Abstract
BACKGROUND AND PURPOSE: Occipital neuralgia syndrome can cause severe refractory headaches. In a small percentage of people, these headaches can be devastating and debilitating, with the potential for complete relief following surgical rhizotomy. We describe CT fluoroscopy–guided percutaneous C2–C3 nerve block for the confirmation of diagnosis of occipital neuralgia and for demonstrating to patients the sensory effects of intradural cervical dorsal rhizotomy before the definitive surgical procedure.
METHODS: Seventeen patients with occipital neuralgia underwent 32 CT fluoroscopy–guided C2 or C2 and C3 nerve root blocks. Of the 17 patients, nine had occipital neuralgia following prior neck or skull base surgeries. On the basis of the positive results of the nerve blocks in terms of temporary pain relief, all 17 patients underwent unilateral (n = 16) or bilateral (n = 1) intradural C1 (n = 9), C2 (n = 17), C3 (n = 17), or C4 (n = 7) dorsal rhizotomies. All patients were followed up for a mean of 20 months (range, 5–37 months) for assessment of pain relief. Sixteen patients were assessed for degree of satisfaction with and functional state after surgery.
RESULTS: All patients had temporary relief of symptoms after percutaneous CT-guided block (positive result) and felt that occipital numbness was an acceptable alternative to pain. Immediately after surgery, all patients had complete relief from pain. At follow-up, 11 patients (64.7%) had complete relief of symptoms, two (11.8%) had partial relief, and four (23.5%) had no relief. Seven of eight (87.5%) patients without prior surgery had complete relief of symptoms and one (12.5%) patient had partial relief, as opposed to complete relief in four of nine (44.4%), partial relief in one of nine (11.2%), and no relief in four of nine (44.4%) patients with a history of prior surgery. Because of the small number of patients, this difference was not statistically significant (P = .110). Eleven of 16 (68.8%) patients stated that the surgery was worthwhile. Eight of 16 (50%) patients felt they were more active and functional after surgery, whereas 25% felt they were either unchanged or less functional than before surgery. None of the patients without a history of prior surgery reported a decreased sense of functional activity following rhizotomy.
CONCLUSION: CT fluoroscopy–guided percutaneous cervical nerve block is useful for the confirmation of occipital neuralgia, for demonstrating to patients the sensory effects of nerve sectioning, and possibly as a guide for selection of patients for intradural cervical dorsal rhizotomy. Although not statistically significant, there was a trend toward better response to rhizotomy in patients without prior head or neck surgery.
Headache is one of the most common symptoms affecting the general population. Occipital neuralgia is an uncommon cause of headache described in 1821 by Beruto and Ramos (1). It is a specific syndrome of paroxysmal severe lightening-like sharp headache in the distribution of the occipital nerve. Although most cases of occipital neuralgia are idiopathic, they may be related to specific causes such as trauma, prior skull base surgery, rheumatoid arthritis, nerve entrapment by hypertrophied atlantoepistrophic (C1–C2) ligament, compression by an anomalous ectatic vertebral artery, or degenerative C1–C2 arthrosis (2–6). A wide variety of treatments have been tried, including cervical collars, transcutaneous nerve stimulation, analgesics and antimigraine drugs, occipital nerve block with or without glucocorticoid injection, chemical or radio-frequency occipital nerve ablation, atlantoaxial arthrodesis, and dorsal cervical rhizotomy (2, 3, 6–11), each with variable degrees of success. We describe a group of patients with severe occipital neuralgia refractory to medical treatment. These patients were screened for potential surgery by using CT fluoroscopy–guided percutaneous C2- and C3-selective nerve blocks. If these blocks abated the symptoms, the patients then underwent intradural surgical sectioning of the upper cervical nerve roots (rhizotomy). Patients who did not respond to CT-guided nerve block were not offered surgical sectioning. We present our technique of selective nerve root block and the long-term follow-up of patients undergoing rhizotomy.
