Abstract
Summary: Extracranial vertebral artery (VA) dissection may lead to significant arterial stenosis, occlusion, or pseudoaneurysm formation with subsequent hemodynamic and embolic infarcts. To prevent thromboembolic complications, anticoagulation with intravenous heparin followed by oral warfarin has been recommended for all patients with acute dissections, regardless of the type of symptoms. Nevertheless, anticoagulation is not innocuous and may be associated with hemorrhagic transformation of a cerebral infarction or may be ineffective to prevent symptoms or dissection progression. We present a case of a bilateral spontaneous extracranial VA dissection presenting with multiple embolic infarctions. The dominant VA was reconstructed with multiple in-tandem stents and the contralateral VA, proved to be the source of emboli, was occluded with coils. Stent-assisted VA angioplasty has rarely been reported in the management of spontaneous dissections and appears to be a safe, effective and immediate method of restoring vessel lumen integrity and should be considered in the therapy of selected cases of VA dissection.
Vertebral artery (VA) dissection may occur spontaneously or in vessels weakened by a primary arteriopathy; however, dissection more commonly occurs after trauma or even after less violent activities such as coughing, chiropractic manipulation, or endovascular or surgical procedures (1–3).
Ischemic symptoms occur in more than 90% of patients in whom a VA dissection is diagnosed and may involve the brain stem as well as the thalamus and the cerebral or cerebellar hemispheres. To prevent VA occlusion or distal embolization, anticoagulation and antiplatelet therapy have gained widespread acceptance, although, because reported series are too small, it is difficult to establish firmly the benefits of any particular therapeutic technique (3–5). Nevertheless, anticoagulation is not innocuous and may be contraindicated or be ineffective to prevent symptomatic progression (3, 6, 7). We present a case of a bilateral spontaneous extracranial VA dissection presenting with multiple symptomatic embolic infarctions. The dominant VA was reconstructed with multiple in-tandem stents, and the contralateral VA, which proved to be the source of emboli, was occluded with coils.
Case Report
A 42-year-old woman was admitted to the emergency room after a sudden-onset coma that persisted for 4 hours that was followed by slow spontaneous recovery of consciousness. Hemodynamic and respiratory parameters remained stable for the next 36 hours, and the patient was transferred to our institution. On admission, the patient was alert and oriented, with persistent backache, internuclear ophthalmoplegia, left homonymous hemianopsia, blurred vision (finger-count vision), and a mild left hemiparesis. MR diffusion-weighted images disclosed bilateral occipital infarctions corresponding to the territory of the posterior cerebral arteries (PCA) and multiple infarctions in the cerebellar hemisphere and brain stem.
Digital angiography of the left VA revealed a long, severe subintimal dissection with associated pseudoaneurysms extending through the cervical segment (Fig 1A). The right VA presented a severe subocclussive dissection with multiple free-intraluminal thrombi (Figs 2A–C). Intracranially, a complete embolic occlusion origin of the right PCA was diagnosed.
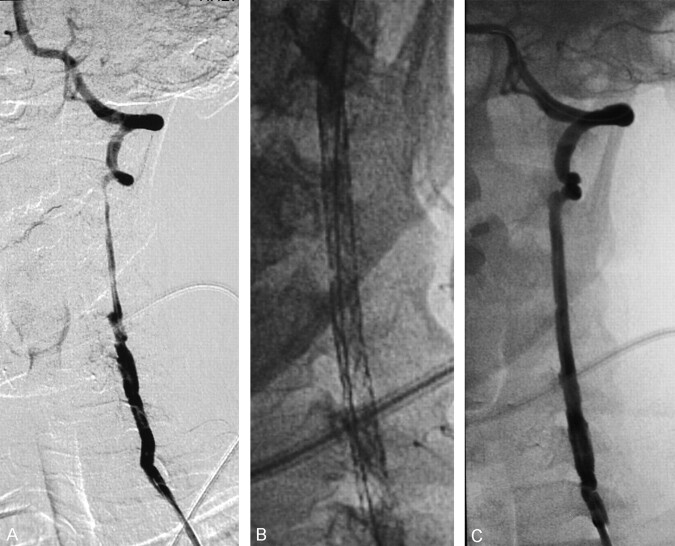
Fig 1.
