 0.37 (0.15) | 0.51 (0.14) | 0.48 (0.19) | 0.51 (0.23) | 0.52 (0.22) |
Note. Data are the mean. Numbers in parentheses are SD.
Developmental Comparisons.
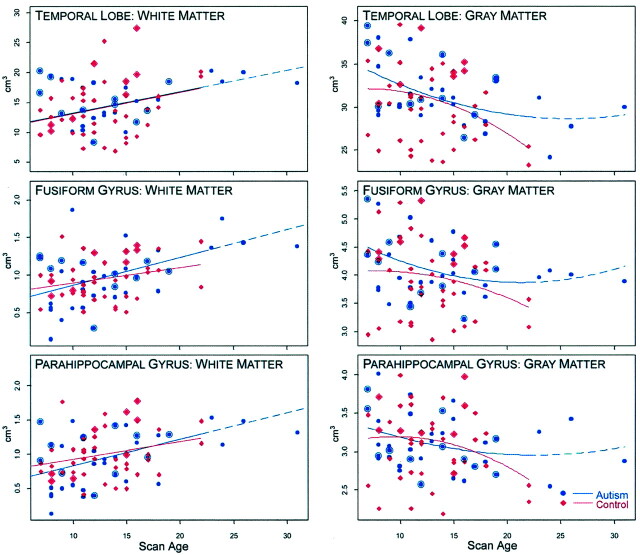
Selected structures depicted in Figures 4 and 5 summarize the findings representing cross-sectional developmental changes by age. As shown in Figure 4, when viewed as the average total temporal lobe white matter volume, the autism and control groups had strikingly similar patterns. For the average total gray matter of the temporal lobes, the fit was curvilinear and somewhat different between control and autistic subjects, but the difference was not statistically significant. Generally, gray matter volumes for individual gyri also decreased in a curvilinear fashion with age, whereas white matter volumes increased in a linear fashion. Although the patterns for each gyrus differed somewhat by age between autism and control groups, they were not significantly different. Because the fusiform and parahippocampal gyri are of particular interest in autism, growth plots for these structures are presented in Figure 4. As can been seen from viewing this pattern, the growth curves for these individual gyri were similar to overall temporal lobe patterns for both white matter and gray matter; the gray matter quadratic fits were different but not significantly so. Figure 5 depicts growth plots (total volume) for the hippocampus, amygdala, white matter stem, and the area dentata and CAS regions of the hippocampus. Although in some cases subtle trajectory differences were observed, none was significant. Significant developmental changes in temporal lobe CSF (not shown) were also noted within each group but no across-group differences were significant; in all cases CSF volume increased with age.
Fig 4.
Growth plots by age for total temporal lobe white matter and gray matter, fusiform gyrus white and gray matter, and parahippocampal gyrus white and gray matter. Controls include typically developing subjects with or without macrocephaly, and subjects with reading disorder. The nature of the relationship of structure, development, and age was tested by either a linear or quadratic fit. As can be seen for all white matter structures, the better fit was linear. For gray matter structures, the fit was quadratic. The dash-line represents the extension of the quadratic for the autistic subjects older than 23 years. Since there were no controls to compare beyond that age, the actual regression line stops with the solid line and the projected regression is represented by the dash. None of these growth plots were significantly different, indicating generally similar growth patterns between autistic and control subjects, even though the curves showed some differences. Large blue ‘bulls eye’ points denote macrocephalic (both selected and unselected) autistic subjects, and the large red diamonds indicate macrocephalic control subjects. Age is shown on the x axis and volume (cm3) on the y axis.
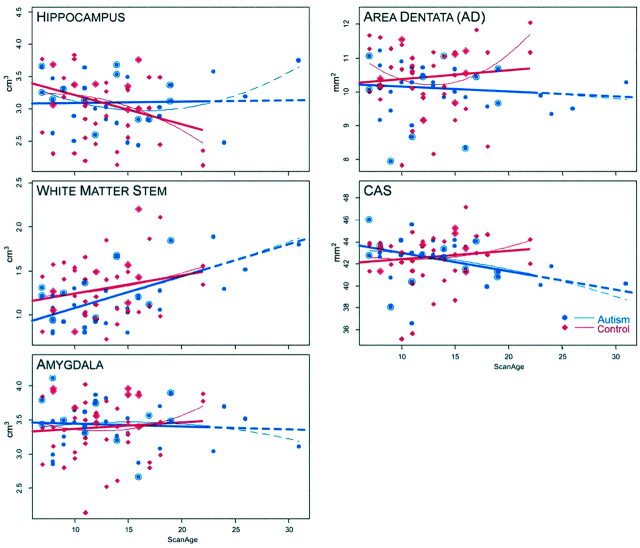
Fig 5.