Methods
Patients
The medical records of 17 consecutive patients who had undergone CT fluoroscopy–guided cervical nerve block and surgical dorsal rhizotomy for occipital neuralgia, between December 1999 and September 2002, were retrospectively reviewed. Patient ages ranged from 17 to 76 years (mean age, 43 years). Six patients were male, and eleven were female. All patients had refractory occipital neuralgia. Nine patients developed occipital neuralgia following neck or skull base surgery that included surgery for trigeminal neuralgia (n = 4); retromastoid vestibular neurectomy (n = 2); resection of neck neuroma (n = 1); cervical diskectomy (n = 1); and occipital osteoma resection (n = 1). Seven patients did not have an identifiable cause of occipital neuralgia, and in one patient it commenced after an automobile accident.
CT Fluoroscopy–Guided Nerve Block
No anesthesia or sedation was used for any patient, and a total of 32 (C2 = 18 and C3 = 14) nerve root blocks were performed. One patient underwent bilateral C2 and C3 nerve root block. An axial scout CT from C1 to C3 without intravenous contrast material was performed in all patients. Thereafter, the anticipated site of needle entry between C1–C2 and C2–C3 was marked. Needle placement for C2 block was between posterior arches of C1 and C2, just behind the inferior aspect of the lateral mass of C2 (Figs 1 and 2). For C3 block, needle placement was at the lateral aspect of the C2–C3 foramen, just anterior to the base of C3 superior facet (Figs 3 and 4). Using sterile technique, a 25-gauge 8.89-cm straight spinal needle (Becton Dickinson, Franklin Lakes, NJ) was advanced medially under intermittent CT fluoroscopy toward the C2 (or C3) nerve root. After confirming the placement of needle tip adjacent to the expected location of the exiting C2 (or C3) nerve root (Fig 5), a short connector tubing was attached to the spinal needle. Approximately 0.5–2 mL of 0.25% bupivacaine was injected after making sure that no blood was aspirated before injection. Injection of 1 mL of nonionic iodinated contrast material (Optiray 350, Mallinckrodt, St. Louis, MO) was performed in two patients to document accurate placement of needle tip (Fig 6). The patient’s response to bupivacaine injection was documented, and at the completion of the procedure trigger points were palpated in an attempt to elicit the pain and assess the nerve block’s effectiveness. A positive response was considered complete relief of pain with numbness in the distribution of the occipital nerves and no pain on stimulation of trigger points. A negative response was considered pain persistence following the nerve block or after pain during trigger point stimulation after the nerve block. The total duration of anesthesia was not determined.
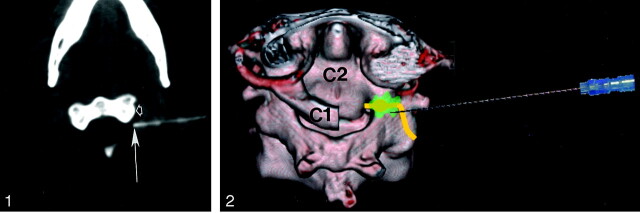
Fig 1.
Left C2 nerve root block. Axial CT at level of C2 vertebral body demonstrates final location of tip of 25-gauge spinal needle (arrow) posterior to lateral mass (open arrow) of C2 vertebra.
Fig 2.
Three-dimensional illustration of left C2 nerve root block, posterosuperior view. Posterior arch of C1 has been removed for clarity. C2 nerve is depicted in yellow, vertebral artery in red and injected medication in green.
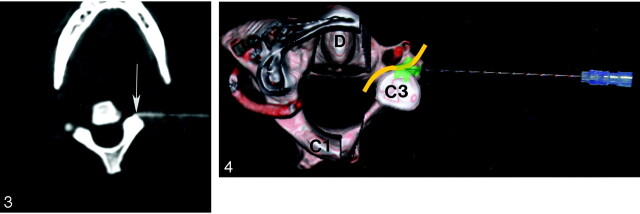
Fig 3.
