Abstract
Summary: Infantile Refsum disease is a rare inborn error of phytanic acid metabolism. It is inherited in an autosomal recessive manner and frequently causes signs and symptoms in the neonate period. The only source of phytanic acid in humans is exogenous, from diet. We report the MR imaging findings in two cases of infantile Refsum disease and note the MR imaging changes that occurred over time because of further progression of the disease. The initial diagnosis in both patients was made on basis of history, clinical findings, and biochemical studies.
Refsum disease is a rare autosomal recessive disorder characterized biochemically by accumulation of phytanic acid in blood and tissues (1), including fat and neurons (2). Also termed “heredopathia atactica polyneuritiformis,” it was first identified as a clinical entity by Refsum in the 1940s (3). A variant of this disease that occurs in children is called infantile Refsum disease. Clinically, it manifests by severe sensorineural deafness, atypical retinitis pigmentosa, mental and growth retardation, facial dysmorphism, hepatomegaly (4), peripheral neuropathy, cerebellar ataxia, and high concentrations of protein in CSF (3). We report the MR imaging findings in two patients with infantile Refsum disease.
Case Reports
Patient 1
A 2-year 9-month-old girl with nystagmus, visual and hearing impairment, and developmental delay was evaluated for the possibility of multisystem genetic disorder. The nystagmus was the first symptom with onset in the first few months of life. Her medical history was otherwise unremarkable with a normal pregnancy and delivery. There was no history of genetic disorders in the family. Electroretinogram (ERG) showed retinal pigmentation abnormalities in both fundi and a flat ERG. She had hyperopia and astigmatism bilaterally. She also had bilateral sensorineural hearing loss and developmental delay. No dysmorphic facial features were seen. She had complaints of frequent nose bleeds. The musculoskeletal examination was normal. Examination of the central nervous system revealed a wide-based gait with some unsteadiness.
Biochemical abnormalities included elevation of very long chain fatty acids, with lower than normal long chain polyunsaturated fatty acids, arachidonic and docosahexaenoic acid. There was elevation of C26:0 to 1.33 μg/mL (normal [n], 0.22), phytanic acid to 5.66 μg/ ml (n, 0.12), and pristanic fatty acid to 0.44 μg/mL (n, 0.33) and elevation of C26/22 to 0.132 (n, 0.01), consistent with the peroxisomal disorder of infantile Refsum disease.
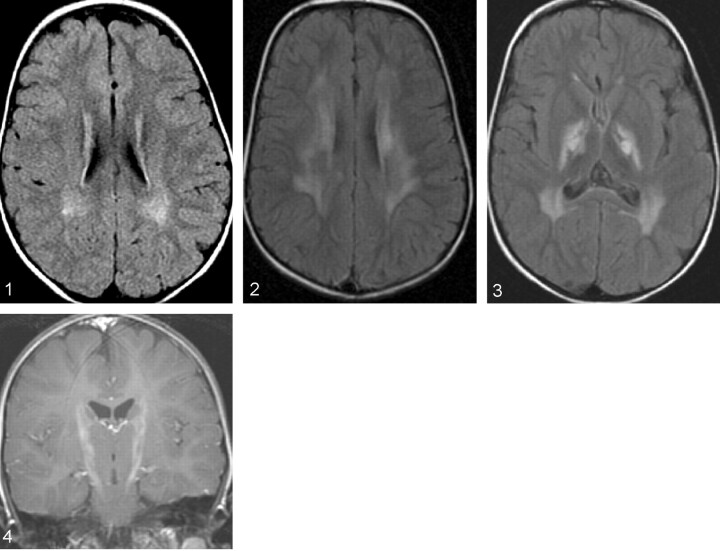
MR images obtained at the time of diagnosis revealed patchy periventricular white matter hypintensities on T2-weighted and fluid-attenuated inversion recovery (FLAIR) images (Fig 1). Increased T2 signal intensity was also identified in the cerebellar dentate nuclei. Follow-up MR imaging of the brain performed when the patient was 6 years of age showed further progression of the signal intensity changes in the periventricular white matter (Fig 2) and dentate nuclei. In addition, there were new areas of abnormally increased T2 and FLAIR signal intensity showing mild enhancement, along the course of the cortical spinal tracts from the upper pons through the midbrain, into the internal capsules (Figs 3 and 4). There was also interval development of mild supratentorial volume loss. Clinically the patient had deteriorated neurologically since her last MR imaging study, with worsening of oral motor skills including diminished language, increased drooling, and increased swallowing difficulty with choking and gagging during eating. She also had increasing difficulty with walking, along with increased nystagmus.
Fig 1.
FLAIR image in the initial MR imaging series, showing patchy periventricular white matter hyperintensities.
Fig 2.
Follow-up MR image, revealing increase in the periventricular white matter hyperintensities.
Fig 3.
Follow-up FLAIR image, revealing increased signal intensity abnormality along the corticospinal tract in the internal capsules bilaterally along with hyperintense signal intensity in the periventricular white matter.
Fig 4.
T1-weighted image in the recent MR imaging series, revealing mild enhancement along the corticospinal tracts.
