Abstract
Summary: Despite new developments in the treatment of cerebral aneurysms, recurrent hemorrhage of an aneurysm is the most feared complication of subarachnoid bleeding. The prognosis of patients with early reruptured aneurysms is discouraging. A rerupture of an aneurysm with active bleeding is extremely rare, and the mechanisms involved remain unclear. We report the management of active bleeding of reruptured aneurysms during diagnostic angiography. In two patients, emergency treatment of the active hemorrhage was performed by means of neurosurgical intervention and by an endovascular approach in one other case. The causes and management of reruptured aneurysms with active bleeding are also discussed within the context of a review of the literature.
Advances in diagnostic and therapeutic techniques, together with the improvement of peri- and post-therapeutic care, have resulted in a much safer treatment and better outcome of patients after subarachnoid hemorrhage (SAH). Nevertheless, a rerupture of aneurysms with demonstration of active bleeding is rare, and the outcome is extremely poor.
In a cooperative study of 5484 patients with SAH, only one case of extravasation of contrast medium was noted during diagnostic angiography (1, 2). Recently, Saitoh et al (3) reported a 4.8% incidence of rerupture during angiography within 6 hours after initial intracranial aneurysm rupture. Rerupture caused by angiography was estimated to occur in 1.4% (2/144) of cases. Komiyama et al (4) revealed a rebleeding rate of 3.3% during diagnostic angiography within 6 hours after SAH. The mortality rate was 80% (3, 4). Taking these facts together, it is assumed that the overall risk of an aneurysm rerupture during diagnostic conventional angiography within 6 hours after ictus is 1–2% (5).
The question of whether rebleeding occurs coincidentally as a natural course, by the angiographic procedure itself, or by other mechanisms remains unclear. It is assumed that rerupture of an aneurysm that has recently bled can be caused by a single or several factors such as increased blood pressure, decreased intracranial pressure, vasospasm, and quick injection of a large volume of contrast medium (1). A review of the literature revealed no significant correlation between rerupture rate and injected volume of contrast medium; aneurysm size, shape, or site; CT findings of intracerebral or intraventricular hemorrhage; and patient age or sex (3, 4). Angiography within 6 hours after the first SAH and the state of consciousness, however, appear to be significant risk factors for aneurysm rerupture (3). Rerupture of an aneurysm with an active hemorrhage is very rare but needs an interdisciplinary strategy of emergency treatment. We report our experience with the management of reruptured SAH in three patients.
Case Reports
Case 1
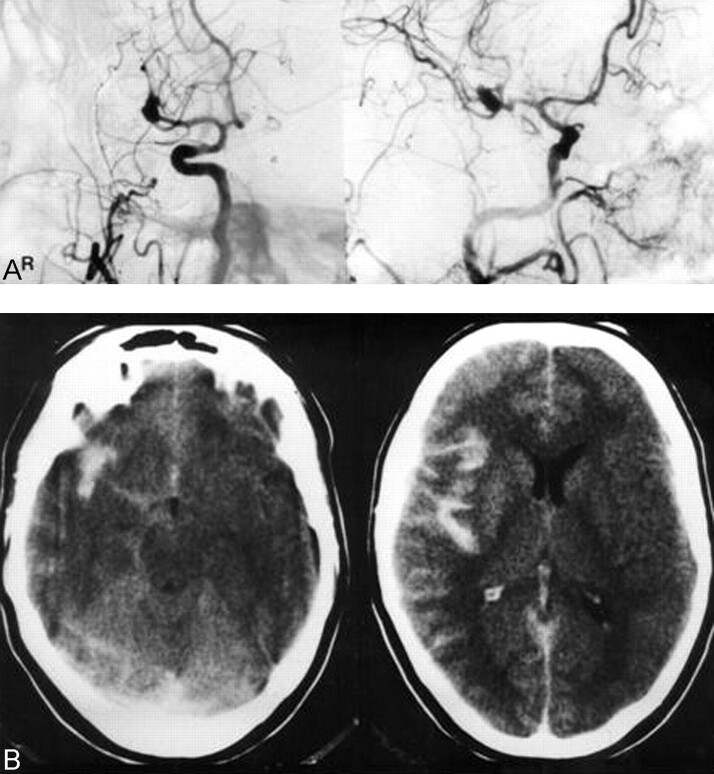
A 31-year-old woman had an acutely severe headache, nausea, and tinnitus (Fig 1). Initial CT showed an SAH in the right sylvian fissure. At admission to the neurosurgery department 2 days later, angiography revealed a left middle cerebral artery (MCA) aneurysm. Because angiographic findings and bleeding type seen on the CT scan were different, angiography was repeated, showing a right broad-necked MCA aneurysm. During angiography there was a drop of blood pressure and an episode of bradycardia. A mild local vasospasm of proximal MCA branches developed, indicating rebleeding of the MCA aneurysm (Fig 1A) without visible extravasation of contrast medium. The localization of the MCA aneurysm and its broad neck as well as already existing vasospasms were arguments against endovascular treatment in this case. The patient underwent surgical clipping the following day. She recovered and was discharged 4 weeks later without neurologic deficit.
