Abstract
BACKGROUND AND PURPOSE: Professional boxing is associated with chronic, repetitive head blows that may cause brain injuries. Diffusion-weighted imaging is sensitive to microscopic changes and may be a useful tool to quantify the microstructural integrity of the brain. In this study, we sought to quantify microscopic alterations associated with chronic traumatic brain injury in professional boxers.
METHODS: MR and diffusion-weighted imaging were performed in 24 boxers and in 14 age- and sex-matched control subjects with no history of head trauma. Using distribution analysis, the average diffusion constant of the entire brain (BDav) and diffusion distribution width (σ) were calculated for each subject; findings in professional boxers were compared with those of control subjects. In the boxer group, correlations between diffusion changes and boxing history and diffusion changes and MR imaging findings were assessed.
RESULTS: The measured diffusion values in the boxer group were significantly higher than those measured in the control group (BDav, P < .0001; σ, P < .01). In the boxer group, a robust correlation was found between increased BDav and frequency of hospitalization for boxing injuries (r = 0.654, P < .05). The most common MR finding in the boxer group was volume loss inappropriate to age followed by cavum septum pellucidum, subcortical white matter disease, and periventricular white matter disease.
CONCLUSION: Boxers had higher diffusion constants than those in control subjects. Our data suggest that microstructural damage of the brain associated with chronic traumatic brain injury may elevate whole-brain diffusion. This global elevation can exist even when routine MR findings are normal.
Brain injury from repetitive head blows has been reported in the boxer population (1–8). Diffuse axonal injury has been described as a major form of primary damage to the brain in blunt head injury. Chronic traumatic encephalopathy (CTE) and traumatic brain injury (TBI) often result in dementia pugilistica, a neurologic abnormality caused by repetitive exposure to head blows in contact sports such as soccer and boxing (2–4, 6, 7). A prevalence study of retired professional boxers revealed that 20% of those assessed developed CTE (8).
Commonly, CT and MR imaging findings of CTE are negative or nonspecific. Imaging findings of brain atrophy and hyperintensity are associated with periventricular white matter disease (PWMD) with or without cavum septum pellucidum (CSP) (3, 9, 10). None of these findings are definitive signs of early CTE (11).
MR diffusion-weighted imaging is sensitive to changes of microscopic Brownian motion of water molecules in brain tissue. The underlying microstructure of the brain tissue imposes restriction on the diffusion of water. Changes in the microstructure of the brain can be inferred from increased diffusion. This information cannot be obtained from routine MR imaging (12–14).
Experimental models of TBI show that diffusion initially decreases in the acute phase after trauma and subsequently increases (15). Some data suggest that diffusion was changed at the site of trauma and surrounding tissue even when T2-weighted findings were unremarkable (16). We hypothesize that the global diffusion of the brains exposed to chronic trauma is different from that of normal age-matched controls.
Methods
Study Participants
We studied 24 professional boxers (aged 21–53 years, average 32.3 years ± 7.2) and 14 age-matched healthy volunteers as controls (aged 23–45 years, average 32.2 years ± 7.3). Control subjects were free of neurologic disease and had no boxing history. All participants were male. Informed consent was obtained from all participants. Imaging protocols were approved by our institutional review board.
MR Imaging Protocol
The following sequences were performed in all the subjects by using a 1.5-T clinical MR system (Signa; GE Medical Systems, Milwaukee, WI) equipped with a quadrature head coil. Imaging parameters were: axial T1-weighted imaging (TR/TE, 500/14), axial T2-weighted imaging (TR/TE, 4000/102), fluid-attenuated inversion recovery imaging (TR/TE/TI,10,000/162/2200; matrix, 256 × 192), and diffusion-weighted imaging (TR/TE, 10500/106; matrix, 128 × 128). For all imaging protocols, section thickness was 0.5 cm, (no intersection gap); field of view, 22 cm; and number of excitations, 1. We used 30 sections covering the entire brain from the top convexity to the brain stem.