Left C3 nerve root block. Axial CT at level of C2–C3 intervertebral foramen demonstrates final location of tip of 25-gauge spinal needle (arrow) anterior to superior articular facet of C3.
Fig 4.
Three-dimensional illustration of left C2 nerve root block viewed from above. Posterior arches of C1 and C2 have been removed for clarity. C3 nerve is depicted in yellow and injected medication in green. Note vertebral artery (red) just anterior to the nerve. D, dens.
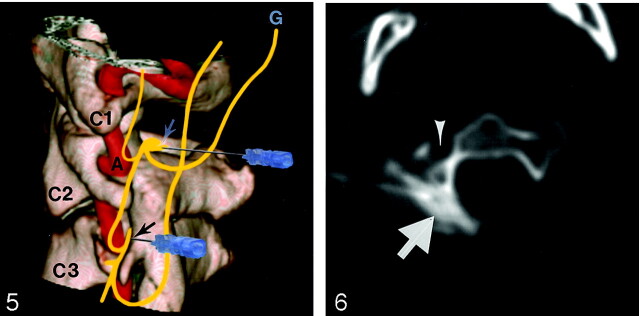
Fig 5.
Composite 3D illustration of left C2 (white arrow) and C3 (black arrow) nerve root block viewed from left. Vertebral artery (A) runs close to C3 nerve. Note connections between C1, C2, and C3 nerves. G, greater occipital nerve.
Fig 6.
Right C2 nerve root block. Iodinated contrast medium (arrow) is injected before injection of bupivacaine to confirm accurate placement of needle tip. Arrowhead marks foramina transversaria that transmits vertebral artery and veins.
Surgery
Seventeen patients with a positive response to C2 or C2 and C3 nerve blocks underwent unilateral (n = 16) or bilateral (n = 1) intradural C1 (n = 9), C2 (n = 17), C3 (n = 17), or C4 (n = 7) dorsal rhizotomies. C2 and C3 rhizotomies were performed in isolation or in combination with C1 or partial C4 rhizotomies. The number of levels was dependent on presence of C1 dorsal rootlets and access to upper C4 rootlets after C1, C2, and partial C3 laminectomies. All rhizotomies were performed by using general anesthesia with the patient prone. A midline cervical incision was used to bilaterally expose the ring of C1 and lamina of C2, C3 and C4. Following resection of ring of C1 and lamina of C2 and upper C3, the dura and arachnoid were opened and the cord with its exiting nerves exposed. The C1–C4 dorsal nerve roots were identified. All dorsal nerve rootlets on the side of the pain for C1, C2, C3, and upper C4 were sectioned. A careful search for small interconnecting branches between C1–C4 dorsal nerves was made, and they too were sectioned. Following completion, the cervical dorsal rhizotomies the dura and wound were closed in a watertight fashion. The patients were discharged home within 2–3 days.
All patients were followed up for a mean of 20 months (range, 5–37 months) for relief of pain. Sixteen patients were additionally assessed for satisfaction with surgery and subjective functional state following surgery. One patient died 8 months after rhizotomy of complications related to preexisting liver disease.
Results
All 17 patients had occipital numbness for at least 1–4 hours after the nerve blocks with complete relief of their usual pain; during this period no pain could be elicited with trigger-point stimulation. One patient experienced a vasovagal response to the injection but recovered uneventfully. There were no other complications related to CT fluoroscopy–guided occipital nerve block. Following surgery, 11 of 17 (64.7%) patients had complete relief of symptoms at follow-up, two (11.8%) patients reported partial relief, and four (23.5%) patients experienced no relief despite permanent numbness. Of the eight patients without history of prior surgery, seven (87.5%) had complete relief of symptoms and one (12.5%) patient had partial relief. Complete relief was found in four of nine (44.4%), partial relief in one of nine (11.2%), and no relief in four of nine (44.4%) patients with a history of prior surgery. This difference was not statistically significant (P = .110). Six patients without complete relief had diagnostic C2 or C3 nerve blocks before surgery. Of these six patients two each had C2, C3 and C2, C3, of C4 rhizotomies, respectively, and the remaining two patients each had C1, C2, C3 and C1, C2, C3, or C4 rhizotomies. There was no correlation between levels of surgery and pain relief. Eleven of 16 (68.8%) patients stated that the surgery was worthwhile, whereas five of 16 (31.2%) patients did not feel it was beneficial. Subjective functional state was improved in eight of 16 (50%) patients and unchanged or worse in four (25%) patients each. No patients without a history of prior surgery had decrease in functional status following rhizotomy.