Angiography of the cervical left VA reveals a multiple and irregular subintimal dissection with associated pseudoaneurysms extending through the cervical segment (A, left). Magnified radiographic view of the implanted stents (B, center). Angiography of the reconstructed artery (C, right).
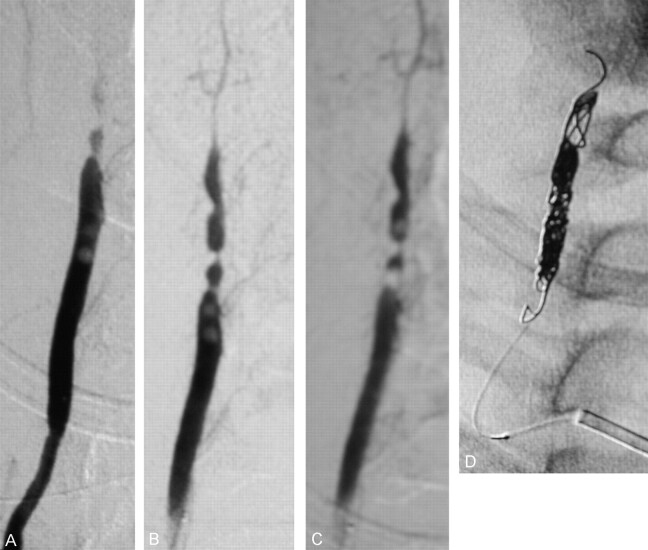
Fig 2.
Angiography of the cervical right VA reveals a severe hemodynamically significant arterial dissection with multiple intraluminal thrombi. Magnified angiographic view shows migration of the intraluminal thrombi (A–C, left to right). Rapid occlusion of the artery was performed by using detachable coils (D).
Considering the presence of multiple acute infarctions, the severity of the dissections, and especially the presence of intraluminal thrombi in the right VA, anticoagulation was considered insufficient to prevent embolic events and even relatively contraindicated (potential for hemorrhagic transformation). Therefore, endovascular reconstruction of the dominant left VA and occlusion of the right VA was proposed.
Endovascular Procedure
Under sedation, a 6F introducer sheath was placed on the right femoral artery. At the start of the procedure, the patient received a heparin bolus (10,000 IU), which was flushed with saline and heparin (1 U heparin per milliliter of isotonic sodium chloride solution at a rate of 100 mL/h) with the use of a rotating hemostatic valve. Heparin was administered to achieve an activated clotting time of greater than 250 s, and a bolus of 8 mg of glycoprotein IIb/IIIa antagonist (Integrilin [Eptifibatide], Schering-Plough Laboratories, Heist-op-den-Berg, Belgium) followed by an infusion rate of 2.25 mg/h was administered.
A 6F guiding catheter (Envoy; Cordis Endovascular, Miami Lakes, FL) was placed at the origin of the left VA. Selective angiography was performed, and the targeted segment was outlined in multiple projections with rotational tridimensional angiography. The narrowed arterial segment was then crossed under road mapping with a 2.3F microcatheter (Rapid Transit; Cordis) over a 0.014-inch, 300-cm-long exchange microguidewire. The wire was positioned at the distal intracranial VA and the microcatheter was exchanged for a stent-delivery catheter. A 3.5 × 12 mm balloon-mounted stent (AVE inx, Medtronic AVE, Minneapolis, MN) was then advanced over the 0.014-inch guidewire across the lesion site and placed at the distal end of the dissection. The balloon was inflated up to the maximum recommended pressure to deploy and implant the stent into the inner arterial wall. Then, two 3.5 × 12 mm balloon-mounted stents (AVE inx, Medtronic AVE) followed by four BX Sonic stents (Cordis Neurovascular), ranging 3.5 to 4.5 mm in diameter by 12 mm length were sequentially implanted until complete reconstruction of the vessel was achieved (Fig 1 A–C).
