Abstract
REVEAL is the first large, prospective observational study aimed at examining the contemporary demographics, burden of disease, clinical management, patient-reported outcomes, and health care resource utilization in patients with polycythemia vera in the United States.
Background:
Polycythemia vera (PV) has a prevalence of 44 to 57 per 100,000 people in the United States. Prospective data concerning the demographics, clinical characteristics, and treatment patterns of patients with PV in the United States are lacking.
Patients and Methods:
The ongoing, prospective, observational REVEAL study evaluates demographics, disease burden, clinical management, patient-reported outcomes, and health care resource utilization of adult patients with PV in the United States. This report summarizes the demographics and clinical characteristics of patients at enrollment (baseline).
Results:
Patients (n = 2510) were a median age of 67.0 years, 54.2% were male, and 89.1% were white. The median time from PV diagnosis to study enrollment was 4.0 (range, 0–56.3) years. Most patients (89.7%) were diagnosed after an abnormal blood test. Less than half (49.2%) underwent JAK2 mutation analysis, of whom 95.8% were JAK2 V617F mutation positive; < 1% were positive for JAK2 exon 12 mutations. At enrollment, 47.7% of patients had elevated hematocrit (> 45%), 35.8% had elevated platelets (> 400 × 109/L), and 37.0% had elevated leukocytes (> 10 × 109/L). Most patients (94.5%) were receiving active PV treatment, predominantly therapeutic phlebotomy alone (33.6%), hydroxyurea monotherapy (29.0%), or hydroxyurea plus phlebotomy (23.7%). Thrombotic events occurred in 11.9% of patients before PV diagnosis (venous, 6.7%; arterial, 5.7%), and 8.3% between diagnosis and enrollment. Hypertension (70.6%) was the most common previous medical condition.
Conclusion:
REVEAL enrollment data inform our understanding of the baseline demographics, diagnostic approach, disease characteristics, and treatment patterns of patients with PV in the United States. Longitudinal real-world data collected in this study will complement information collected during randomized controlled clinical trials.
Keywords: Blood tests, Demographics, JAK2 V617F mutation, Myeloproliferative neoplasm, Population characteristics
Introduction
Polycythemia vera (PV) is a myeloproliferative neoplasm in which clonal proliferation of a hematopoietic progenitor cell results in erythrocytosis, leukocytosis, and thrombocytosis.1 With respect to the epidemiology of PV in the United States, analyses of administrative claims from 2008 to 2010 reported a prevalence of approximately 44 to 57 per people.2 Most patients with PV have a Janus kinase 2 (JAK2) mutation that results in constitutive activation of hematopoietic signal transduction, thus explaining the exaggerated hematopoiesis. More than 95% of patients with PV carry the JAK2 V617F mutation; ~4% have mutations in JAK2 exon 12.3–7
Patients with PV have an increased mortality risk relative to the general population, primarily due to thrombotic events, transformation to leukemia or myelofibrosis, and solid tumors. 8–12 Patients also experience a broad range of symptoms that may lead to decreased quality of life and productivity.13–16
Large multicenter observational studies of PV have focused primarily on European patients,13,14,16–21 whereas US studies have been limited to single-center settings.22,23 Patterns of care can be characterized with administrative claims databases and electronic medical record audits. However, these data sources frequently lack important clinical information and do not capture patient-reported outcomes (PROs).
The Prospective Observational Study of Patients With Polycythemia Vera in US Clinical Practices (REVEAL; ClinicalTrials.gov, NCT02252159) was designed to collect contemporary demographics and data on the burden of disease, clinical management, PROs, and health care resource utilization of US patients with PV being seen at community and academic medical centers. This report describes the demographics as well as the clinical and disease characteristics of patients in the REVEAL study at the time of study enrollment.
Patients and Methods
Study Design
REVEAL is a multicenter, noninterventional, prospective observational study of patients with PV conducted in accordance with the Declaration of Helsinki. Approval of all study materials by central (Sterling IRB; Atlanta, GA) and local institutional review boards and provision of informed consent by all patients was required, including a separate informed consent form documenting willingness to participate in an optional biological substudy to examine the molecular features of PV. Patients with a clinical diagnosis of PV were enrolled from community practices and academic centers in the United States over a 24-month enrollment period. Physician assessments and PRO outcomes (previously published24) were recorded during routine care visits (approximately every 6 months). Blood samples were collected at enrollment and annually for patients agreeing to participate in an optional molecular substudy. All treatment decisions during the study were made by patients’ treating physicians.