Growth plots by structure and age, for amygdala, temporal lobe, white matter stem, hippocampus, area dentata, and the CAS region of the hippocampus. As with Figure 4, the fit was tested for quadratic and linear, and, in the case of these structures, several showed no superiority of one fit over the other, therefore both are presented. Legend is otherwise the same as that in Figure 4. Of interest was that as a group, the autistic subjects had smaller white matter stem volumes than those of the control subjects earlier in life (younger than 17 years), although the slopes of the regression lines were not different. Note that area dentata and CAS values are in mm2 as they represent the surface area at that level.
Temporal Lobe Comparisons by TICV and TBV.
Table 4 depicts white and gray matter ratios of the temporal lobe by TICV and TBV. As for gray matter, there were no ratio differences. However, autistic subjects had the least white matter volume ratio of all groups, but the differences were not significant.
TABLE 4:
Temporal lobe gray and white matter ratios
| Group | Gray Matter |
White Matter |
||
|---|---|---|---|---|
| TICV Ratio* | TBV Ratio† | TICV Ratio* | TBV Ratio† | |
| Autism (n = 26) | 13.98 (7.96) | 15.22 (8.61) | 7.66 (4.40) | 8.35 (4.81) |
| Autism with macrocephaly (n = 12) | 15.70 (7.54) | 16.84 (8.07) | 9.19 (4.51) | 9.86 (4.88) |
| Control (n = 19) | 17.36 (4.43) | 18.67 (4.67) | 9.92 (2.65) | 10.68 (2.86) |
| Benign macrocephaly (n = 8) | 13.46 (8.56) | 14.77 (9.38) | 8.42 (5.78) | 9.23 (6.30) |
| Reading disorder (n = 17) | 15.13 (7.29) | 16.24 (7.83) | 9.05 (4.42) | 9.70 (4.73) |
| F statistic | 0.76 | 0.67 | 0.86 | 0.75 |
| P value | .56 | .62 | .49 | .56 |
Note.—Data are the mean. Numbers in parentheses are SD.
TICV Ratio = (Temporal Gray or White Matter/Total Intracranial Volume) × 100
TBV Ratio = (Temporal Gray or White Matter/Total Brain Volume) × 100
Discussion
If there were striking or even reliable differences in gross temporal lobe morphology distinctive of autism, they would have undoubtedly been consistently reported by now. The current study demonstrates that volume of the temporal lobe in autism, whether the individual is normocephalic or macrocephalic, is generally proportional to TICV and TBV, in alignment with that observed in controls. We found no major dissimilarity in regional temporal lobe volume between autism with and that without macrocephaly and no differences with controls, even those with benign macrocephaly, when temporal lobe volume was examined in the context of TBV or TICV. Thus, temporal lobe size in individuals with autism with or without macrocephaly is proportional to the volume of the cranial vault and brain. However, there were some intriguing observations that will require further scrutiny. For example, as a group, normocephalic subjects with autism had smaller white matter stem volume than that of normocephalic controls, a difference that approached significance (P = .06; Fig 2). This could have implications for the organization of white matter pathways within the temporal lobe in autism. Likewise, although not significant, the ratio of temporal lobe white matter to TBV or TICV was smallest in the autistic subjects with normal head size.
From a developmental cross-sectional perspective, growth patterns were generally found to be similar in autistic and control subjects. White matter volumes demonstrated a generally robust linear increase with maturation, presumably an indication of increased myelination (5, 56–58). Inversely, gray matter volumes generally demonstrated a linear to curvilinear decrease in volume with age. The decrease in gray matter volume is thought to represent cellular pruning (5, 56). Despite functional neuroimaging studies that have implicated abnormalities in the parahippocampal and fusiform regions in autism (59), from a general morphology perspective the volumes of these regions appeared to be similar in autistic and comparison subjects. However, in both the parahippocampal and fusiform gyri, the direction of the quadratic fit for gray matter was different for autistic and control subjects. Unfortunately, the sample sizes of the autism and comparison groups limit power and further speculation, at this time, as to the significance of this observation.