The guiding catheter was then repositioned at the origin of the right VA, and the artery was completely occluded by using detachable coils (Micrus Corporation, Mountainview, CA; Fig 2D).
Heparin was discontinued after the procedure. Integrilin was maintained for 12 hours and then discontinued, and the patient received aspirin (300 mg/day) and clopidogrel (75 mg/day) for a period of 3 months. The patient was discharged to a rehabilitation institute after 5 days. On discharge, the patient was fully alert and oriented, with a partial left homonymous hemianopsia and able to walk independently. Follow-up angiography at 2 months revealed patency of the stented artery with no signs of in-stent de novo stenosis (Fig 3).
Fig 3.
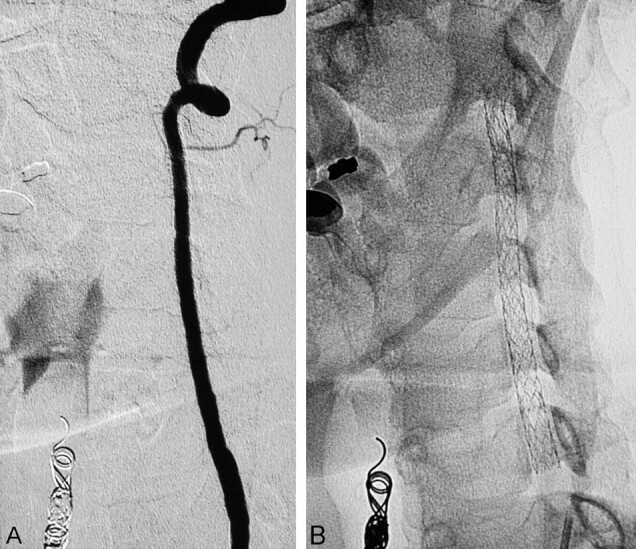
Digital angiography follow-up after 2 months. The left VA is patent and shows no signs of in-stent de novo stenosis (A). The plain unsubtracted image shows the unchanged continuous string of stents (B).
Discussion
Dissection of the VA is a cause of embolic stroke, particularly in younger patients (3, 8). Imaging studies suggest that more than 90% of infarcts due to dissection are thromboembolic rather than hemodynamic in origin, and transcranial Doppler studies show a high frequency of intracranial microemboli (9–11). Dissection is a dynamic process, and although the angiographic appearance may worsen during the acute phase of dissection, about 90% of stenoses eventually resolve and two-thirds of occlusions are recanalized (12–14). This healing process may take 2–3 months after the dissection, and rarely longer.
Although antithrombotic therapy has been advocated since the 1970s, no randomized studies have been reported, and the validity of such treatment has never been established (3–5). Anticoagulation with a target international normalized ratio of 2.0–3.0 is generally used for 3–6 months; nevertheless, this approach is not innocuous and may be contraindicated in cases with extensive intracranial infarctions or intracranial extension of the dissection or because of risk of pseudoaneurysm rupture. This case presented a partial contraindication for anticoagulation, because a masseffect infarction developed in the right PCA territory.
Anticoagulation, although critical to prevent thromboembolic events in initial stages of the disease, may be insufficient to overcome the flow-limiting nature of the dissecting lesion or to limit embolic phenomena when free intraluminal emboli are detected.
