Abstract
Summary: We report a case of primary diffuse meningeal melanomatosis, a rare variant of primary malignant melanoma of the CNS, in a 68-year-old woman. The disease mimicked intracranial hypotension syndrome and was diagnosed only at autopsy (CSF cytologic results were negative). CT revealed hydrocephalus with effacement of the cerebral convexity sulci and abnormal contrast enhancement in the right sylvian and frontoparietal fissures, whereas MR imaging showed diffuse marked dural and leptomeningeal contrast enhancement. In retrospect, these nonspecific findings correlated with the extensive leptomeningeal invasion in the cerebral hemispheres, brain stem and spinal cord. The clinical, radiologic, and pathologic features of diffuse meningeal melanomatosis are reviewed.
Primary diffuse meningeal melanomatosis is a rare variant of malignant melanoma of the CNS that seems to arise directly from melanocytes within the leptomeninges (1). Despite the use of CT, contrast-enhanced MR imaging, and CSF cytology, the disease can be difficult to recognize. In fact, meningeal melanomatosis may be radiologically and clinically confused with diffuse leptomeningeal neoplasms or, as in the present case, with intracranial hypotension syndrome.
Case Report
Clinical History
A 68-year-old woman without any relevant clinical history was admitted to the hospital in October 1996 because of headache, vomiting, mental deterioration, and a gait disturbance. Neurologic examination showed mental slowness, poor concentration and memory, a short-stepped gait, and lower limb weakness with bilateral Babinski signs. Fundoscopy revealed ill-defined optic disks. Cranial CT scans revealed hydrocephalus with effacement of the cerebral convexity sulci and abnormal contrast enhancement in the right sylvian and frontoparietal fissures (Fig. 1A and B). Intracranial monitoring revealed increased ventricular CSF pressure. Analysis of the CSF did not reveal neoplastic cells. Obstructive hydrocephalus was diagnosed. A right-sided parietal ventriculoperitoneal CSF shunt was created in December 1996.
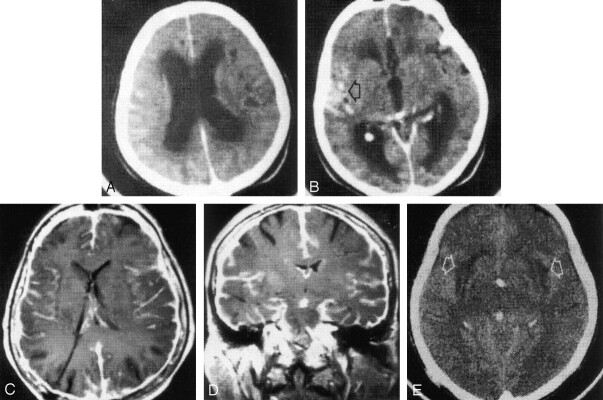
Fig 1.
Primary diffuse meningeal melanomatosis in a 68-year-old woman.
A and B, At presentation, contrast-enhanced cranial CT scans show dilatation of the lateral (A) and third (B) ventricles, with effacement of the cerebral convexity sulci (in A). Abnormal contrast enhancement is present in the right sylvian fissure (arrow in B) and in the right frontoparietal region.
C and D, Twelve months after the placement of a ventricular CSF shunt with a right parietal approach, cranial axial (C) and coronal (D) T1-weighted spin-echo MR images (TR/TE, 580/14) obtained after the intravenous administration of gadopentetate dimeglumine show small lateral and third ventricles and diffuse dural and leptomeningeal enhancement.
E, Plain cranial CT scan obtained 1 month before the patient’s death shows tiny ventricles and symmetric areas of hypoattenuation (arrows) in the subinsular white matter and external capsules. This finding was probably due to neoplastic encasing of the penetrating arteries.
In November 1997, cranial MR imaging showed no abnormalities, although the T1- and T2-weighted images showed small ventricles. After the intravenous administration of contrast material, diffuse marked dural and leptomeningeal contrast enhancement was observed (Fig 1C and D). Five months later, she complained of episodic headaches that were relieved by lying down. Because of the suspicion of intracranial hypotension, the CSF shunt valve was substituted with a programmable valve in February 1999. Despite the disappearance of the headaches, cranial MR images still showed small ventricles and abnormal diffuse enhancement of the dura mater and leptomeninges. The patient’s mental faculties and gait progressively deteriorated, and sporadic partial seizures occurred. In August 1999, she fractured her left femur. In September 1999, plain cranial CT scans (Fig 1E) revealed tiny ventricles and bilateral symmetric hypoattenuating areas in the subinsular white matter and external capsules. The patient died of bronchopneumonia 1 month later.