Recent studies by Courchesne et al (5) and Sparks et al (25) implicate that volumetric differences between autistic and control subjects occur early in life. Our youngest subject was aged 7 years. Early differences in growth could become volumetrically nonsignificant with maturation and may be one reason behind the lack of significance of the current findings. For example, as shown in Figure 5, although not significant at the level of the temporal stem, white matter volume was initially less in the autistic subjects. Weidenheim et al’s (10) histologic examination of two cases of individuals with autistic behavior found dystrophic axons in limbic regions of the temporal lobe. Casanova et al (60) found that cell columns in individuals with autism, while more numerous, were smaller, less compact, and with less neuropil space in the periphery. Since gross brain abnormalities do not characterize autism, subtle white matter anomalies may turn out to be a more defining feature of this disorder. Improper timing of myelination, cell or axonal packing may significantly alter the function of a neural system without necessarily resulting in volumetric differences. If these differences occur early in brain development, followed by some stabilization, thereafter there may not be corresponding volumetric findings within the age range studied in this investigation.
Studies have implicated abnormal volume of the amygdala and hippocampus in autism (6, 25). We did not find significant volumetric differences in these structures once head, brain size, PIQ, and age were controlled for. Likewise, starting at age 7 years, no significant developmental differences were observed in these structures. Since these structures remain candidate areas for abnormalities associated with autism, the current findings suggest that it may be very important to study younger individuals with autism. Also, given the current findings, it may be that important abnormalities of the temporal lobe will be found with functional rather than structural neuroimaging methods.
The clinical interpretation of the images requires some comment. The overall frequency of incidental findings was consistent with that of other studies (61, 62). Although most of the images were read as normal, there were more abnormal findings in the autistic subjects (Table 2). As already reviewed, Courchesne et al (5) and Sparks et al (25) both implicated very early aberrant brain growth in autism by age 3 years, which may then stabilize thereafter. Since the brain is in a dynamic growth pattern with the skull, accelerated early brain growth could stimulate skull development, whereas cessation of brain growth could lead to presence of more CSF space in some individuals—as a subtle reflection of brain-intracranial mismatch. This could take on the appearance of mild atrophy or CSF prominence (including ventricular dilatation) and was observed in seven of the subjects with autism, but was also observed in four of the control subjects. Elia et al (63), in an autism sample that contained lower functioning individuals, found that 27.5% had clinical findings, with the most frequent being CSF prominence in subarachnoid or ventricular space. Since such prominence may give rise to classification of atrophy, the findings of Elia et al (64) are also consistent with our observations that there may be a higher frequency of clinical findings in autism.
The current study has several limitations. Sample sizes of the macrocephalic groups were relatively small. Accordingly, statistical power may be an issue in lack of significant differences. Also, we examined only higher functioning individuals with autism. It may be that more meaningful brain differences can be elucidated by examining a broader spectrum of autistic subjects, including those with moderate to severe mental retardation. Finally, the growth curves presented used cross-sectional, rather than longitudinal, data.
Conclusion
The findings of this study continue to demonstrate the complexity and enigma of autism. By carefully matching control and autistic subjects by important cognitive (ie, IQ) and physical (height and head size) measurements along with age, we demonstrated no obvious differences in the main structures of the temporal lobe, including selected cases of autism with macrocephaly. In autism with macrocephaly, temporal lobe structures are proportional to head size and do not differ from those of a control comparison group with benign macrocephaly.
Acknowledgments
We wish to thank the other staff of the Utah Autism Project, BYU Brain and Behavior Lab, and the University of Utah Center for Advanced Medical Technology for their assistance. In particular, we would like to acknowledge the technical assistance of Tracy Abildskov and the manuscript assistance of Jo Ann Petrie. We express our sincere gratitude to the children and adults who participated in this study and their families. We also gratefully acknowledge the contributions of Brian Chong, MD, and Joseph Piven, MD, during the early stages of this project.
Footnotes
Supported in part by National Institutes of Health contract grant number 2U19 HD3547606 and by the Ira Fulton Foundation. Additional financial aid ‘in-kind’ support was provided by the Utah Autism Foundation and Valley Mental Health.