When dealing with flow-limiting dissections, the endovascular approach may be a valuable option. This treatment consists of stent-assisted angioplasty at the level of the injured vessel; the stent covers the dissected arterial segment and reconstructs the arterial diameter, limiting the occurrence of hemodynamic and embolic complications (15, 16). As in every stent procedure, antiplatelet medication is required. Because of the emergent treatment, a rapid-acting intravenous antiplatelet medication (Integrilin) was instituted. As recent studies have shown, this is not accompanied by an increased rate of hemorrhagic complications (17). Stent-assisted angioplasty is a well-established therapeutic alternative to operative reconstruction of proximal and, more recently, in distal VA stenosis and seems to be relatively safe (16, 18–20).
From a technical point of view, the most demanding step is the selective microcatheterization of the true lumen of the artery. We recommend the use of a coaxial microcatheter that assists the safe navigation of a soft-tip microguidewire. After crossing the dissected segment with the coaxial system, contrast material injection through the microcatheter should demonstrate a normal arterial segment; slow contrast material washout is related to limited antegrade flow. If a regular length guidewire was used, this is then exchanged for a 300-cm microguidewire and the microcatheter is removed (a magnet-assisted exchange may also be of value). It is of paramount importance to firmly maintain the position of the microguidewire; attempting to reposition the microguidewire through a dissection that may have progressed or may have associated vasospasm may be frustrating and unsuccessful. Stents allow the apposition of the dissected segment to the vessel wall, obliterating the false lumen, limiting the emboli source, and resolving the stenosis.
Ideally, flexible, self-expandable, low-profile microstents are desired to treat VA dissections. To date, however, the only type of available stents in adequate calibers and sizes for VA implantation are the short and relatively rigid, balloon-expandable coronary-type stents.
When dealing with a severely injured, flow-limiting artery that presents free intraluminal thrombi, deliberate occlusion may be desired, avoiding the imminent risk of embolic phenomena.
Surgical or endovascular treatment has been reserved for patients who have persistent symptoms of ischemia despite adequate anticoagulation or for patients in whom anticoagulation is contraindicated. Surgical treatment consists of ligation of the dissected artery combined with an in situ or extracranial to intracranial bypass; these procedures are technically demanding and are associated with a substantial morbidity rate (3, 21, 22).
Spasm, immediate occlusion secondary to dissection, procedural emboli, and restenosis have, however, been described. Distal embolization on the cerebral/cerebellar/brain stem circulation is always a major concern when dealing with a diseased vessel, and, especially when intraluminal thrombi are discovered, embolic protection devices have not been approved for VA use. The major factors contributing to restenosis—elastic recoil and vessel-wall remodeling—are addressed by stent placement; however, in light of the fact that drug-eluting stents and intravascular radiation have not yet been approved for general use, there is currently no effective therapy for the prevention of neointimal proliferation. We found stent-assisted VA reconstruction to be a feasible treatment for the subgroup of patients with symptomatic dissections of the extracranial VA who are poor candidates for systemic anticoagulation or for patients who hemodynamically depend on the patency of that artery (ie, contralateral VA occlusion with incomplete circle of Willis).