Fig 1.
Case 1. A, Right ICA angiogram (anteroposterior view [left] and lateral-oblique view [right], arterial phase) shows an MCA aneurysm. During angiography, blood pressure dropped and an episode of bradycardia and mild local vasospasm of proximal MCA branches developed without visible extravasation.
B, CT scans obtained immediately after angiography shows an SAH located in the right sylvian fissure.
Case 2
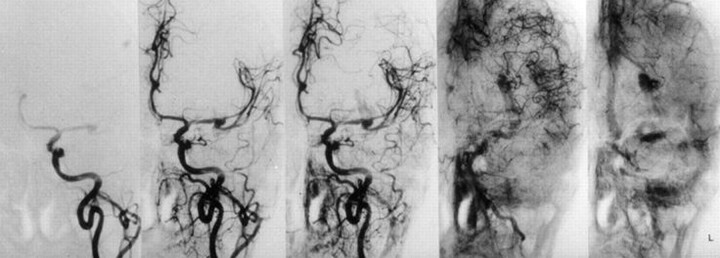
A 60-year-old man with a long history of chronic headache had an acute onset of severe headache and was found by his wife with complete aphasia and right hemiparesis several hours after having a generalized seizure (Fig 2). CT showed an SAH in the left sylvian fissure. After intubation for angiography, the patient developed an acute bradycardia and arrhythmia. During diagnostic angiography, acute rebleeding of a left MCA aneurysm with extravasation of contrast medium into the sylvian fissure could be demonstrated (Fig 2). CT was performed immediately, showing a massive intracerebral hemorrhage (ICH) with mass effect and moderate midline shift. Because of the ICH, emergency surgery was indicated. The aneurysm was clipped successfully, and after a long hospital course complicated by vasospasm and a wound infection, the patient was discharged with significant permanent neurologic deficits.
Fig 2.
Case 2. Left ICA angiograms (anteroposterior view, early arterial, and early venous phases) demonstrates active bleeding into the lentiform nucleus from an MCA aneurysm and mild local vasospasm.
Case 3
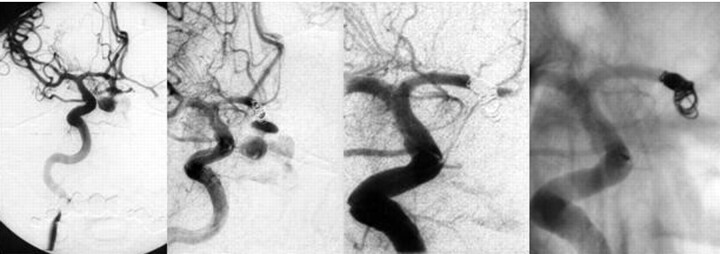
A 52-year-old man was admitted 10 hours after onset of SAH with symptoms of severe headache and nausea (Fig 3). Three months earlier, the patient had an episode of acute headache, but MR imaging at that time revealed no SAH or aneurysm but signs of vascular encephalopathy. After a generalized seizure indicating a first rebleed, he deteriorated from Hunt and Hess grade III to grade V. After intubation and initiation of deep anesthesia, isocoria returned, and the patient was transported to the neurosurgery department. Initial CT showed a massive SAH with hydrocephalus due to intraventricular blood. A ventricular drain was placed immediately showing more than 50 cm water pressure. Systemic blood pressure was 180/100 mm Hg at admission and increased suddenly to 240/100 mm Hg, indicating a second rebleed with concomitant anisocoria after 1 hour. A few minutes later, arterial blood was drained from the ventricle. Emergency angiography of the right ICA revealed an anterior communicating artery aneurysm (4.5 × 2.8 × 1.9 mm) with active bleeding into the subarachnoid space and the chiasmal cistern (Fig 3). Because of the massive active bleeding, extended brain resection would have been necessary to control bleeding surgically. Endovascular treatment was carried out by using a series of detachable coils. Because of the morphology of the aneurysm and the large tear in the aneurysm dome, occlusion of the parent artery along with the aneurysm was necessary. Over the next 4 days, elevations of the intracranial pressure could not be controlled, and the patient died.