References
- 1.Hauser SL, DeLong GR, Rosman NP. Pneumographic findings in the infantile autism syndrome: a correlation with temporal lobe disease. Brain 1975;98:667–688 [DOI] [PubMed] [Google Scholar]
- 2.Bachevalier J. Medial temporal lobe structures and autism: a review of clinical and experimental findings. Neuropsychologia 1994;32:627–648 [DOI] [PubMed] [Google Scholar]
- 3.Kates WR, Mostofsky SH, Zimmerman AW, et al. Neuroanatomical and neurocognitive differences in a pair of monozygous twins discordant for strictly defined autism. Ann Neurol 1998;43:782–791 [DOI] [PubMed] [Google Scholar]
- 4.Saitoh O, Karns CM, Courchesne E. Development of the hippocampal formation from 2 to 42 years: MRI evidence of smaller area dentata in autism. Brain 2001;124:1317–1324 [DOI] [PubMed] [Google Scholar]
- 5.Courchesne E, Karns CM, Davis HR, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology 2001;57:245–254 [DOI] [PubMed] [Google Scholar]
- 6.Aylward EH, Minshew JH, Goldstein G, et al. MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology 1999;53:2145–2150 [DOI] [PubMed] [Google Scholar]
- 7.Piven J, Bailey J, Ranson BJ, Arndt S. No difference in hippocampus volume detected on magnetic resonance imaging in autistic individuals. J Autism Dev Disord 1998;28:105–110 [DOI] [PubMed] [Google Scholar]
- 8.Asano E, Chugani DC, Muzik O, et al. Autism in tuberous sclerosis complex is related to both cortical and subcortical dysfunction. Neurology 2001;57:1269–1277 [DOI] [PubMed] [Google Scholar]
- 9.Bolton PF, Griffiths PD. Association of tuberous sclerosis of temporal lobes with autism and atypical autism. Lancet 1997;349:392–395 [DOI] [PubMed] [Google Scholar]
- 10.Weidenheim KM, Goodman L, Dickson DW, Gillberg C, Rastam M, Rapin I. Etiology and pathophysiology of autistic behavior: clues from two cases with an unusual variant of neuroaxonal dystrophy. J Child Neurol 2001;16:809–819 [DOI] [PubMed] [Google Scholar]
- 11.DeMeyer W. Microcephaly, micrencephaly, megalocephaly, and megalencephaly. In: Manning S, ed. Pediatric Neurology: Principles and Practice. 2nd ed. St. Louis: Mosby;1994. :205–218
- 12.Gillberg C, de Souza L. Head circumference in autism, Asperger syndrome, and ADHD: a comparative study. Dev Med Child Neurol 2002;44:296–300 [DOI] [PubMed] [Google Scholar]
- 13.Lainhart JE, Piven J, Wzorek M, et al. Macrocephaly in children and adults with autism. J Am Acad Child Adolesc Psychiatry 1997;36:282–290 [DOI] [PubMed] [Google Scholar]
- 14.Miles JH, Hadden LL, Takahashi TN, Hillman RE. Head circumference is an independent clinical finding associated with autism. Am J Med Genet 2000;95:339–350 [PubMed] [Google Scholar]
- 15.Fombonne E, Roge B, Claverie J, Courty S, Fremolle J. Microcephaly and macrocephaly in autism. J Autism Dev Disord 1999;29:113–119 [DOI] [PubMed] [Google Scholar]
- 16.Stevenson RE, Schroer RJ, Skinner C, Fender D, Simensen RJ. Autism and macrocephaly. Lancet 1997;349:1744–1745 [DOI] [PubMed] [Google Scholar]
- 17.Woodhouse W, Bailey A, Rutter M, Bolton P, Baird G, LeCouteur A. Head circumference in autism and other pervasive developmental disorders. J Child Psychol Psychiatry 1996;97:665–671 [DOI] [PubMed] [Google Scholar]
- 18.Fidler DJ, Bailey J, Smalley SL. Macrocephaly in autism and other pervasive developmental disorders. Dev Med Child Neurol 2000;42:737–740 [DOI] [PubMed] [Google Scholar]
- 19.Hardan AY, Minshew NJ, Mallikarjuhn M, Keshavan MS. Brain volume in autism. J Child Neurol 2001;16:421–424 [DOI] [PubMed] [Google Scholar]