References
- 1.Opeskin K. Traumatic carotid artery dissection. Am J Forensic Med Pathol 1997;18:251–257 [DOI] [PubMed] [Google Scholar]
- 2.Hufnagel A, Hammers A, Schonle PW, et al. Stroke following chiropractic manipulation of the cervical spine. J Neurol 1999;246:683–688 [DOI] [PubMed] [Google Scholar]
- 3.Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med 2001;344:898–906 [DOI] [PubMed] [Google Scholar]
- 4.Fisher CM, Ojemann RG, Robertson GH. Spontaneous dissection of cervico-cerebral arteries. Can J Neurol Sci 1978;5:9–19 [PubMed] [Google Scholar]
- 5.Lucas C, Moulin T, Deplanque D, et al. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke 1998;29:2646–2648 [DOI] [PubMed] [Google Scholar]
- 6.Mokri B, Sundt TM Jr, Houser OW, Piepgras DG. Spontaneous dissection of the cervical internal carotid artery. Ann Neurol 1986;19:126–138 [DOI] [PubMed] [Google Scholar]
- 7.Martin PJ, Humphrey PR. Disabling stroke arising five months after internal carotid artery dissection [letter]. J Neurol Neurosurg Psychiatry 1998;65:136–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caplan LR, Tettenborn B. Vertebrobasilar occlusive disease: review of selected aspects. Cerebrovasc Dis 1992;2:226–272 [Google Scholar]
- 9.Droste DW, Junker K, Stögbauer F, et al. Clinically silent circulating microemboli in 20 patients with carotid and vertebral dissection. Cerebrovasc Dis 2001;12:181–185 [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan J, Newell DW, Sturzenegger M, et al. Transcranial Doppler in the evaluation of internal carotid artery dissection. Stroke 1996;27:1226–1230 [DOI] [PubMed] [Google Scholar]
- 11.Koennecke HC, Trocio SH Jr., Mast H, Mohr JP. Microemboli on transcranial doppler in patients with spontaneous carotid dissection. J Neuroimaging 1997;7:217–220 [DOI] [PubMed] [Google Scholar]
- 12.Houser OW, Mokri B, Sundt TM Jr, et al. Spontaneous cervical cephalic arterial dissection and its residuum: angiographic spectrum. AJNR Am J Neuroradiol 1984;5:27–34 [PMC free article] [PubMed] [Google Scholar]
- 13.Djouhri H, Guillon B, Brunereau L, et al. MRI angiography for the long-term follow-up of dissecting aneuryms of the extracranial internal carotid artery. AJR Am J Roentgenol 2000;174:1137–1140 [DOI] [PubMed] [Google Scholar]
- 14.Kasner SE, Hankins LL, Bratina P, Morgenstern LB. Magnetic resonance angiography demonstrates vascular healing of carotid and vertebral artery dissections. Stroke 1997;28:1993–1997 [DOI] [PubMed] [Google Scholar]
- 15.Malek AM, Higashida RT, Phatouros CC, et al. Endovascular management of extracranial carotid artery dissection achieved using stent angioplasty. AJNR Am J Neuroradiol 2000;21:1280–1282 [PMC free article] [PubMed] [Google Scholar]
- 16.Lylyk P, Cohen JE, Ceratto R, et al. Angioplasty and stenting of intracranial atherosclerotic stenoses and dissections. AJNR Am J Neuroradiol 2002;23:430–436 [PMC free article] [PubMed] [Google Scholar]
- 17.Kapadia SR, Bajzer CT, Ziada KM, et al. Initial experience of platelet glycoprotein IIb/IIIa inhibition with abciximab during carotid stenting: a safe and effective adjunctive therapy. Stroke 2001;32:2328–32 [DOI] [PubMed] [Google Scholar]
- 18.Lylyk P, Cohen JE, Ceratto R, et al. Angioplasty and stent placement in intracranial atherosclerotic stenoses and dissections. AJNR Am J Neuroradiol 2002;23:430–436 [PMC free article] [PubMed] [Google Scholar]
- 19.Lylyk P, Cohen JE, Ceratto R, et al. Endovascular reconstruction of intracranial arteries by means of stenting and combined techniques. J Neurosurg 2002;97:1306–1313 [DOI] [PubMed] [Google Scholar]
- 20.Piotin M, Spelle L, Martin JB, et al. Percutaneous transluminal angioplasty and stenting of the proximal vertebral artery for symptomatic stenosis. AJNR Am J Neuroradiol 2000;21:727–731 [PMC free article] [PubMed] [Google Scholar]
- 21.Schievink WI, Piepgras DG, McCaffrey TV, Mokri B. Surgical treatment of extracranial internal carotid artery dissecting aneurysms. Neurosurgery 1994;34:809–816 [DOI] [PubMed] [Google Scholar]
- 22.Morgan MK, Sekhon LHS. Extracranial-intracranial saphenous vein bypass for carotid and vertebral dissections: a report of six cases. J Neurosurg 1994;80:237–46 [DOI] [PubMed] [Google Scholar]