Pathologic Findings
Skin examination did not reveal abnormal pigmented lesions. General necroscopy showed bilateral pneumonia. The postfixed brain weighed 1140 g. No gross abnormality of the intracranial or spinal dura was seen. The cerebral hemispheres were swollen, with mild transtentorial uncus herniation. On macroscopic examination, the leptomeninges over the base of the brain were diffusely thickened and embedded in a dark tissue that covered the circle of Willis (Fig 2A) and extended bilaterally to the temporal lobes and orbitofrontal cortex. A thick brown coat of tissue filled the entire spinal subarachnoid spaces, obscuring the spinal cord and cauda roots (Fig 2B). Coronal sections of the brain revealed small ventricles and diffuse, almost symmetric areas of brown pigmentation in the cortical layer under the leptomeninges coating (Fig 2C). Removal of the spinal cord revealed a small, dark nodule within the dorsal column. No evidence of a primary melanoma was found elsewhere.
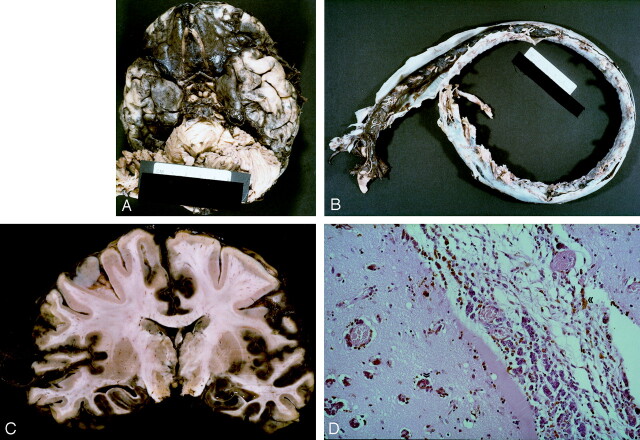
Fig 2.
Brain images.
A, Basal view of the brain. The leptomeninges over the base of the brain are dark and thickened. The left temporal lobe shows several discrete black nodules of varying size. Mild uncus herniation indicates the presence of brain edema.
B, Opening of the dura mater of the spinal cord reveals brown neoplastic tissue. This tissue is visible through the arachnoid membrane and fills the entire subarachnoid space, completely obscuring the underlying spinal cord from view.
C, Coronal section of the cerebrum at the level of the mammillary bodies reveals extensive laminar brown coloration of the cortex. The overlying leptomeninges appears thickened and brown.
D, Spreading of neoplastic cells along subpial, perivascular, and Virchow-Robin spaces into the cerebral cortex (arrows). The cells are polygonal with a dusty cytoplasmic pigment (hematoxylin-eosin, original magnification × 25).
Microscopic examination of routinely fixed and stained brain specimens revealed diffuse leptomeningeal infiltration by small epithelioid cells in the cerebral hemispheres, brain stem, and spinal cord. The tumor cells showed vesicular nuclei with prominent nucleoli and dusty cytoplasmic pigment. The neoplastic cells invaded the underlying brain tissue, spreading along the Virchow-Robin spaces around the cortical blood vessels (Fig 2D).
The immunohistochemical findings were diagnostic of malignant melanoma. In particular, the neoplastic cells were strongly positive for S-100, vimentin, and HMB-45, but they were negative for glial fibrillary acid protein and cytokeratins. Because of the absence of other systemic localizations, primary diffuse meningeal melanomatosis was diagnosed.
Discussion
The CNS and meningeal coverings can be affected by a wide variety of melanocytic lesions ranging from diffuse melanosis and well-differentiated melanocytomas to primary malignant melanomas (2). The latter can occur either as a diffuse leptomeningeal proliferation or as discrete masses anywhere in the CNS (most often in the posterior fossa and in the spinal canal).
Primary diffuse meningeal melanomatosis, a rare variant of primary malignant melanoma, results from the spread of malignant melanocytes into the leptomeninges and Virchow-Robin spaces, with superficial invasion of the brain. The tumor is derived from leptomeningeal melanocytes, which have a neuroectodermal origin (3), as do cutaneous melanocytes. Leptomeningeal melanocytes are normally found in the pia arachnoid that covers the base of the brain, the caudal medulla, and cervical spinal cord. Primary diffuse meningeal melanomatosis is more common in adults (3) than in children (in whom it is sometimes associated with multiple or extensive pigmented skin lesions and often transmitted as an autosomal dominant trait [4]).