Patient 2
A 1-year-old boy presented with motor retardation. Pregnancy and delivery were uncomplicated, although there was a history of second-degree consanguinity between the parents. By the age of 2.5 years, the child had loss of motor capabilities and frequent nosebleeds and was pale. On physical examination, the skin was pale, there was hepatomegaly, a 2/6° of systolic murmur, and strabismus. There was generalized hypotonia with stereotypic movements such as shaking and bending forward. Deep tendon reflexes were decreased in the upper and normal in the lower extremities. Laboratory findings included a decreased hemoglobin of 4.6 g/dL (n,12.1–17.2 g/dL) and an elevated ammonium level of 400 mg/dL (n, 0–75 mg/dL). Concomitant cranial MR imaging performed at the time revealed bilateral increased signal intensity on T2-weighted images in the substantia nigra, subthalamic nucleus, and globus pallidus. Because the serum ammonium levels were very high, the signal intensity pattern was thought to be related to hepatolenticular degeneration; however, very long-chain fatty acid studies revealed C24/C22 of 1.35 (n, 0–1.01), C26/C22 of 0.085 (n, 0–0.026), phytanic acid of 65.82 1 umol/L (n, 0–15), pristanic acid of 21.42 umol/L (n, 0–2), consistent with the peroxisomal disorder of infantile Refsum disease.
Over the next 5 years, the child had progressive mental and motor retardation. Subsequent MR imaging studies (in 1997 and 2000) revealed increased signal intensity changes on T2-weighted and FLAIR images within cerebellar dentate nuclei, corticospinal tracts, substantia nigra, corpus callosum, and periventricular deep white matter. In the last follow-up, at age of 7.5 years, physical examination showed severe weight loss and weakness, with the child needing assistance to sit. He was still able to recognize his parents.
Discussion
Infantile Refsum disease is a peroxisomal biogenesis disorder. It differs from classic Refsum disease in that Infantile Refsum disease is a generalized peroxisomal disorder resulting from failure of the peroxisomes to form or maintain themselves so that functional defects are present in more than one enzyme of this organelle (5). Other disorders in this group include neonatal adrenoleukodystrophy, Zellweger syndrome, and rhizomelic chondrodysplasia. Plasma levels of phytanic acid are consistently elevated in infantile Refsum disease (2). Clinically, infantile Refsum disease varies from classic Refsum disease in that the former has an infantile onset with minor dysmorphism, mental retardation, hepatomegaly, sensorineural hearing loss, retinal pigmentary degeneration, and hypocholesterolemia (5). Skeletal abnormalities are part of the clinical syndrome in Refsum disease (6). Refsum disease is treated with a strict diet low in phytanic acid, although the long-term effect of dietary treatment is not yet known because this disease produces many biochemical abnormalities other than elevated plasma phytanic acid (7).
The autopsy findings that have been reported in the brain include mild diffuse reduction of axons and myelin within the corpus callosum, periventricular white matter, optic nerves, and corticospinal tracts. Lipid-laden macrophages are seen in the cerebral white matter. Severe hypoplasia of the cerebellar granule layer and ectopic Purkinje cells in the molecular layer has been shown (8).
Previously described MR imaging changes in infantile Refsum disease include changes in the white matter with symmetrical signal intensity abnormalities in the region of the dentate nuclei (4). Our cases showed symmetric changes in not only the cerebellum, but also in the periventricular white matter, with interval progression over time. We also saw signal intensity changes within the corticospinal tracts in both patients and the corpus callosum in the second patient, as has been described at autopsy. This may represent more severe forms of the disease than have previously been described.
Conclusion
Although rare, this diagnosis should be considered in the appropriate clinical setting. Disorders attributable to peroxisomal dysfunction are well recognized on the basis of their clinical and biochemical characteristics. MR imaging showing symmetrical signal intensity abnormalities in the cerebellum in the region of dentate nucleus and in advanced cases in the periventricular white matter, corticospinal tracts, and corpus callosum appear to be highly suggestive of infantile Refsum disease.
References
- 1.Fertl E, Foldy D, Auff E, et al. Refsum’s disease in an Arabian family. J Neurol Neurosurg Psychiatry 2001;70:564–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wills AJ, Manning NJ, Reilly MM. Refsum’s disease. QJM 2001;94:403–406 [DOI] [PubMed] [Google Scholar]
- 3.Jansen GA, Wanders RJA, Watkins PA, Mihalik SJ. Phytanoyl–coenzyme a hydroxylase deficiency: the enzyme defect in Refsum’s disease. N Engl J Med 1997;337:133–134 [DOI] [PubMed] [Google Scholar]
- 4.Dubois J, Sebag G, Argyropoulou M, Brunelle F. MR findings in infantile Refsum disease: case report of two family members AJNR Am J Neuroradiol 1991;12:1159–1160 [PMC free article] [PubMed] [Google Scholar]
- 5.Naidu S, Moser H. Infantile Refsum disease. AJNR Am J Neuroradiol 1991;12:1161–1163 [PMC free article] [PubMed] [Google Scholar]
- 6.Plant GR, Hansell DM, Gibberd FB, Sidey MC. Skeletal abnormalities in Refsum’s disease (heredopathia atactica polyneuritiformis). Br J Radiol 1990;63:537–541 [DOI] [PubMed] [Google Scholar]
- 7.Valk J, Van Der Knaap MS. Magnetic resonance of myelin myelination and myelin disorders. New York: Springer-Verlag,1989;113–118
- 8.Torvik A, Torp S, Kase BF, et al. Infantile Refsum disease: a generalized peroxisomal disorder: report of a case with postmortem examination. J Neurol Sci 1988;85:39–53 [DOI] [PubMed] [Google Scholar]