Diffusion was measured in three orthogonal directions (x, y, z) at b = 1000s/mm2. Another set of images (So) were obtained at b = 0. By using the diffusion-weighted images in three orthogonal directions, an orientation-independent diffusion image related to trace of diffusion tensor was obtained as
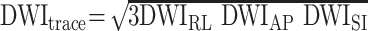 |
1) |
where DWIRL is the diffusion-weighted image with a diffusion gradient in the right-left direction (anteroposterior and superoinferior). The diffusion trace images and b = 0 images were transferred to a computer workstation for further data processing. The Dav maps were calculated from the DWItrace and So images by using the following equation:
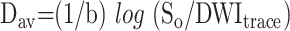 |
2) |
Distribution Analysis and BDav Calculation
In each subject, a computer program was used to calculate the whole-brain diffusion distribution histogram (Fig 1). This program distributed the pixels into 250 bins with a bin width of 0.02 10−5 cm2/s. This histogram was then fitted to a triple Gaussian curve by using commercial software (KaleidaGraph, Adelbeck Software, Reading PA). This curve (C1e-[(Dav-BDav)/σ]2 + C2e-[(Dav-D2)/σ 2]2 + C3e-[(Dav-D3)/σ 3]2) represents a two-compartment model with mixing (three compartments): 1) brain tissue compartment, 2) brain tissue mixed with CSF, 3) high diffusion compartment of CSF and nonbrain tissue. The mean of the brain tissue distribution is recognized as a mean diffusion constant for the whole brain (BDav). Peak locations and peak widths were determined from the fitted data. The peak location of the brain tissue compartment was interpreted to be BDav, and the Dav distribution width to be σ.
Fig 1.
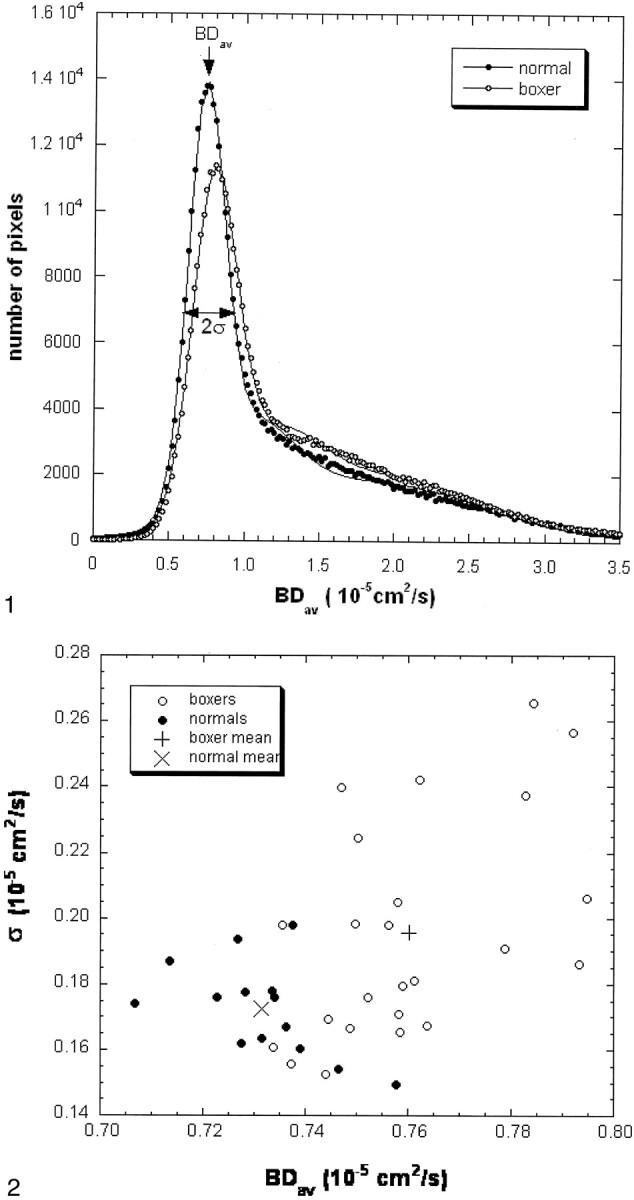
Normalized brain diffusion distribution histograms in a control subject and a boxer (case 15). The areas under the two curves are the same. The Dav data (dots and circles) are fitted with a triple Gaussian function to represent the two-compartment nature and the mixing between the two compartments (lines). The narrow peak represents the distribution of the brain tissue about its mean. The second and the third compartments have a broader distribution. The mean of the brain tissue pixel distribution is recognized as a mean diffusion constant for the entire brain (BDav). The distribution width (σ) of the brain tissue compartment is also recorded. The fitted curve of the boxer (circles) shifts to the right as compared with the curve of the control subject (dots). The second compartment level of the boxer’s curve is higher than that of the control subject.