Clinically, the antemortem diagnosis of primary diffuse meningeal melanomatosis is notoriously difficult. It may be established by means of cytologic examination of the CSF (5). Nevertheless, in the absence of a tumoral lesion on CT or MR images, the isolated neoplastic cells in the CSF can easily escape recognition (6), as in our case. Furthermore, primary diffuse meningeal melanomatosis can clinically mimic a wide variety of other conditions, including lymphoma, leukemia, metastatic carcinoma, subacute meningitis, viral encephalitis (5), and idiopathic hypertrophic cranial pachymeningitis (7).
The clinical presentation of our patient was consistent with her obstructive idiopathic hydrocephalus. The postural headache that occurred after CSF shunt placement was presumably related to intracranial hypotension secondary to hypoperfunctioning CSF drainage. Despite effective ventricular CSF drainage, as demonstrated with MR imaging and CT, mental and motor deterioration and partial seizures developed. In retrospect, these manifestations can clearly be attributed to diffusion of the disease in the subarachnoid spaces and invasion of the subpial cerebral cortex (as appreciable in Fig 1D). Brain or meningeal biopsy is often the only definitive diagnostic procedure in cases of suspected primary diffuse meningeal melanomatosis (8). Despite the deterioration of our patient’s condition, such an invasive procedure was not deemed appropriate in the absence of cytologic evidence of malignancy. In cases in which a biopsy sample is obtained, immunohistochemical and electron microscopic procedures can facilitate the identification of malignant melanoma cells and therefore the antemortem diagnosis (9). Nevertheless, recognition of HMB-45 or S-100 protein or both do not in themselves allow a definitive diagnosis, because their expression can also be observed in several nonmelanotic tumors of the nervous system, including malignant Schwannomas, gliosarcomas, and many metastatic carcinomas (10).
The radiologic features of dural and leptomeningeal contrast enhancement in our patient were completely nonspecific. Radiologically, the differential diagnosis is extensive and includes the following: meningeal carcinomatosis and diffuse leptomeningeal oligodendrogliomatosis (clinically identical but with contrast enhancement restricted to the leptomeninges) (11, 12); infectious conditions (radiologically similar but clinically different) (11); transthyretin amyloidosis (with contrast enhancement in the leptomeninges and distinct clinical features [eg, polyneuropathy, vitreal deposits, cardiomyopathy, and a much slower evolution]) (13); idiopathic hypertrophic cranial pachymeningitis (contrast enhancement restricted to the dura but with similar clinical features) (7); and skull and dural metastasis (contrast enhancement restricted to the dura and similar clinical features) (11). In our case, the MR imaging findings in the intracranial dural and leptomeningeal enhancement were erroneously interpreted as correlating with the clinical features of intracranial hypotension syndrome; however, the contrast enhancement pattern remains confined to the dura mater (11, 14, 15).
Thus, the diagnosis of primary diffuse meningeal melanomatosis was established at postmortem examination (no clinical, radiographic, or necroscopic evidence of primary melanoma was found elsewhere). On pathologic grounds, the distinction of primary diffuse meningeal melanomatosis from diffuse melanosis and well-differentiated melanocytoma must be made on the basis of topographic, gross, and histologic features. In diffuse melanosis, proliferating melanocytes diffusely involve the leptomeninges but without frank invasion of the brain. On the other hand, melanocytoma appears as a solitary, variably pigmented mass of the spinal and basilar leptomeninges resembling pigmented meningioma with minimal cytologic atypia. In our case, postmortem examination revealed diffuse infiltration of the leptomeninges and the underlying cerebral cortex by malignant neoplastic melanocytes (Fig 2D).
Histologically, primary diffuse meningeal melanomatosis can mimic a wide variety of other lesions (16), including brain metastases from a malignant melanoma of the skin. The diffuse pattern of growth commonly found in primary melanomas of the CNS (eg, diffuse meningeal melanomatosis) sharply contrasts with the localized presentation most often found in metastatic melanoma (6). Nevertheless, the diagnosis of metastatic malignant melanoma is generally based on the clinical finding of an extracerebral primary melanoma. The polygonal neoplastic cells with dusty cytoplasmic pigment seen in our case are typical of malignant melanoma (Fig 2D). The diagnosis of meningeal melanomatosis depended on the diffuse growth pattern and the absence of other malignant melanomas elsewhere.
Little is known about the radiologic-pathologic correlation in primary diffuse meningeal melanomatosis. The correlation that we recorded in the brain was striking. Indeed, a substantial match was found between the extent of neoplastic spread at autopsy and both the superficial extension and the depth of the leptomeningeal contrast enhancement.