Eligibility Requirements
At enrollment, patients were ≥ 18 years old; had a clinical diagnosis of PV per physician judgment; were willing and able to provide informed consent and complete patient assessments and questionnaires, either alone or with minimal assistance from a caregiver or trained site personnel; and were currently under physician supervision for management of PV. Patients were excluded if they were participating in an active, blinded clinical trial; had a life expectancy < 6 months; had a diagnosis of myelofibrosis, acute myeloid leukemia, or myelodysplastic syndrome; had a history of or active plan (within 3 months of enrollment) to proceed to allogeneic hematopoietic stem-cell transplantation; or had undergone splenectomy.
Assessments
This analysis describes patient demographics as well as clinical and disease characteristics at time of diagnosis and around the time of enrollment. Historic data pertaining to the evaluations and disease characteristics at time of diagnosis and physician-reported data from usual care visits, including information from the 6 months before enrollment, were collected and abstracted from electronic medical records into an electronic data capture system. For the purpose of this analysis, physician-reported data in the following categories were examined: demographics and PV disease characteristics; education, employment, insurance, and residency characteristics; laboratory tests and procedures used to determine a diagnosis of PV; signs and symptoms at time of PV diagnosis; laboratory values at time of enrollment; PV-directed therapy; comorbid conditions; thrombotic event history; concomitant medications; and health care resource utilization.
The Charlson Comorbidity Index (CCI) was used to assess morbidity.25 The CCI uses a 6-point scale to rate diagnosis codes from the International Classification of Diseases for each comorbid condition, which are summed to generate a total index score for each patient; higher scores correspond with more severe morbidity.
Statistical Analysis
Descriptive statistics were used. Missing data were not imputed; however, missing dates for temporal end points were imputed when applicable. If the entire date (ie, day, month, and year) was missing, all corresponding data were excluded from analyses involving dates. If the day was missing but the month and year were available, the date was imputed as the first day of the month. If the day and month were missing but the year was available, the date was imputed as January 1.
Results
Patient Demographics and PV Disease Characteristics
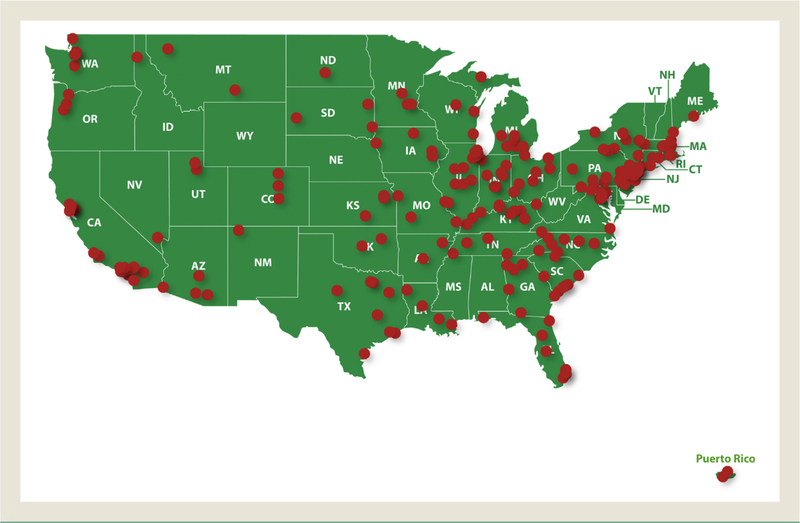
In this ongoing study, 2510 patients were enrolled from July 22, 2014, to August 3, 2016, from 227 US sites (39 academic and 188 community [self-identified]; Figure 1). Patients had a mean (SD) age of 66.3 (12.3) years (Table 1), and 2.9% were young (aged < 40 years). Most patients were male (54.2%) and white (89.1 and 48.1% had a history of smoking; 11.1% were active smokers at time of enrollment. Family history of PV was documented in 132 patients (5.3%).
Figure 1.
IRB-Approved Study Sites Participating in REVEAL. A Total of 2510 Patients Were Enrolled From 227 Sites Across the United States (39 Academic and 188 Community [Self-identified])
Abbreviations: IRB = institutional review board; REVEAL = Prospective Observational Study of Patients With Polycythemia Vera in US Clinical Practices.
Table 1.