Details of this brain model and distribution analysis have been described elsewhere (17).
Scoring of MR Findings
For each boxer, volume loss, CSP, SWM, PWMD, and negative findings were independently scored. Any of the findings above were scored as one if listed in any of the MR findings in the boxer group; otherwise, the score was 0. Volunteers did not have any positive MR signs.
Statistical Analysis
The means and standard deviations of the measured diffusion parameters were calculated for the boxer and control groups. A Student t test was then used to determine the significance of the results. P < .05 was considered to be statistically significant. Pearson product-moment correlations were conducted on calculated diffusion parameters, BDav and σ; measures of boxing performance and history; and routine MR imaging findings.
Results
BDav and σ and MR findings in boxers are summarized in Table 1. BDav values in boxers and control subjects were 0.760 ± 0.018 (10−5cm2/s) and 0.732 ± 0.013 (10−5cm2/s), respectively. Average BDav in control subjects was consistent with that cited in a previous report (17). Thirteen boxers had BDav values more than two SD above the controls’ mean, and five boxers had BDav values more than four SD above the controls’ mean. The statistical analysis is summarized in Table 2. No statistically significant difference existed between the age of boxers and that of control subjects (P > .05). BDav and σ values were significantly higher in the boxers compared with those in control subjects (P < .0001and P < .01, respectively). The difference between boxers and control subjects remained significant even when the measured diffusion results (BDav and σ) of boxers with normal MR findings were compared with those of control subjects, the diffusion values of boxers with normal MR images were still significantly different from those of control subjects (P < .01).
TABLE 1:
Comparison of diffusion values and MR findings in boxers
| Case (No.) | Age (y) | BDav | σ | MR Findings |
|---|---|---|---|---|
| 1 | 20.84 | 0.7562 | 0.1980 | Normal |
| 2 | 22.52 | 0.7502 | 0.2245 | Left minimal hippocampal atrophy, otherwise normal |
| 3 | 24.68 | 0.7933 | 0.1861 | Volume loss, CSP |
| 4 | 26.00 | 0.7637 | 0.1672 | CSP, mild atrophy, non-specific SWM |
| 5 | 26.51 | 0.7622 | 0.2419 | Normal |
| 6 | 27.04 | 0.7372 | 0.1558 | Normal |
| 7 | 27.09 | 0.7590 | 0.1795 | Normal |
| 8 | 27.71 | 0.7613 | 0.1812 | Normal |
| 9 | 27.72 | 0.7486 | 0.1667 | Normal |
| 10 | 30.00 | 0.7580 | 0.2048 | Normal |
| 11 | 30.07 | 0.7497 | 0.1984 | CSP, nonspecific SWM |
| 12 | 30.17 | 0.7586 | 0.1655 | Nonspecific SWM in left frontal lobe |
| 13 | 32.29 | 0.7920 | 0.2564 | Mild volume loss inappropriate to age |
| 14 | 33.64 | 0.7828 | 0.2376 | CSP, SWM, mild volume loss |
| 15 | 33.72 | 0.7947 | 0.2061 | Normal |
| 16 | 34.13 | 0.7842 | 0.2655 | CSP |
| 17 | 35.14 | 0.7444 | 0.1695 | Normal |
| 18 | 36.10 | 0.7439 | 0.1526 | Normal |
| 19 | 36.12 | 0.7337 | 0.1606 | Cerebellar atrophy, mild non-specific PWMD |
| 20 | 38.05 | 0.7584 | 0.1711 | Atrophy in left inferior cerebella, mild dilatation of sulci |
| 21 | 38.52 | 0.7522 | 0.1759 | Nonspecific SWM, atrophy inappropriate to age |
| 22 | 40.00 | 0.7469 | 0.2397 | PWMD |
| 23 | 42.92 | 0.7355 | 0.1979 | Normal |
| 24 | 53.09 | 0.7786 | 0.1909 | Normal |
Note.—CSP indicates cavum septum pellucidum; PWMD, nonspecific periventricular white matter disease; and SWM, subcortical white matter disease.