- 20.Orstavik KH, Strmme P, Ek J, Torvik A, Skjeldal OH. Macrocephaly, epilepsy, autism, dysmorphic features, and mental retardation in two sisters: a new autosomal recessive syndrome? J Med Genet 1997;34:849–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davidovitch M, Patterson B, Gartside P. Head circumference measurements in children with autism. J Child Neurol 1996;11:389–393 [DOI] [PubMed] [Google Scholar]
- 22.Kanner L. Autistic disturbances of affective contact. Nerv Child 1943;2:217–250 [PubMed] [Google Scholar]
- 23.Nellhaus G. Head circumference from birth to eighteen years: practical composite international and interracial graphs. Pediatrics 1968;41:106–114 [PubMed] [Google Scholar]
- 24.Petersson S, Pedersen NL, Schalling M, Levebratt C. Primary megalencephaly at birth and low intelligence level. Neurology 1999;53:1254–1259 [DOI] [PubMed] [Google Scholar]
- 25.Sparks BF, Friedman S, Shaw DW, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology 2002;59:184–192 [DOI] [PubMed] [Google Scholar]
- 26.Bigler ED. Brain morphology and intelligence. Dev Neuropsychol 1995;11:377–403 [Google Scholar]
- 27.Eliez S, Blasey CM, Fruend LS, Hastie T, Reiss AL. Brain anatomy, gender and IQ in children and adolescents with fragile X syndrome. Brain 2001;124:1610–1618 [DOI] [PubMed] [Google Scholar]
- 28.Guerin P, Lyon G, Barthelemy C, et al. Neuropathological study of a case of autistic syndrome with severe mental retardation. Dev Med Child Neurol 1996;38:203–211 [DOI] [PubMed] [Google Scholar]
- 29.Pinter JD, Eliez S, Schmitt E, Capone GT, Reiss AL. Neuroanatomy of Down’s syndrome: a high-resolution MRI study. Am J Psychiatry 2001;158:1659–1665 [DOI] [PubMed] [Google Scholar]
- 30.Pinter JD, Brown WE, Eliez S, Schmitt JE, Capone GT, Reiss AL. Amygdala and hippocampal volumes in children with Down syndrome: a high-resolution MRI study. Neurology 2001;56:972–974 [DOI] [PubMed] [Google Scholar]
- 31.Reiss AL, Abrams MT, Singer HS, Ross JL, Denkla MB. Brain development, gender and IQ in children: a volumetric imaging study. Brain 1996;119:1763–1774 [DOI] [PubMed] [Google Scholar]
- 32.Rapin I. Autism in search of home in the brain. Neurology 1999;52:902–904 [DOI] [PubMed] [Google Scholar]
- 33.Wechsler D. Wechsler Intelligence Scales for Children-Third Edition (WISC-III). San Antonio: The Psychological Corporation;1991
- 34.Wechsler D. Wechsler Adult Intelligence Scale-Third Edition (WAIS-III). San Antonio: The Psychological Corporation;1997
- 35.Habib M. The neurological basis of developmental dyslexia: an overview and working hypothesis. Brain 2000;123:2373–2399 [DOI] [PubMed] [Google Scholar]
- 36.Richards TL. Functional magnetic resonance imaging and spectroscopic imaging of the brain: application of fMRI and fMRS to reading disabilities and education. Learn Disabil Q 2001;24:189–203 [Google Scholar]
- 37.Heim S, Eulitz C, Kaufmann J, et al. Atypical organization of the auditory cortex in dyslexia as revealed by MEG. Neuropsychologia 2000;38:1749–1759 [DOI] [PubMed] [Google Scholar]
- 38.Alarcon M, Pennnington BF, Filipek PA, DeFries JC. Etiology of neuroanatomical correlates of reading disability. Dev Neuropsychol 2000;17:339–360 [DOI] [PubMed] [Google Scholar]
- 39.Leonard CM, Eckert MA, Lombardino LJ, et al. Anatomical risk factors for phonological dyslexia. Cereb Cortex 2001;11:148–157 [DOI] [PubMed] [Google Scholar]
- 40.Duvernoy HM. TheHhuman Hippocampus: Functional Anatomy, Vascularization and SerialSsections with MRI. 2nd ed. Berlin: Springer-Verlag;1998
- 41.Szatmari P. Heterogeneity and the genetics of autism. J Psychiatry Neurosci 1999;24:159–165 [PMC free article] [PubMed] [Google Scholar]