Demographics and PV Disease Characteristics at Enrollment for 2510 Patients
| Characteristic | Value |
|---|---|
| Age (y) | |
| Mean (SD) | 66.3 (12.3) |
| Median (range) | 67.0 (22.0–95.0) |
| Male sex | 1360 (54.2) |
| BMI (kg/m2) (n = 2502) | |
| Mean (SD) | 28.7 (5.8) |
| Median (range) | 28.0 (13.7–56.6) |
| Smoking | |
| Ever smoked | 1208 (48.1) |
| Smoking at baseline | 279 (11.1) |
| Race | |
| White | 2237 (89.1) |
| Black or African American | 143 (5.7) |
| Asian | 37 (1.5) |
| Other | 19 (0.8) |
| No information/missing | 74 (2.9) |
| Ethnicity | |
| Non-Hispanic/Latino | 2293 (91.4) |
| Hispanic/Latino | 98 (3.9) |
| No information/missing | 119 (4.7) |
| Time from PV diagnosis to enrollment (y) (n = 2485), median (range) | 4.0 (0–56.3) |
| Spleen Palpation | |
| Spleen palpation performed | 1601 (63.8) |
| Patients with palpable spleen | 282 (11.2) |
| PV Risk | |
| Low | 570 (22.7) |
| Higha | 1940 (77.3) |
| History of thrombosis (venous or arterial) | 500 (19.9) |
| Positive family history of PV | 132 (5.3) |
Data are presented as n (%) unless otherwise indicated. Sample size is 2510 unless otherwise indicated.
Abbreviations: BMI = body mass index; PV = polycythemia vera.
Aged ≥ 60 years and/or history of thrombotic events.
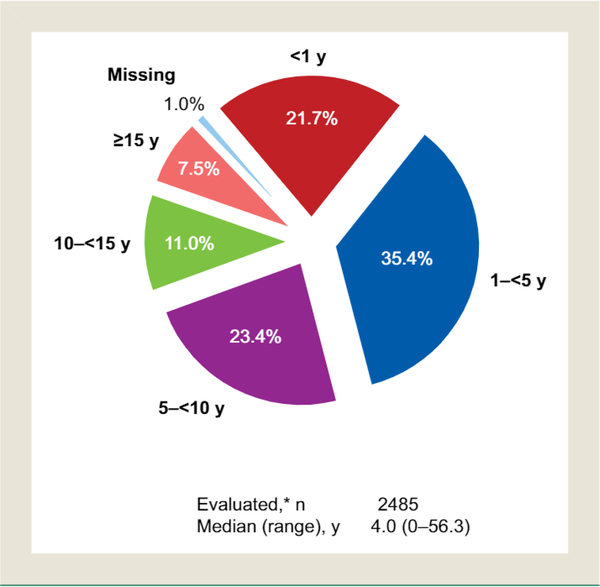
Median (range) disease duration was 4.0 (0–56.3) years (Figure 2), 77.3% of patients were classified as having high-risk disease (aged ≥ 60 years and/or history of thrombotic events), and 72.6% were obese or overweight. Spleen assessment by physical examination was performed in 1601 patients (63.8%) at time of enrollment. Of these patients, 282 (17.6%) had palpable splenomegaly. Spleen length measurements were available for 122 patients, 51.6% of whom had spleen length ≥ 5 cm from the left costal margin to the point of greatest splenic extension.
Figure 2.
Duration of Disease at Enrollment. *Excluding 25 Patients With Missing Diagnosis Date
Patient Education, Employment, Insurance, and Residency Characteristics
Most patients (62.7%) had at least some college education, 33.5% were employed full time or part time, 51.1% were retired, and 4.4% were disabled or unable to work (Supplemental Table 1). The vast majority of patients (99.1%) had health insurance (public only, 47.8%; private only, 40.4%; both public and private, 10.9%). Most patients (94.9%) were living independently at home, 3.7% were living as a dependent at home, and 0.8% were living in assisted housing; 0.1% and 0.4% were living in long-term care facilities or other residency settings, respectively.
PV Diagnosis
The majority of patients were reported to have abnormal hemoglobin (59.0%) or hematocrit (57.5%) results at time of diagnosis (Table 2). An abnormal platelet count or erythropoietin level was reported in only 28.7% and 20.1% of patients at time of diagnosis, respectively. In almost half (49.2%) of patients, JAK2 mutational testing results were reported; most of these patients were positive for JAK2 V617F (95.8%), with only 0.8% positive for JAK2 exon 12 mutations. A JAK2 test was reported for 19.9% of patients diagnosed before January 1, 2006, compared to 54.3% of patients diagnosed after January 1, 2006. Bone marrow biopsy/aspiration results were reported in 24.4% of patients. Palpable splenomegaly was reported as a factor in the diagnosis of 7.0% of patients. With respect to PV-specific symptoms, the most commonly reported symptoms noted at diagnosis were tiredness (22.0%), itching (13.9%), muscle aches and/or bone pain (8.8%), and difficulty sleeping (7.8%; Table 2).
Table 2.