TABLE 2:
Distributions of BDav and σ in boxer group versus those in control group
| Age (y) | BDav (10−5 cm2/s) | σ (10−5 cm2/s) | |
|---|---|---|---|
| Boxers | 32.3 ± 7.2* | 0.760 ± 0.018* | 0.197 ± 0.033* |
| Control subjects | 32.2 ± 7.3* | 0.732 ± 0.013* | 0.173 ± 0.014* |
| Increase (%) | 0.3 | 3.68 | 12.18 |
| Significance | P > .05 | P < .0001 | P < .01 |
Mean ± SD.
Figure 1 shows diffusion distribution histograms from a control subject and a boxer (case 15). The diffusion distribution of the boxer has a tissue peak that is wider and lower than that of the control subject. The peak is also shifted to the higher diffusion value. Figure 2 displays the diffusion data from all boxers and control subjects. Overall, BDav is higher and σ wider in the boxers as compared with control subjects.
Fig 2.

BDav versus σ for boxers and control subjects: Overall, the boxer group shows elevated BDav and σ.
We compared increased BDav with times of hospitalization for boxing injury and found a robust correlation (r = 0.65, P < .05). Similar analyses between BDav and age when boxing was started, total rounds, years of performance, or number of wins and losses did not reveal any significant correlation (P > .05). MR findings in boxers are summarized in Table 3. General or focal volume loss inappropriate for age was found in eight boxers, CSP in five, nonspecific PWMD in two, and SWM in four (Fig 3). Scores of the positive MR findings for the boxers were not correlated to BDav (P > .05).
TABLE 3:
MR findings in boxers
| Premature Volume Loss | CSP | PWMD | SWM | Normal | |
|---|---|---|---|---|---|
| Number (n) | 8 | 5 | 2 | 4 | 13 |
| Percentage | 33.3% | 20.8% | 8.3% | 16.7% | 54.2% |
Note.—Some boxers had more than one positive finding. CSP indicates cavum septum pellucidum; PWMD, nonspecific periventricular white matter disease; and SWM, subcortical white matter disease.
Fig 3.
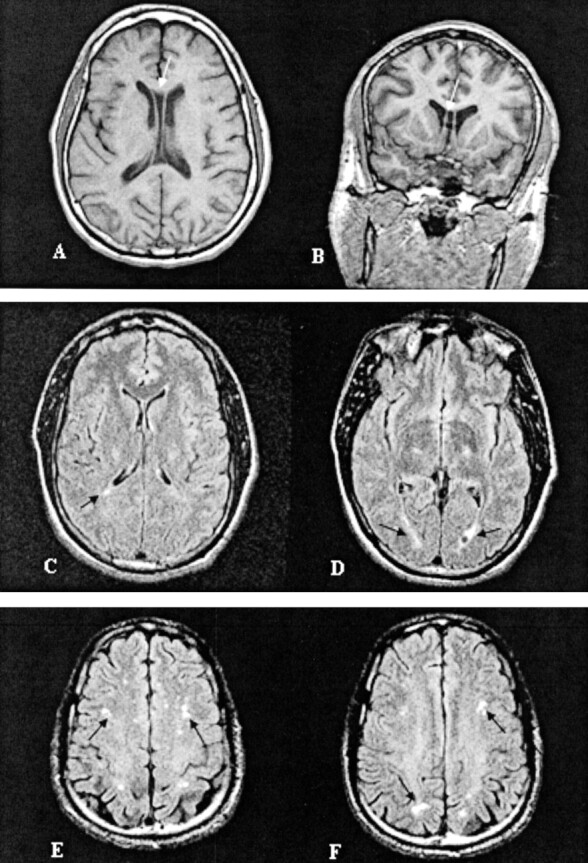
Representative images of MR findings in boxers: A, Cavum septum pellucidum (case 14); B, nonspecific periventricular white matter disease (case 22); and C, mild subcortical white matter demyelination (case 21).