Fig 3.
Right ICA angiogram, anteroposterior view. Active bleeding from the reruptured anterior communicating artery aneurysm during the angiography is visible (left two pictures). The dome of the aneurysm is completely lacerated. Extravasation of contrast material into the chiasmal cistern is visible. The diameter of the neck is 1.9 mm, and the diameter of the fundus 4.5 mm. After detachment of the first coil, bleeding continued, and protrusion of the coil into the subarachnoid space was imminent. Closure of the aneurysm with parent vessel occlusion of the A1-A2 segment of the right anterior cerebral artery (right two pictures).
Discussion
Rerupture of previously bled aneurysms, as described in our three cases, has high morbidity and mortality rates. The estimated mortality rate is between 50% and 80% (3–9). There are only a few reports on the management of patients with documented active bleeding after rerupture of an aneurysm (8, 9). Rerupture during diagnostic angiography occurs in about 1–2% of cases (5), and it often remains unclear whether this is a coincidental event or caused by the procedure. In the literature, various studies analyzing risk factors for ultra-early rebleeding have revealed inconsistent results. Saitoh et al (3) and Komiyama et al (4) found no significant correlation between rerupture rate and injected volume of contrast medium; size, shape, and site of aneurysm; presence of intracerebral or intraventricular hemorrhage; or patient age or sex (3, 4). In their study of 179 patients with SAH, however, Fuji et al (6) stated that the incidence of rebleeding depends on high systolic blood pressure, intracerebral or intraventricular hemorrhage, and poor neurologic condition on admission. Furthermore, Fuji et al (6) found three factors to be independently associated with ultra-early rebleeding: the level of enhancement of platelet sensitivity, the time interval between last attack and admission, and the plasma level of the thrombin-antithrombin complex.
Komiyama et al (4) reviewed 14 of their own cases and 202 cases in the literature with rebleeding during angiography and found that most rebleedings occurred within 6 hours after the last SAH. Fujii et al (6) could not verify that emergency angiography was an independent risk factor for ultra-early rebleeding.
Karhunen et al (10) performed an autopsy series of 76 patients with fatal outcome following SAH. The main cause of sudden death or rapid clinical deterioration was intraventricular hemorrhage from an MCA aneurysm, complicated later in many cases by cerebral artery vasospasm. There is no definite proof of an acute phase of arterial spasm following rupture of an aneurysm; however, this vasospasm is usually related to a prior episode of bleeding in most cases (11).
In the acute phase of rerupture, stabilization of blood pressure, together with ventricular drainage, seems to be the treatment of choice. After an aneurysm rupture, there is a sudden elevation of intracranial pressure to a level approaching systemic blood pressure. This tamponade effect prevents further bleeding and allows formation of a platelet plug at the site of the rupture. Then, over the next hours, this plug is reinforced by fibrin (12). Aoyagi et al (13) assumed that a gradual and progressive thinning of the wall of bump-type aneurysms and the fibrin net covering the brittle dome, which does not tolerate slight increases in pressure, are responsible for early rerupture. Therefore, in Hunt and Hess grade III or IV patients, breath holding during CT examination and lumbar puncture with evacuation of large amounts of CSF should be avoided during the first hours after the first hemorrhage (13).