Abnormal Laboratory Tests and Polycythemia Vera—Related Signs and Symptoms at Time of Diagnosis in 2510 Patients
| Characteristic | Value |
|---|---|
| Abnormal Laboratory Testa | |
| Hemoglobin | 1482 (59.0) |
| Hematocrit | 1443 (57.5) |
| Platelet count | 720 (28.7) |
| White blood cell count | 499 (19.9) |
| Erythropoietin level | 505 (20.1) |
| Red blood cell count | 454 (18.1) |
| Red cell mass | 105 (4.2) |
| Serum LDH | 88 (3.5) |
| EEC growth | 8 (0.3) |
| Palpable splenomegaly | 175 (7.0) |
| Mutational Test | 1236 (49.2) |
| JAK2 V617F, n/N (%)b | 1184/1236 (95.8) |
| JAK2 other, n/N (%)b | 10/1236 (0.8) |
| Missing, n/N (%)b | 42/1236 (3.4) |
| Bone marrow test | 612 (24.4) |
| Symptoms (Documented by Physician) | |
| Tiredness | 552 (22.0) |
| Itching | 349 (13.9) |
| Muscle aches and/or bone pain | 220 (8.8) |
| Difficulty sleeping | 195 (7.8) |
Data are presented as n (%) unless otherwise indicated.
Abbreviations: EEC = endogenous erythroid colony; JAK2 = Janus kinase 2; LDH = lactate dehydrogenase.
As reported by survey respondents; definitions of “abnormal” were not available. A patient could be counted in > 1 category.
Denominator of 1236 is number of patients who had mutational analysis data from peripheral blood test or other test.
Laboratory Values
At time of enrollment, 5.3% of patients had an elevated hemoglobin level (> 18.5 g/dL in men or > 16.5 g/dL in women), 47.7% had elevated hematocrit (> 45%), 35.8% had elevated platelets (> 400 × 109/L), and 37.0% had elevated leukocytes (> 10 × 109/L). Mean hemoglobin, hematocrit, platelet, and leukocyte values at enrollment were 14.5 g/dL, 45%, 367.5 × 109/L, and 10.4 × 109/L, respectively (Table 3).
Table 3.
Polycythemia Vera—Related Laboratory Values at Enrollment in 2510 Patients
| Characteristic | Value |
|---|---|
| Hemoglobin | |
| No. evaluated | 2241 |
| Mean (SD; range) (g/dL) | 14.5 (1.9; 8.2–22.1) |
| Elevateda | 118 (5.3%) |
| Hematocrit | |
| No. evaluated | 2242 |
| Mean (SD; range) (%) | 45 (5.7; 25–70) |
| > 45% | 1070 (47.7%) |
| Platelet Count | |
| No. evaluated | 2212 |
| Mean (SD; range) (×109/L) | 367.5 (186.4; 15.0–1542.0) |
| > 400 × 109/L | 792 (35.8%) |
| Leukocyte Count | |
| No. evaluated | 2219 |
| Mean (SD; range) (×109/L) | 10.4 (7.2; 0.70–151.3) |
| > 10 × 109/L | 821 (37.0%) |
Defined as > 18.5 g/dL for men and > 16.5 g/dL for women.
PV-Directed Therapy at Time of Enrollment
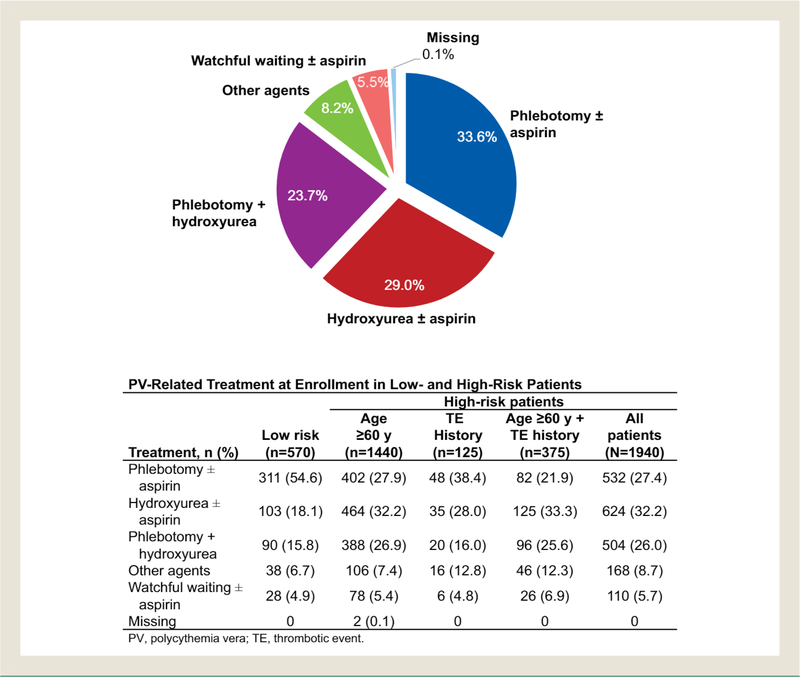
At time of enrollment, the majority (54.6%) of patients with low-risk disease were being managed with therapeutic phlebotomy. However, 18.1% and 15.8% of low-risk patients were being managed with hydroxyurea alone and phlebotomy plus hydroxyurea, respectively. Only 4.9% of patients with low-risk PV were managed with watchful waiting. In contrast, hydroxyurea alone (32.2%) and phlebotomy alone (27.4%) were the most commonly reported PV-directed therapies in patients with high-risk PV. Management with watchful waiting was reported for 5.7% of patients with high-risk disease (Figure 3). The most common hydroxyurea daily doses were 500 mg (35.4%) and 1000 mg (30.2%). The mean hydroxyurea daily doses were 769.5 and 834.9 mg for patients undergoing and not undergoing phlebotomy, respectively. Among patients who were treated with therapeutic phlebotomy, mean number of phlebotomies was 2.5 (median, 2) during the 6 months before enrollment. A small proportion of patients were treated with other agents (8.2%), including ruxolitinib (3.8%), anagrelide (2.0%), and interferon (1.4%).