Conclusion
The sequence of radiologic findings recorded in our patient may provide tentative indications regarding the natural history of primary diffuse meningeal melanomatosis, at least as far as the present case is concerned. We initially observed diffuse enhancement in the leptomeninges covering the right cerebral hemisphere. Subsequently, contrast enhancement extended first to the entire brain (12 months after presentation) and then to the spinal leptomeningeal coating (at 29 months). Finally, symmetric hypoattenuation appeared in the region of the basal forebrain (at 35 months), presumably as a result of neoplastic encasing of the penetrating arteries.
Acknowledgments
The authors are grateful to Mr. Robin M. T. Cooke for helping with the manuscript.
Footnotes
Supported by a grant from the Centro Interdipartimentale di Ricerche sul Cancro G. Prodi and Università degli Studi di Bologna, Italy.
References
- 1.Savitz M, Anderson P. Primary melanoma of the leptomeninges: a review. Mt Sinai J Med NY 1974;41:774–791 [PubMed] [Google Scholar]
- 2.Painter TJ, Chaljub G, Sethi R, Singh H, Gelman B. Intracranial and intraspinal meningeal melanocytosis. AJNR Am J Neuroradiol 2000;21:1349–1353 [PMC free article] [PubMed] [Google Scholar]
- 3.Allcutt D, Michowiz S, Weitzman S, Becker L, Blaser S, Hoffmann HJ, et al. Primary leptomeningeal melanoma: an unusually aggressive tumor in childhood. Neurosurgery 1993;32:721–729 [DOI] [PubMed] [Google Scholar]
- 4.Makin GW, Eden OB, Lashford LS, Moppett J, Gerrard MP, Davies HA, et al. Leptomeningeal melanoma in childhood. Cancer 1999;86:878–886 [PubMed] [Google Scholar]
- 5.Nicolaides P, Newton RW, Kelsey A. Primary malignant melanoma of meninges: atypical presentation of subacute meningitis. Pediatr Neurol 1995;12:172–174 [DOI] [PubMed] [Google Scholar]
- 6.Grant DN. Primary meningeal melanomatosis: limitations of current diagnostic techniques. J Neurol Neurosurg Psychiatry 1983;46:874–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bang OY, Kim DI Yoon SR, Choi IS. Idiopathic hypertrophic pachymeningeal lesions: correlation between clinical patterns and neuroimaging characteristics. Eur Neurol 1998;39:49–56 [DOI] [PubMed] [Google Scholar]
- 8.Crisp DE, Thompson JA. Primary malignant melanomatosis of the meninges. Clinical course and computed tomographic findings in a young child. Arch Neurol 1981;38:528–529 [DOI] [PubMed] [Google Scholar]
- 9.Aichner F, Schuler G. Primary leptomeningeal melanoma: diagnosis by ultrastructural cytology of cerebrospinal fluid and cranial computed tomography. Cancer 1982;50:1751–1756 [DOI] [PubMed] [Google Scholar]
- 10.Zimmer C, Gottschalk J, Goebel S, Cervos-Navarro J. Melanoma-associated antigens in tumours of the nervous system: an immunohistochemical study with the monoclonal antibody HMB-45. Virchows Archiv A Pathol Anat 1992;420:121–126 [DOI] [PubMed] [Google Scholar]
- 11.River J, Schwartz A, Gomoi JM, Soffer D, Siegal T. Clinical significance of diffuse dural enhancement detected by magnetic resonance imaging. J Neurosurg 1996;85:777–783 [DOI] [PubMed] [Google Scholar]
- 12.Armao DM, Stone J, Castillo M, Mitchell KM, Bouldin TW, Suzuki K. Diffuse leptomeningeal oligodendrogliomatosis: radiologic/pathologic correlation. AJNR Am J Neuroradiol 2000;21:1122–1126 [PMC free article] [PubMed] [Google Scholar]
- 13.Herrick MK, DeBruyne K, Horoupian DS, Skare J, Vanefsky MA, Ong T. Massive leptomeningeal amyloidosis associated with Val30Met transthyretin gene. Neurology 1996;47:988–992 [DOI] [PubMed] [Google Scholar]
- 14.Fishman RA, Dillon WP. Dural enhancement and cerebral spinal displacement secondary to intracranial hypotension. Neurology 1993;43:609–611 [DOI] [PubMed] [Google Scholar]
- 15.Pannullo SC, Reich JB, Krol G, Deck MDF, Poster JB. MRI changes in intracranial hypotension. Neurology 1993;43:919–926 [DOI] [PubMed] [Google Scholar]
- 16.Nakhleh RE, Wick MR, Rocamora A, Swanson PE, Dehner LP. Morphologic diversity in malignant melanomas. Am J Clin Pathol 1990;93:731–740 [DOI] [PubMed] [Google Scholar]