Discussion
The earliest reference to occipital neuralgia was in 1821 by Beruto and Ramos (1). Numerous sporadic reports of this condition and its treatment have been described since (2, 3, 7–13). The syndrome’s clinical features, described by Hammond and Danta in 1978, (3) included severe paroxysmal or continuous pain in occipital nerve distribution with localized tenderness overlying the nerve trunk as it crosses the superior nuchal line, altered sensation in the form of hypo-, para-, or dysesthesia in the distribution of the nerve during or following the acute event, and relief of symptoms by local treatment such as nerve block with local anesthesia or occipital neurectomy. More recently, the International Headache Society defined occipital neuralgia (14) as a paroxysmal, sharp pain in the distribution of the lesser or greater occipital nerve with associated paraesthesia or dysesthesia in the same region. There is usually tenderness over the affected nerve with persistent aching between the paroxysms and temporary relief of the condition by local anesthetic block.
Anatomy of the greater and lesser occipital nerves provides explanation of etiology and treatment options available for occipital neuralgia. The greater occipital nerve is formed by the medial branch of the dorsal ramus of C2 that runs between the posterior arch of the atlas and lamina of the axis. The greater occipital nerve ascends between the inferior oblique and the semispinalis capitis muscles. It pierces the semispinalis capitis and the trapezius (adjacent to their insertion into the occipital bone between the superior and inferior nuchal lines) to run along the occipital artery (15). It receives a filament from the medial branch of the third dorsal cervical (C3) ramus after it pierces the trapezius. A cutaneous branch of the suboccipital nerve [first cervical (C1) dorsal ramus] will occasionally join the greater occipital nerve as it accompanies the occipital artery. The greater occipital nerve frequently connects with the lesser occipital nerve, which arises from the cervical plexus (formed by the upper four ventral cervical rami), and uncommonly with the superficial auriculotemporal nerve (16). The greater occipital nerve divides into several branches and supplies the skin of the back of the scalp as far forward as the vertex of the skull. Radiation of pain to the retro-orbital and other facial regions is believed to be due to sensory connections between the principal sensory nucleus of trigeminal nerve and substantia gelatinosa of the upper cervical spinal cord via the nucleus of spinal tract of the trigeminal nerve (3, 15, 17, 18) which explains the frequent occurrence of occipital neuralgia with trigeminal neuralgia and for the occurrence of retro-orbital pain. Seven of 17 patients in our series had coexistent trigeminal and occipital neuralgia. Seven patients also described numbness of retro-orbital, posterior auricular, temporal, facial, frontal, or parietal regions after CT fluoroscopy nerve blocks, thus reinforcing the above hypothesis. Moreover, anatomic dissections by Zander et al (19) showed terminal fibers of upper cervical nerves in all but the central pure trigeminal field of the face (20).
In our series, four of the nine patients with prior head and neck surgeries had been operated on for trigeminal neuralgia and two patients for Meniere disease (retromastoid vestibular neurectomy). Of the four patients without any relief from occipital neuralgia following rhizotomies, two each had previously undergone surgery for trigeminal neuralgia and Meniere disease, respectively. Whether there is an association between these two conditions and occipital neuralgia is not clear; however, connections between trigeminal nerve sensory neucleus and upper cervical spinal cord substance as described above may play a role.