The role of intracarotid pressure changes during injection of contrast material is also not fully understood. These pressure changes depend on many interacting factors, such as vasospasm in proximity to the catheter tip, size and branching of the arteries, peripheral anastomoses, autoregulation, pulsation, and blood viscosity. Saitoh et al (14) concluded from experimental data in dogs that standard injection rates and doses might also contribute to some elevation of intracranial blood pressure in humans. This confirms Stoeter et al’s (15) observation of an elevation of mean ICA blood pressure during cerebral angiography. Therefore, it can be assumed that the intraaneurysmal pressure is increased by the injection of contrast medium (16). Komiyama (4) pointed out that there is a higher incidence of angiographic rupture for ICA and MCA aneurysms, because a significantly higher intravascular pressure is measurable far from the site of injection. In addition to this, however, Hinshaw et al (17) showed a nonlinear pressure-volume relationship inside the aneurysms during injection of contrast medium, which leads to a sudden enlargement of the aneurysm and subsequent rupture. Obviously, in clinical routines the small volume (6 mL) of contrast material at an injection rate of 3–6 mL per second is able to rupture an aneurysm (18). Yousem et al (19) recently reported the prevailing opinion that the rate of intraangiographic aneurysm ruptures is negligible. On the other hand, some authors advocate avoiding conventional angiography during the first 3–6 hours after the first hemorrhage because of the high incidence (4.8–32%) of aneurysm rerupture during emergency cerebral angiography (3, 20). Although there is a significantly higher rate of rerupture within 6 hours after the initial aneurysm rupture (3), emergency angiography should not be discouraged, because several studies revealed that early surgery reduces mortality and morbidity (21, 22). In addition, on the basis of a study of 317 patients treated by coil embolization within 30 days after aneurysm rupture, Byrne (23) concluded that patients should be treated as soon as possible after an SAH, because the technique appears less susceptible to the adverse effects of vasospasm during the initial 3–10-day period after SAH. This strategy may be supported by Amagasa’s (24) analysis of 197 cases with early angiography after aneurysm rupture reporting only one case of rerupture.
In two of our three cases of rerupture, an extravasated saccular pooling of contrast medium was clearly visualized in the arterial phase and in the late venophase during angiography, indicating active bleeding. During this acute phase, CT angiography may be an alternative, noninvasive tool to localize the bleeding aneurysm (eg, in cases of SAH and acute subdural hematoma [25–27]).
Conclusion
The management of active bleeding from reruptured aneurysms during diagnostic angiography is interdisciplinary. The strategy of individual emergency treatment depends on the localization and configuration of the aneurysm, the type of bleeding (SAH, ICH, subdural hematoma), and associated complications such as thrombosis of the parent vessel. Despite new treatment modalities, however, the prognosis of patients with reruptured aneurysms during diagnostic angiography is still very poor.
References
- 1.Liliequist B, Lindqvist M, Probst F. Rupture of intracranial aneurysm during carotid angiography. Neuroradiology 1976;11:185–190 [DOI] [PubMed] [Google Scholar]
- 2.Kassell NF, Torner JC, Haley EC Jr, et al. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1. Overall management results. J Neurosurg 1990;73:18–36 [DOI] [PubMed] [Google Scholar]
- 3.Saitoh H, Hayakawa K, Nishimura K, et al. Rerupture of cerebral aneurysms during angiography. AJNR Am J Neuroradiol 1995;16:539–542 [PMC free article] [PubMed] [Google Scholar]
- 4.Komiyama M, Tamura K, Nagata Y, et al. Aneurysmal rupture during angiography. Neurosurgery 1993;33:798–803 [DOI] [PubMed] [Google Scholar]
- 5.van Gijn J, Rinkel GJE. Subarachnoid hemorrhage: diagnosis, causes and management. Brain 2001;2:249–278 [DOI] [PubMed] [Google Scholar]