Figure 3.
PV-Related Treatment at Enrollment. For “Other Agents,” Patients Could Be Counted in More Than One Category; Treatment Included Ruxolitinib (3.8%), Anagrelide (2.0%), Interferons (1.4%), and Other (1.2%). Treatment Was Unknown for 2 Patients
Comorbid Conditions
The most common comorbid conditions were hypertension (70.6%), hyperlipidemia (30.4%), obesity (17.5%), and diabetes mellitus (15.7%). Mean (SD) CCI was 3.4 (0.83). A history of skin cancer (not otherwise specified) and nonmelanoma skin cancer was documented in 3.5% and 3.3% of patients, respectively. Malignant melanoma was noted in 1.1% of patients. A history of other malignancies included prostate (2.9%), breast (1.9%), and colon (1.0%) cancers (Supplemental Table 2).
History of Thrombosis
At time of study enrollment, for patients with a known PV diagnosis date (n = 2485), 468 patients (18.8%) had a documented thrombotic event (Table 4), and 4.6% had at least 2 thrombotic events. Venous thrombotic events had occurred in 279 patients (11.2%); the most common were deep vein thrombosis (6.1 and pulmonary embolism (2.6%). Arterial thrombotic events had occurred in 218 patients (8.8%); the most common were cerebrovascular arterial thrombosis (5.1%) and acute myocardial infarction (1.8%). A total of 29 patients (1.2%) experienced both a venous and arterial thrombotic event. Before PV diagnosis, 296 patients (11.9%) experienced thrombotic events (venous, 6.7%; arterial, 5.7%). Between diagnosis and enrollment, 207 patients (8.3%) experienced a thrombotic event (venous, 5.2%; arterial, 3.5%). Of the patients who experienced thrombotic events before PV diagnosis, 35 (11.8%) had a subsequent thrombotic event after PV diagnosis. The exposure-adjusted thrombotic event rate between diagnosis and enrollment was 1.55 per 100 patient-years. Among patients who did not have a history of thrombotic events at time of diagnosis, 7.0% had a thrombotic event after diagnosis.
Table 4.
History of Thrombotic Events in 2485 Patients With Evaluable Data
| Thrombotic Event | N (%) |
|---|---|
| Any Events Before Enrollmenta | 468 (18.8) |
| Before diagnosisb | 296 (11.9) |
| Venous | 166 (6.7) |
| Arterial | 142 (5.7) |
| Between diagnosis and enrollmentb | 207 (8.3) |
| Venous | 130 (5.2) |
| Arterial | 87 (3.5) |
| More than one thrombotic event | 115 (4.6) |
| Venous Events Before Enrollment | 279 (11.2) |
| Deep-vein thrombosis | 151 (6.1) |
| Pulmonary embolism | 64 (2.6) |
| Superficial venous thrombosis | 30 (1.2) |
| Portal vein thrombosis | 20 (0.8) |
| Cerebral vein/sinus thrombosis | 17 (0.7) |
| Splenic vein thrombosis | 9 (0.4) |
| Retinal vein thrombosis | 7 (0.3) |
| Visceral/splanchnic vein thrombosis | 1 (<0.1) |
| Other | 42 (1.7) |
| Arterial Events Before Enrollment | 218 (8.8) |
| Cerebrovascular arterial thrombosis | 126 (5.1) |
| Acute myocardial infarction | 44 (1.8) |
| Peripheral arterial thrombosis | 14 (0.6) |
| Splenic infarction | 6 (0.2) |
| Retinal artery thrombosis | 4 (0.2) |
| Visceral arterial thrombosis | 1 (<0.1) |
| Other | 38 (1.5) |
Patients without known PV diagnosis date were excluded from evaluable patient population.