Discussion
Diffusion-weighted imaging is sensitive to microscopic Brownian movement (18–20). Microscopic change occurs in the structure and diffusion of the brain after TBI (21, 22). Decreased diffusion has been reported in association with diffuse brain injury (23). Animal studies have shown reductions in diffusion after acute spinal cord injury involving both gray and white matter as well as abnormalities in tissues beyond the site of injury (13, 15). In this study, the average diffusion in brain tissue, as measured by BDav, was higher in the brains of professional boxers as compared with the brains of nonboxing control subjects (P < .0001). In addition, the distribution of Dav in brain (σ) was wider in boxers than that in control subjects (P < .01) (Figs 1 and 2). These phenomena may be explained by less restricted diffusion of water molecules in the brains of boxers as compared with control subjects. The widened distribution (σ) within the brain tissue compartment may indicate greater heterogeneity of diffusion within the brain. Factors such as damage to the integrity of cells and microvasculature of brain, which increase intercellular space and decrease restriction of diffusion, may contribute to the increased diffusion in the brains of boxers. It is, however, difficult to correlate, noninvasively and in vivo, these diffusion changes to damage to the cells and microvasculature of the brain. Future investigation is needed to explore the histologic detail and pathologic evolution occurring in the brain after traumatic injury.
In this study, five boxers had high BDav and σ values, similar values reported in previous studies of dementia (24, 25). Of these five cases, case 15 had no positive MR findings, whereas three of the remaining four cases had CSP revealed by MR imaging. This may suggest that increased BDav is highly associated with CSP because of CSF fluid dynamics. It is believed that CSP in boxers is acquired rather than congenital and results from rotational injuries with tearing of the septum pellucidum. A larger study sample may further elucidate the correlation between history of trauma and pathologic findings. It would be of interest to track the changes in diffusion in the brain associated with repetitive trauma and the corresponding clinical correlation. A robust correlation exists between increased BDav values in boxers and number of times hospitalized for boxing-related injuries (r = 0.65, P < .05). This supports our assumption that accumulative exposure to severe head trauma in boxing causes brain injury and increases entire brain diffusion. Similar analyses revealed no significant correlation between BDav or σ and the total rounds of performance, years of performance, age when boxing began, years of boxing, or number of losses.
Eleven boxers were found to have abnormalities on brain MR images. Volume loss inappropriate for age was the most frequent finding (n = 8), followed by CSP (n = 5) and SWM (n = 4); some boxers had more than one positive MR finding. This is in agreement with previous studies (3, 4, 8, 9). None of these factors were independently correlated with an increase in BDav and summed scores. BDav values in boxers with negative MR findings were significantly different from those in control subjects (P < .01). This supports the theory that BDav increase occurs before abnormalities appear on MR images.
Four of our cases had nonspecific SWM either with or without CSP and mild volume loss. Etiology could include infarct, demyelination, or gliosis. Myelination is among the major sources for restricted diffusion of water in brain, and demyelination may explain increases in BDav. MR spectroscopy may help detect the focal or general integrity of myelin in injured brains. Previous studies reported increase in choline- and myo-inositol-containing compounds increase after trauma (26–28). A recent MR spectroscopy study revealed that myelin degradation occurred 2 days after blunt head trauma, which likely evolves during the postinjury period (27). This increase may reflect membrane disruption and the consequent release of choline-containing compounds. Membrane disruption leads to damage of cell integrity; thus, increased diffusion of inter- and intracellular water molecules could lead to a higher measured BDav.
Changes in BDav in boxers’ brains may be explained by cumulative exposure to TBI, and cerebral self-restoration may lead to complex diffusion changes in the brain. BDav increase may appear before positive MR findings; thus, BDav could be a useful tool to help predict neurologic impairment from boxing and dynamically monitor results of treatment for brain injury. We have previously reported increases in BDav values and σ in patients with dementia (24, 25) and therefore speculate that these observations in boxers may represent a preclinical sign of cognitive decline.