- 42.Piven J, Arndt S, Bailey J, Andreasen NC. Regional brain enlargement in autism: a magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry 1996;35:530–536 [DOI] [PubMed] [Google Scholar]
- 43.Lord M, Rutter A, LeCouteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994;24:659–685 [DOI] [PubMed] [Google Scholar]
- 44.Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000;30:205–223 [PubMed] [Google Scholar]
- 45.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4th ed. Washington, D C: American Psychiatric Association;1994
- 46.Woodcock RW, Johnson MB. Woodcock-Johnson Psycho-Educational Battery-Revised. Itasca, IL: Riverside Publishing;1990
- 47.Wilkinson GS. Wide Range Achievement Test 3. Wilmington: Wide Range;1993
- 48.Wiederholt JL, Bryant BR. Gray Oral Reading Test-4th Ed (GORT-4). Austin: PRO-ED;1992
- 49.Bolton P, Macdonald H, Pickles A, et al. A case-control family history study of autism. J Child Psychol Psychiatry 1994;35:877–900 [DOI] [PubMed] [Google Scholar]
- 50.Folstein SE, Santangelo SL, Gilman SE, et al. Predictors of cognitive test patterns in autism families. J Child Psychol Psychiatry 1999;40:1117–1128 [PubMed] [Google Scholar]
- 51.Farkas LG, Hreczko TA, Katie MJ. Craniofacial norms in North American Caucasians from birth to young adulthood. In: Farkas LG, ed. Anthropometry of the Head and Face. New York: Raven Press;1994
- 52.Bigler ED, Anderson CV, Blatter DD. Temporal lobe morphology in normal aging and traumatic brain injury. AJNR Am J Neuroradiol 2002;23:255–266 [PMC free article] [PubMed] [Google Scholar]
- 53.Bigler ED, Blatter DD, Anderson CV, et al. Hippocampal volume in normal aging and traumatic brain injury. AJNR Am J Neuroradiol 1997;18:11–23 [PMC free article] [PubMed] [Google Scholar]
- 54.Robb RA. ANALYZE: the biomedical imaging resource at Mayo Clinic. IEEE Trans Med Imaging 2001;20:854–867 [DOI] [PubMed] [Google Scholar]
- 55.Robb R. ANALYZE: Three-Dimensional Biomedical Imaging. New York: VCH Publishers;1995
- 56.De Bellis MD, Keshavan MS, Beers SR, et al. Sex differences in brain maturation during childhood and adolescence. Cerebral Cortex 2001;11:552–557 [DOI] [PubMed] [Google Scholar]
- 57.Giedd JJ, Blumenthal J, Jeffried NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 1999;2:861–863 [DOI] [PubMed] [Google Scholar]
- 58.Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain 1991;114:2037–2049 [DOI] [PubMed] [Google Scholar]
- 59.Schultz RT, Gauthier I, Klin A, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry 2000;57:331–340 [DOI] [PubMed] [Google Scholar]
- 60.Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology 2002;58:428–432 [DOI] [PubMed] [Google Scholar]
- 61.Katzman GL, Dagher AP, Patronas NJ. Incidental findings on brain magnetic resonance imaging from 1000 asymptomatic volunteers. JAMA 1999;282:36–39 [DOI] [PubMed] [Google Scholar]
- 62.Kim BS, Illes J, Kaplan RT, Reiss A, Atlas SW. Incidental findings on pediatric MR images of the brain. AJNR Am J Neuroradiol 2002;23:1674–1677 [PMC free article] [PubMed] [Google Scholar]
- 63.Elia M, Ferri R, Musumeci SA, Panerai S, Bottitta M, Scuderi C. Clinical correlates of brain morphometric features of subjects with low-functioning autistic disorder. J Child Neurol 2000;15:504–508 [DOI] [PubMed] [Google Scholar]
- 64.Elia M, Ferri R, Amato C, et al. Neuroimaging and autism. In: Elia M, Romano V, Curatolo P, eds. Consensus in Child Neurology: Biological Bases and Clinical Perspectives in Autism. Hamilton, Ontario: BC Decker, Inc.;2001. :69–73