Thrombotic events without event dates were excluded.
Patient groups reporting events “before diagnosis” and “between diagnosis and enrollment” are not mutually exclusive.
Concomitant Medications
The 3 most common categories of concomitant medications were statins (26.3%), selective β-blocking agents (18.9%), and angiotensin-converting enzyme inhibitors (16.6%). Aspirin use was reported in 62.9% of patients.
Health Care Resource Utilization
There were 667 patients (26.6%) who reported at least one hospital, urgent care, emergency room, or outpatient visit within the 6 months before enrollment. Most of these patients (82.6%) reported having outpatient medical care visits. PV was a causal factor for 39.9% of all hospital, urgent care, emergency room, and outpatient visits.
Discussion
The REVEAL study is the first large, prospective observational study to examine the contemporary demographics, burden of disease, clinical management, PROS, and health care resource utilization among US patients with PV. Enrolled patients represent a broad, real-world segment of the PV population who are actively being managed at community or academic centers. In this analysis of clinical and disease characteristics at time of enrollment to REVEAL, patients were mostly male (54.2%) and had a mean (SD) age of 66.3 (12.3) years, with a median (range) PV disease duration of 4.0 (0–56.3) years. In the Cytoreductive Therapy in Polycythemia Vera (CYTO-PV) study, which had similar eligibility criteria, 62.2% were male, mean (SD) age at recruitment/enrollment was 65 (12) years, and mean (SD) time from diagnosis to enrollment was 4.3 (5.4) years.26 Baseline data reported from the 1638 patients included in the database collected during the observational study of the European Collaboration on Low-Dose Aspirin in Polycythemia Vera (ECLAP) project revealed that 57.5% were male, mean (SD) age at recruitment/enrollment was 60 (13) years, and mean (SD) time from diagnosis to enrollment was 5.0 (5.0) years.27 Demographic statistics from REVEAL are similar to those reported by other highly regarded studies.
Bone marrow biopsy and JAK2 mutation testing are important to differentiate between a PV diagnosis and myelofibrosis or essential thrombocythemia; confirmation of diagnosis by bone marrow biopsy may affect treatment selection and potentially outcomes. At the time of diagnosis, it was reported that < 25% of patients underwent bone marrow biopsy/mspiration, and < 50% underwent JAK2 mutation testing. Of the patients who did undergo JAK2 mutation testing, approximately 96% had a JAK2 V617F mutation, and approximately 1% had other JAK2 mutations (eg, exon 12). Because the majority of patients did not have JAK2 mutation testing to verify diagnosis, it is possible that some do not truly have PV. Importantly, JAK2 mutation testing is increasing over time. According to data reported in this study, of the patients diagnosed in the 3-year intervals of 2005–2007, 2008–2010, 2011–2013, and 2014–2016, 38%, 57%, 57%, and 61% underwent JAK2 mutation testing, respectively. However, even when taking into consideration that the JAK2 mutation was discovered in 2005 and the time needed for this mutation testing to become widely available to all practice settings, the rates of JAK2 mutation testing reported in this study seem low. Similarly, the percentage of patients who reportedly underwent bone marrow biopsy and/or erythropoietin testing seems low. These low rates may be related to the various ways in which these data are acquired, recorded, and abstracted from electronic medical records.28
Regarding the management of patients with low-risk disease, in line with National Comprehensive Cancer Network clinical practice guidelines,29 the majority (54.6%) of patients were managed with phlebotomy with or without aspirin. However, > 40% of low-risk patients received hydroxyurea or some other cytoreductive therapy with or without aspirin. Conversely, approximately one third of high-risk patients were managed without cytoreductive medications with or without aspirin. These data suggest variance from guideline-defined risk-adapted treatment of PV, which was published after many of these patients were diagnosed and enrolled.30 It is important to note that treatment with aspirin was reported in 62.9% of patients, but it is likely that, in some patients, over-the-counter use of aspirin was not reported or recorded.