Unlike other cervical nerve roots, pedicles and articular facets do not protect the dorsal rami of C1 and C2 nerves as they pass above the arches of the atlas and axis, respectively (Figs 2 and 5). Because there is little motion between atlas and occipital bone, it is unlikely that C1 nerve root plays a major role in the syndrome of occipital neuralgia (13). The marked degree of movement (rotary and extension) between the posterior arches and articular facets of atlas and axis, however, has the potential of traumatizing C2 dorsal ramus (13). With rotary motion, the inferior atlantal facet strikes the arch of axis on the side of rotation of the chin, whereas on the opposite side the arches of atlas and axis are in contact. During neck extension, the posterior arches of atlas and axis (Fig 7) come close to each other and may actually be in contact. Hence, there is potential for compression and injury to the posterior C2 ramus on either side with normal or excessive head rotation and extension (13). Although the greater occipital nerve receives contributions from dorsal rami of C3 and C4 (via lesser occipital nerve), they are less likely to be traumatized because their nerve roots are protected by pedicles or articular facets. Numerous other conditions—such as developmental or congenital anomalies of the cranviovertebral junction (2), rheumatoid arthritis (3), osteoarthritis (3–5), gout, infections, and, rarely, ectatic vertebral artery (6)—can compress C1–C4 dorsal nerve roots and cause occipital neuralgia.
Fig 7.
Illustration of occipital region and upper cervical vertebrae viewed from behind showing course of greater occipital nerve (G) with possible sites of compression: F, due to atlantoaxial joint disease; M, as it penetrates tendinous portion of the trapezius muscles (Z); T, as occipital nerve pierces posterior atlantoaxial membrane (depicted in light semitransparent blue); and between posterior arches of C1 and C2 vertebrae. Note connections between greater occipital nerve (G) and C1 (i) and C3 (ii) nerves. O, occipital bone.
The other site where the greater occipital nerve is vulnerable to injury (Fig 7) is as it pierces the posterior atlantoaxial membrane (3, 7). This membrane stretches with even minor degrees of C1–C2 subluxation or with loss of normal cervical lordosis of the lower cervical spine with resultant narrowing of the point of exit of the greater occipital nerve causing possible occipital nerve injury. Finally, the greater occipital nerve maybe damaged as it penetrates the tendinous portion of the trapezius muscles (Fig 7) close to its attachment to the occipital bone (3, 7).
Pain in patients with occipital neuralgia is typically in the distribution of the greater occipital nerve. Recurring bouts of severe pain are usually localized to one side. Trigger points can be identified in some patients along with worsening of pain with rotation of head toward the side of pain or with extension. Pain usually starts in the suboccipital area with subsequent radiation to the skull vertex and temporal areas. In some patients, the pain may primarily be peri- or retro-orbital (7). Frequently tenderness of the occipital nerve along the superior nuchal line can be elicited as the greater occipital nerve crosses the superior nuchal line midway between the mastoid process and external occipital protuberance.
Numerous treatments for occipital neuralgia have been attempted with varying degrees of success, such as conservative treatment with a cervical collar, antimigraine drugs, transcutaneous nerve stimulation (2, 3), percutaneous nerve blocks, chemical and radio-frequency ablation (7, 8, 10, 11), and surgical sectioning or decompression of the upper dorsal cervical nerve roots (9, 12, 13, 21). Percutaneous occipital nerve block by using a local anesthetic agent such as lidocaine with or without steroids is valuable not only for the diagnosis of occipital neuralgia but also almost always provides immediate pain relief with varying long-term success (3). Permanent relief of symptoms in some patients following a single injection may be explained by interruption of the cycle of pain, reflex muscle spasm, and more pain (22). Unfortunately, this is uncommon. In one large series of 92 patients treated with percutaneous lidocaine and steroid injection improvement was seen in 87% of patients, with recurrence in 31.5% of those with initial improvement occurring between 1 and 8 months (7). All patients in this series treated with cervical manipulation had worsening of symptoms. Another series by Blume (8) used radio-frequency denaturation of the occipital nerve in the treatment of occipitocervical pain syndrome. Follow-up after 6–24 months showed that patients without spinal abnormalities did better (90.7% excellent results) than those with spinal abnormalities (74.4% good to excellent results) such as spondylosis and disk disease. A similar trend was seen in our study, although the results were not statistically significant, because of the small number of patients. It would require a larger cohort with randomization to preoperative CT-guided block or without guided block before surgical rhizotomy. Surgical decision making in those without CT guided block might be made on the basis of history and examination alone or history and examination combined with palpable scalp block at trigger point or free-hand block of the occipital nerve by using fluoroscopic guidance.