There are limitations to our study. None of the boxers had clinical evidence of CTE. This is not surprising given that CTE typically develops after the cessation of boxing. Further studies will be needed to determine the predictive value of increased diffusion in boxers. If this finding is indeed associated with subsequent development of CTE, it may be possible to prevent or decrease the incidence and severity of the disorder. In this study, diffusion-weighted imaging was performed at a single time point. A larger study sample with multiple examinations at several time points may further elucidate the correlation between history of trauma and pathologic findings. It would be of interest to track the changes in diffusion associated with repetitive trauma and to identify correlations between diffusion changes and cognitive function. It may be possible in the future to use diffusion-weighted imaging to track brain damage, spontaneous healing, and treatment response in boxers.
Conclusion
Quantitative diffusion-weighted imaging revealed statistically significant increases in BDav and σ in the brains of professional boxers compared with diffusion measures in age-matched, nonboxing control subjects. These increased diffusion values were observed even when routine MR imaging results were negative or nonspecific. Our data suggest that diffusion tensor imaging can show early pathologic changes in the cellular and microvascular structures of the brain in the boxer population, and because these changes have also been reported in demented subjects, increased BDav and σ may represent preclinical signs of cognitive decline. Correlation between increased BDav and frequency of hospitalization for boxing-related injury was significant; thus, BDav may be a useful index for monitoring the neurologic health of boxers.
Acknowledgments
We thank the technologists in the MR Department for acquiring high-quality images in this study and the New York State Athletic Commission for their support and assistance with subject recruitment.
References
- 1.Corsellis JAN. Boxing and the brain. BMJ 1989;298:105–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meythaler JM, Peduzzi JD, Eleftheriou E, Novack TA. Current concepts: diffuse axonal injury–associated traumatic brain injury. Arch Phys Med Rehabil 2001;82:1461–1471 [DOI] [PubMed] [Google Scholar]
- 3.Moseley IF. The Neuroimaging evidence for chronic brain due to boxing. Neuroradiology 2000;42:1–8 [DOI] [PubMed] [Google Scholar]
- 4.Jordan BD, Jahre C, Hauser WH, et al. CT of 338 active professional boxers. Radiology 1992;2:181–185 [DOI] [PubMed] [Google Scholar]
- 5.Jordan BD, Relkin NR, Ravdin LD, Jacobs AR, Bennett A, Gandy S. Apolipoprotein E epsilon4 associated with chronic traumatic brain injury in boxing. JAMA 1997;278:136–140 [PubMed] [Google Scholar]
- 6.Rabadi MH, Jordan BD. The cumulative effect of repetitive concussion in sports. Clin J Sport Med 2001;11:194–198 [DOI] [PubMed] [Google Scholar]
- 7.Bodensteiner J, Schaefer G. Dementia pugilistica and cavum septi pellucidi: born to box. Sports Med 1997;24:361–365 [DOI] [PubMed] [Google Scholar]
- 8.Jordan BD. Chronic traumatic injury associated with boxing. Semin Neurol 2000;20:179–185 [DOI] [PubMed] [Google Scholar]
- 9.Jordan BD, Zimmerman RD. Magnetic resonance imaging in amateur boxers. Arch Neurol 1988;45:1207–1208 [DOI] [PubMed] [Google Scholar]
- 10.Bigler ED. Quantitive magnetic resonance imaging in traumatic brain injury. J Head Trauma Rehabil 2001;16:1–21 [DOI] [PubMed] [Google Scholar]
- 11.Haglund Y, Bergstrand G. Does Swedish amateur boxing lead to chronic brain damage? 2. A retrospective study with CT and MRI. Acta Neurol Scand 1990;82:297–302 [DOI] [PubMed] [Google Scholar]