With respect to thrombotic events, 11.9% of patients in REVEAL experienced thrombotic events before PV diagnosis (venous, 6.7%; arterial, 5.7%), and 8.3% experienced a thrombotic event between diagnosis and enrollment. In comparison, 14% of patients with PV had thrombotic events before diagnosis, and 19% had a thrombotic event during a 20-year follow-up in a retrospective study of 1213 patients in Italian hematology institutions conducted in 1995. 18
Conclusion
As the first prospective observational study of its size to assess US patients with PV, REVEAL is expected to provide information that will improve our understanding of the demographics, diagnosis, management, and outcomes of these patients. This analysis describes patient demographics as well as clinical and disease characteristics at the time of diagnosis and around the time of enrollment. REVEAL baseline data indicated that the JAK2 V617F mutation rate among patients who underwent molecular testing was consistent with previous reports.4,31 However, the reported percentage of patients who underwent testing was much lower than expected, which could be related to the challenges of capturing retrospective data from medical records. Many patients had poorly controlled hematocrit, platelet, and leukocyte counts at enrollment, with elevated hematocrit values observed in nearly half of all evaluated patients. Additional prospective data from REVEAL (eg, treatment patterns, PROS, and cardiovascular risk assessments) will be important for understanding how the disease progresses and how management changes with time.
Clinical Practice Points
PV is a Philadelphia chromosome—negative myeloproliferative neoplasm that can be diagnosed on the basis of absolute erythrocytosis (hemoglobin > 16.5 g/dL in men, hemoglobin > 16 g/dL in women), bone marrow biopsy, and the presence of JAK2 V617F or JAK2 exon 12 mutation. According to World Health Organization PV diagnostic criteria, bone marrow biopsy may not be required in cases of sustained absolute erythrocytosis (hemoglobin > 18.5 g/dL in men or > 16.5 g/dL in women) in the presence of one of the JAK2 mutations and a subnormal serum erythropoietin level.
In this observational study, the retrospective review and abstraction of data from electronic medical records limits definitive conclusions regarding diagnostic approach.
The primary goal of treatment is prevention of arterial and venous thrombotic events. In general, risk-adapted treatment consists of phlebotomy and aspirin in low-risk patients (< 60 years and no history of thrombosis). In high-risk patients (≥ 60 years and/or with a history of thrombosis), cytoreductive therapy is usually added to phlebotomy and aspirin. Data collected from REVEAL suggest variance from guideline-defined risk-adapted treatment of PV.
During treatment, patients with PV should be monitored every 3 to 6 months for new thrombohemorrhagic events, increased need/intolerance of phlebotomy, progressive/symptomatic splenomegaly, symptomatic thrombocytosis, progressive leukocytosis, and progressive disease-related symptoms. This close clinical surveillance is necessary to determine whether a cytoreductive therapy is indicated for low-risk patients or whether a different medication should be selected for high-risk patients.
Baseline characteristics of patients with PV enrolled in REVEAL are similar to those reported by other highly regarded studies.
Supplementary Material
Acknowledgments
Funded by Incyte Corporation. Writing assistance was provided by Tania Iqbal, PhD (Complete Healthcare Communications, LLC, an ICON plc company, North Wales, PA), whose work was funded by Incyte Corporation.
Disclosure
M.R.G. has served as a consultant and/or advisory board member for Incyte Corporation, Alexion, ARIAD, Amgen, Merck, Pfizer, Cardinal Health, Celgene, and Agios; received research funding from Amgen, Forma Therapeutics, Incyte Corporation, Janssen, and Genentech; and is a stockholder of Medtronic. B.L.S. served as a consultant and member of advisory committees for Incyte Corporation. R.V.B. received honoraria from Incyte Corporation. S.T.O. served as a consultant for Incyte Corporation and received research funding from CTI Biopharma, Gilead, Incyte Corporation, and Janssen. D.P., S.P., and P.C. are employees and stockholders of Incyte Corporation. R.M. served as a consultant for Novartis and received research funding from CTI Biopharma, Genentech, Gilead, Incyte Corporation, NS Pharma, Pfizer, and Promedior.
Footnotes
Supplemental Data
Supplemental tables accompanying this article can be found in the online version https://doi.org/10.1016/j.clml.2018.08.009.