All patients in our series had refractory occipital neuralgia with no significant benefit from previous nonsurgical treatment consisting of nonsteroidal antiinflammatory medications, physical therapy, immobilization, narcotic analgesics, and nerve blocks. Some patients in our series received only C2 nerve root block because the greater occipital nerve is formed mainly by the dorsal ramus of C2 and initially we believed it to be the sole culprit for causing occipital neuralgia; however, in light of the fact that the greater occipital nerve also receives major branches from C3 (as also inconsistently from C1 and C4), we realized that C3 may also contribute to this complex syndrome. Therefore, we now perform blocks at both C2 and C3 levels. Although there were no notable complications related to CT-guided cervical nerve root blocks in our series, serious complications can occur because of injection into the cervical subarachnoid space or into vessels in this region. Of special note is the vertebral artery that runs close to C3 nerve as it exits the neural foramina, and certain variations in its course (23) make this procedure challenging. Furman et al (24) documented intravascular contrast material injection in 19.4% patients undergoing transforaminal epidural steroid injection with low sensitivity of blood in the needle hub to predict intravascular injection. Certain authors advocate routine injection of contrast material before injection of medication to confirm that needle is not in a vessel during epidural steroid injections (25).). Although no major complications occurred following surgical rhizotomies in our series, potential risks include infection, stroke, paralysis, CSF leak, and trapezius or deltoid muscle weakness from spinal accessory nerve injury.
Our study is limited by the absence of a control group (patients with positive CT fluoroscopy–guided cervical nerve block who did not undergo rhizotomy; patients with negative results after CT block who underwent surgery) because it was a retrospective study. A prospective study with a control group, although difficult to achieve in light of the low incidence of patients with refractory occipital neuralgia, may be a subject for further investigation.
Conclusion
Occipital neuralgia is a complex pain syndrome. CT fluoroscopy–guided cervical nerve block is a safe procedure that is useful in diagnosis, confirmation, and surgical result simulation (numbness). Although the results of this small series were not statistically significant, there was a trend toward patients with occipital neuralgia secondary to nonsurgical causes having a better outcome compared with patients who had postsurgical occipital neuralgia.
Acknowledgments
We would like to thank Eric Jablonowski for his assistance with the excellent illustrations.
Footnotes
Presented at American Society of Spine Society, Scottsdale, Arizona, February 2003.