- 6.Fuji Y, Takeuchi S, Sasaki O, et al. Ultra-early rebleeding in spontaneous subarachnoid hemorrhage. J Neurosurg 1996;84:35–42 [DOI] [PubMed] [Google Scholar]
- 7.Houkin K, Kuroda S, Takahashi A, et al. Intra-operative premature rupture of the cerebral aneurysms: analysis of the causes and management. Acta Neurochir 1999;141:1255–1263 [DOI] [PubMed] [Google Scholar]
- 8.Koga H, Kaneko M, Hosaka Y. Extrasvasation from aneurysms during angiography. Surg Neurol 1979;12:453–456 [PubMed] [Google Scholar]
- 9.Hayakawa I, Watanabe T, Tsuchida T, Sasaki A. Periangiographic rupture of intracranial aneurysms. Neuroradiology 1978;16:293–295 [DOI] [PubMed] [Google Scholar]
- 10.Karhunen PJ, Servo A. Sudden fatal or non-operable bleeding from ruptured intracranial aneurysm: evaluation by post-mortem angiography with vulcanizing contrast medium. Int J Legal Med 1993;106:55–59 [DOI] [PubMed] [Google Scholar]
- 11.Wilkins RH. Aneurysm rupture during angiography: does acute vasospasm occur? Surg Neurol 1976;5:299–303 [PubMed] [Google Scholar]
- 12.Nornes H. The role of intracranial pressure in the arrest of hemorrhage in patients with ruptured intracranial aneurysms. J Neurosurg 1973;39:226. [DOI] [PubMed] [Google Scholar]
- 13.Aoyagi N, Hayakawa I. Study on early re-rupture of intracranial aneurysms. Acta Neurochir 1996;138:12–18 [DOI] [PubMed] [Google Scholar]
- 14.Saitoh H, Hayakawa, Nichimura K, et al. Intracarotid blood pressure changes during contrast medium injection. AJNR Am J Neuroradiol 1996;17:51–54 [PMC free article] [PubMed] [Google Scholar]
- 15.Stoeter P, Prey N, Hoffmann C, et al. Doppler sonographic examination of the arterial flow in the carotid and supratrochlear arteries during carotid angiography. Neuroradiology 1984;26:199–207 [DOI] [PubMed] [Google Scholar]
- 16.Sorimachi T, Takeuchi S, Koike T, et al. Intra-aneurysmal pressure changes during angiography in coil embolization. Surg Neurol 1997;48:451–457 [DOI] [PubMed] [Google Scholar]
- 17.Hinshaw DB, Simmons CR, Leech W, et al. Loculated intracranial aneurysms: angiography and possible etiology. Radiology 1974;113:101. [DOI] [PubMed] [Google Scholar]
- 18.Gailloud P, Murphy KJ. Rupture of cerebral aneurysm during angiography. N Engl J Med 1999;340:1442. [DOI] [PubMed] [Google Scholar]
- 19.Yousem DM, Trinh BC. Injection rates for neuroangiography: results of a survey. AJNR Am J Neuroradiol 2001;22:1838–1840 [PMC free article] [PubMed] [Google Scholar]
- 20.Inagawa T, Kamiya K, Ogasawara H, Yano T. Rebleeding of ruptured intracranial aneurysms in the acute stage. Surg Neurol 1987;28:93–99 [DOI] [PubMed] [Google Scholar]
- 21.Kassel NF, Torner JC, Jane JA, et al. The international cooperative study on timing of aneurysm surgery. II. J Neurosurg 1990;73:37–47 [DOI] [PubMed] [Google Scholar]
- 22.Roos Y, Beenen L, Groen R, et al. Timing of surgery in patients with aneurysmal subarachnoid hemorrhage: rebleeding is still the major cause of poor outcome in neurosurgical units that aim at early surgery. J Neurol Neurosurg Psychiatry 1997;63:490–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byrne JV. Acute endovascular treatment by coil embolization of ruptured intracranial aneurysms. Ann R Coll Surg Engl 2001;83:253–256 [PMC free article] [PubMed] [Google Scholar]
- 24.Amagasa M, Yoshimoto T, Mizoi K, Suzuki J. Early cerebral angiography after aneurysm rupture: analysis of 197 cases. J Neurosurg 1986;65:776–778 [DOI] [PubMed] [Google Scholar]
- 25.Gosselin MV, Vieco PT. Active hemorrhage of intracranial aneurysms: diagnosis by CT angiography. J Comput Assist Tomogr 1997;21:22–24 [DOI] [PubMed] [Google Scholar]
- 26.Kinoshita Y, Machi T, Satoh Y, Yahata T, Miyake E. Extravasation of contrast media in acute subdural hematoma during three-dimensional CT angiography: a case report. No Shinkei Geka 1999;27:157–61 [PubMed] [Google Scholar]
- 27.Nakatsuka M, Mizumo S, Uchida A. Extravasation on three-dimensional CT angiography in patients with acute subarachnoid hemorrhage and ruptured aneurysm. Neuroradiology 2002;44:25–30 [DOI] [PubMed] [Google Scholar]