References
- 1.Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood 2013; 122:2176–84. [DOI] [PubMed] [Google Scholar]
- 2.Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma 2014; 55:595–600. [DOI] [PubMed] [Google Scholar]
- 3.Barosi G, Birgegard G, Finazzi G, et al. Response criteria for essential thrombocythemia and polycythemia vera: result of a European LeukemiaNet consensus conference. Blood 2009; 113:4829–33. [DOI] [PubMed] [Google Scholar]
- 4.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005; 365:1054–61. [DOI] [PubMed] [Google Scholar]
- 5.Pmssamonti F, Rumi E, Pietra D, et al. A prospective study of 338 patients with polycythemia vera: the impact of JAK2(V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia 2010; 241574–9. [DOI] [PubMed] [Google Scholar]
- 6.Scott LM. The JAK2 exon 12 mutations: a comprehensive review. Am J Hematol 2011; 86:668–76. [DOI] [PubMed] [Google Scholar]
- 7.Pmssamonti F, Elena C, Schnittger S, et al. Molecular and clinical features of the myeloproliferative neoplasm associated with JAK2 exon 12 mutations. Blood 2011; 117:2813–6. [DOI] [PubMed] [Google Scholar]
- 8.Sonmez M, Saglam F, Karahan SC, et al. Treatment related changes in anti-fibrinolytic activity in patients with polycythemia vera. Hematology 2010; 15:391–6. [DOI] [PubMed] [Google Scholar]
- 9.Hultcrantz M, Kristinsson SY, Andersson TM, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol 2012; 30:2995–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med 2004; 117:755–61. [DOI] [PubMed] [Google Scholar]
- 11.Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol 2005; 23:2224–32. [DOI] [PubMed] [Google Scholar]
- 12.Finazzi G, Caruso V, Marchioli R, et al. Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood 2005; 105:2664–70. [DOI] [PubMed] [Google Scholar]
- 13.Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative Neoplasm (MPN) Symptom Assessment Form Total Symptom Score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol 2012; 30:4098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson P, Mesa R, Scherber R, et al. Association between quality of life and clinical parameters in patients with myeloproliferative neoplasms. Leuk Lymphoma 2012; 53:441–4. [DOI] [PubMed] [Google Scholar]
- 15.Mesa R, Miller CB, Thyne M, et al. Myeloproliferative neoplmsm.s (MPNs) have a significant impact on patients’ overall health and productivity: the MPN Landmark survey. BMC Cancer 2016; 16:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood 2011; 118:401–8. [DOI] [PubMed] [Google Scholar]
- 17.Abelsson J, Andrea.sson B, Samuelsson J, et al. Patients with polycythemia vera have the worst impairment of quality of life among patients with newly diagnosed myeloproliferative neoplasms. Leuk Lymphoma 2013; 54:2226–30 [DOI] [PubMed] [Google Scholar]
- 18.Gruppo Italiano Studio Policitemia. Polycythemia versa: the natural history Of 1213 patients followed for 20 years. Ann Intern Med 1995; 123:656–64. [DOI] [PubMed] [Google Scholar]
- 19.Hultcrantz M, Wilkes SR, Kristinsson SY, et al. Risk and cause Of death in patients diagnosed with myeloproliferative neoplasms in Sweden between 1973 and 2005: a population-bmsed study. J Clin Oncol 2015; 33:2288–95 [DOI] [PubMed] [Google Scholar]
- 20.Kaifie A, Kirschner M, Wolf D, et al. Bleeding, thrombosis, and anticoagulauon In myeloproliferative neoplasms (MPN): analysis from the German SAL-MPN-registry. J Hematol Oncol 2016; 9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia 2013; 27: 1874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stein BL, Williams DM, Wang NY, et al. Sex differences in the JAK2 V617F allele burden in chronic myeloproliferative disorders. Haematologica 2010; 95: 1090–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein BL, Saraf S, Sobol U, et al. Age-related differences in disease characteristics and clinical outcomes in polycythemia vera. Leuk Lymphoma 2013; 54: 1989–95. [DOI] [PubMed] [Google Scholar]
- 24.Mesa R, Boccia RV, Grunwald MR, et al. Patient-reported outcomes data from REVEAL at the time of enrollment (baseline): a prospective observational study of patients with polycythemia vera in the United States. Clin Lymphoma Myeloma Leuk 2018; 18590–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 26.Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular ewents and intensity of treatment in polycythemia vera. N Engl J Med 2013; 368:22–33. [DOI] [PubMed] [Google Scholar]
- 27.Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood 2007; 109:2446–52. [DOI] [PubMed] [Google Scholar]
- 28.Saczynski JS, McManus DD. Goldberg RJ. Commonly used data-collection approaches in clinical research. Am J Med 2013; 126:946–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: myeloproliferative neoplasms, version 2.2018, Available at: https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed: September 6, 2018.
- 30.Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol 2011; 29:761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lippert E, Boissinot M, Kralovics R, et al. The JAK2-V617F mutation is frequently present at diagnosis in patients with essential thrombocythemia and polycythemia vera. Blood 2006; 108:1865–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.