References
- 1.Beruto LJ, Ramos MM. Decades de med y cirug pract. Madrid,1821;3:145–169 [Google Scholar]
- 2.Dugan MC, Locke S, Gallagher JR. Occipital neuralgia in adolescents and young adults. N Engl J Med 1962;267:1166–1172 [Google Scholar]
- 3.Hammond SR, Danta G. Occipital neuralgia. Clin Exp Neurol 1978;48:23–32 [PubMed] [Google Scholar]
- 4.Ehni G, Benner B. Occipital neuralgia and the C1–2 arthrosis syndrome. J Neurosurg 1984;61:961–965 [DOI] [PubMed] [Google Scholar]
- 5.Star MJ, Curd JG, Thorne RP. Atlantoaxial lateral mass osteoarthritis: a frequently overlooked cause of severe occipitocervical pain. Spine 1992;17:S71–S76 [PubMed] [Google Scholar]
- 6.Sharma RR, Parekh HC, Prabhu S, et al. Compression of the C-2 root by a rare anomalous ecstatic vertebral artery. J Neurosurg 1993;78:669–672 [DOI] [PubMed] [Google Scholar]
- 7.Schultz DR. Occipital neuralgia. J Am Osteopath Assoc 1977;72:335–343 [PubMed] [Google Scholar]
- 8.Blume HG. Radiofrequency denaturation on occipital pain: a new approach in 114 cases. In: Bonica JJ, Alse-Fessard D, eds. Advances in pain research and therapy. Vol. 1. New York: Raven Press,1976. :691–698
- 9.Poletti CE. Proposed operation for occipital neuralgia: C-2 and C-3 root decompression. Neurosurgery 1983;12:221–224 [DOI] [PubMed] [Google Scholar]
- 10.Koch D, Wakhloo AK. CT-guided chemical rhizotomy of the C1 root for occipital neuralgia. Neuroradiology 1992;34:451–452 [DOI] [PubMed] [Google Scholar]
- 11.Gawel MJ, Rothbart PJ. Occipital nerve block in the management of headache and cervical pain. Cephalalgia 1992;12:9–13 [DOI] [PubMed] [Google Scholar]
- 12.Horowitz MB, Yonas H. Occipital neuralgia treated by intradural dorsal nerve root sectioning. Cephalalgia 1993;13:354–360 [DOI] [PubMed] [Google Scholar]
- 13.Hunter CR, Mayfield FH. Role of the upper cervical roots in the production of pain in the head. Am J Surg 1949;78:743–751 [DOI] [PubMed] [Google Scholar]
- 14.Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8:1–96 [PubMed] [Google Scholar]
- 15.Berry M, Bannister LH, Standring SM. Nervous system. In: Williams PL, ed. Gray’s anatomy. 38th ed. Edinburgh: Churchill Livingstone,1999;1262–1264
- 16.Becser N, Bovim G, Sjaastad O. Extracranial nerves in the posterior part of the head. Spine 1998;23:1435–1441 [DOI] [PubMed] [Google Scholar]
- 17.Perelson HN. Occipital nerve tenderness: a sign of headache. South Med J 1947;40:653–656 [DOI] [PubMed] [Google Scholar]
- 18.Graff-Radford SB, Jaeger B, Reeves JL. Myofascial pain may present clinically as occipital neuralgia. Neurosurgery 1986;19:610–613 [DOI] [PubMed] [Google Scholar]
- 19.Zander R. Beitrage zur Kenntnis der Hautnerven des Kopfes. Anat Hefte 1897;9:1–74 [Google Scholar]
- 20.Lewy FH. The role of cervical nerves in facial sensations and the quantitative disturbance of sensitivity in major trigeminal neuralgia. Am J Med Sci 1938;196:564–572 [Google Scholar]
- 21.Poletti CE, Sweet WH. Entrapment of the C2 root and ganglion by the atlanto-epistrophic ligament: clinical syndrome and surgical anatomy. Neurosurgery 1990;27:288–290 [DOI] [PubMed] [Google Scholar]
- 22.Knox DL, Mustonen E. Greater occipital neuralgia: an ocular pain syndrome with multiple etiologies. Trans Am Acad Ophthalmol Otolaryngol 1975;267:OP-513–OP-519 [PubMed] [Google Scholar]
- 23.Cox TCS, Stevens JM, Kendall BE. Vascular anatomy in the suboccipital region and lateral cervical puncture. BJR 1981;54:572–275 [DOI] [PubMed] [Google Scholar]
- 24.Furman MB, Giovanniello MT, O’Brien EM. Incidence of intravascular penetration in transforaminal cervical epidural steroid injections. Spine 2003;28:21–25 [DOI] [PubMed] [Google Scholar]
- 25.Johnson BA, Schellhas KP, Pollei SR. Epidurography and therapeutic epidural injections: technical considerations and experience with 5334 cases. AJNR Am J Neuroradiol 1999;20:697–705 [PMC free article] [PubMed] [Google Scholar]