- 12.Le Bihan D, Turner R, Douek P, Patronas N. Diffusion MR imaging: clinical applications. AJR Am J Roentgenol 1992;159:591–599 [DOI] [PubMed] [Google Scholar]
- 13.Le Bihan D. Molecular diffusion, tissue microdynamics and microstructure. NMR Biomed 1995;8:375–86 [DOI] [PubMed] [Google Scholar]
- 14.Uluğ AM, van Zijl PCM. Orientation independent diffusion imaging without tensor diagonalization: anisotropy definitions based on physical attributes of the diffusion ellipsoid. J Magn Reson Imaging 1999;9:804–813 [DOI] [PubMed] [Google Scholar]
- 15.Assaf Y, Holokovsky A, Berman E, Shapira Y, Shohami E, Cohen Y. Diffusion and perfusion magnetic resonance imaging following closed head injury in rats. J Neurotrauma 1999;16:1165–1176 [DOI] [PubMed] [Google Scholar]
- 16.Jones D, Dardis R, Ervine M, et al. Cluster analysis of diffusion tensor magnetic resonance images in human head injury. Neurosurgery 2000;47:306–314 [DOI] [PubMed] [Google Scholar]
- 17.Chun T, Filippi CG, Zimmerman RD, Uluğ AM. Diffusion changes in the aging human brain. AJNR Am J Neuroradiol 2000;21:1078–1083 [PMC free article] [PubMed] [Google Scholar]
- 18.Moseley ME, Cohen Y, Kucharczyk J, et al. Diffusion weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology 1990;176:439–445 [DOI] [PubMed] [Google Scholar]
- 19.Doran M, Hajnal JV, van Bruggen N, King MD, Young IR, Bydder GM. Normal or abnormal white matter tracts shown by MR imaging using directional diffusion weighted sequences. J Comput Assist Tomogr 1990;14:865–873 [DOI] [PubMed] [Google Scholar]
- 20.Moonen CT, Pekar J, de Vleeschouwer MH, van Gelderen P, van zijl PC, DesPres D. Restricted and anisotropic displacement of water in healthy cat brain and in stroke studied by NMR diffusion imaging. Magn Reson Med 1991;19:322–327 [DOI] [PubMed] [Google Scholar]
- 21.Adams JH, Doyle D, Ford I, Genarelli TA, Graham DI, McLellan DR. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology 1989;15:49–59 [DOI] [PubMed] [Google Scholar]
- 22.McGowan JC, McCormack TM, Grossman RI, et al. Diffuse axonal pathology detected with magnetization transfer imaging following brain injury in the pig. Magn Reson Med 1999;41:727–733 [DOI] [PubMed] [Google Scholar]
- 23.Takayama H, Kobayashi M, Sugishita M, Mihara B. Diffusion-weighted imaging demonstrates transient cytotoxic edema involving the corpus callosum in a patient with diffuse brain injury. Clin Neurol Neurosurg 2000;102:135–139 [DOI] [PubMed] [Google Scholar]
- 24.Chun T, Filippi CG, Relkin R, Zimmerman RD, Uluğ AM. Diffusion changes in normal pressure hydrocephalus. In: Proceedings of the Eighth Meeting of the International Society for Magnetic Resonance in Medicine. Denver, Co: ISMRM,2000797
- 25.Uluğ AM, Relkin R, Zimmerman RD. Diagnosis of normal pressure hydrocephalus using diffusion imaging. In: Proceedings of the 30th Annual Meeting of the American Aging Association 2001. Madison, Wisc: American Aging Association,72
- 26.Ng HK, Mahaliyana RD, Poon WS. The pathological spectrum of diffuse axonal injury in blunt head trauma: assessment with axon and myelin stains. Clin Neurol Neurosurg 1994;96:24–31 [DOI] [PubMed] [Google Scholar]
- 27.Garnett MR, Blamire AM, Rajagopalan B, Styles P, Cadoux-Hudson TA. Evidence for cellular damage in normal-appearing white matter correlates with injury severity in patients following traumatic brain injury: a magnetic resonance spectroscopy study. Brain 2000;123:1403–1409 [DOI] [PubMed] [Google Scholar]
- 28.Garnett MR, Blamire AM, Corkill RG, Cadoux-Hudson TA, Rajagopalan B, Styles P. Early proton magnetic resonance spectroscopy in normal-appearing brain correlates with outcome in patients following traumatic brain injury. Brain 2000;123:2046–2054 [DOI] [PubMed] [Google Scholar]


