Abstract
Approximately half of the patients with polycythemia vera experience substantial symptom burdens. We analyzed data from the ongoing Prospective Observational Study of Patients with Polycythemia Vera in US Clinical Practices (REVEAL) study to evaluate the relationship between blood count control and symptoms. The severity of individual symptoms, except for pruritus and night sweats, was not affected by blood count control. Consequently, regular monitoring of symptom burden should be factored when assessing disease control.
Background:
Approximately 50% of patients with polycythemia vera (PV) have PV-related symptoms at diagnosis; these symptoms might develop or worsen with time. Symptoms have been shown to negatively affect quality of life and interfere with daily activities. To our knowledge, an analysis to evaluate the relationship between blood count control and symptoms has not been published.
Patients and Methods:
The Prospective Observational Study of Patients with Polycythemia Vera in US Clinical Practices (REVEAL; NCT02252159) is a multicenter, noninterventional, nonrandomized prospective observational study of patients with PV in the United States. Patients included were required to have a complete blood count result within 30 days before completing the at-enrollment Myeloproliferative Neoplasm Self-Assessment Form Total Symptom Score (MPN-SAF TSS). Symptom severity was compared between those who had blood count control versus those who did not.
Results:
At the time of enrollment, 1714 patients (94.5%) were being managed with cytoreductive therapy; 468 patients (25.8%) had complete hematologic remission (CHR), 1614 patients (89.0%) had ≥1 controlled blood count, and 1122 patients (61.9%) had ≥2 controlled blood counts. Mean MPN-SAF TSSs were similar across patients in different blood count control groups. Fatigue was the most frequently reported symptom. The severity of individual symptoms, except those of pruritus and night sweats, was not affected by CHR or the number of blood counts that were controlled.
Conclusion:
Symptom burden in patients with PV can persist despite control of blood counts, which suggests some discordance between laboratory values and symptom burden. Consequently, regular monitoring of symptom burden should be factored into the assessment of disease control.
Keywords: Blood count control, MPN-SAF TSS, Polycythemia vera, REVEAL, Symptom burden
Introduction
Polycythemia vera (PV) is a myeloproliferative neoplasm (MPN) characterized by erythrocytosis, thrombocytosis, leukocytosis, and splenomegaly.1 There are approximately 148,000 patients with PV in the United States1; the prevalence of PV is 44 to 57 cases per 100,000 individuals,2 and the incidence of PV is 1 to 3 per 100,000 individuals3. Patients with PV are at increased risk of medical complications, notably thrombosis, leukemic transformation, and substantial symptom burden.4–8 Ten years after diagnosis, the risks of transformation to post-PV myelofibrosis (MF) and acute myeloid leukemia (AML) are 10% and 5% to 21%, respectively.4,9
Most patients with PV have a mutation in the Janus kinase 2 (JAK2) gene. Most patients (95%) carry the JAK2 V617F mutation, whereas 3% harbor a JAK2 exon 12 mutation.10–12 The JAK2 V617F mutation results in constitutive activation of hematopoietic signal transduction and exaggerated hematopoiesis, leading to PV-associated symptoms, myeloproliferation, and excessive inflammation.13–15
Approximately 50% of patients present with PV-related symptoms, including fatigue, headache, visual disturbances, and pruritus, at the time of diagnosis.5,11,16–20 A subset of patients with PV are asymptomatic at the time of diagnosis and might be diagnosed because of incidental findings from laboratory blood tests.21–23 Over time, as PV progresses, patients often experience worsening of symptoms and can also experience new symptoms, such as fatigue, headache, early satiety, and inactivity.1,8,11,23,24
The symptom burden associated with PV might negatively affect patient health-related quality of life by interfering with daily activities, family and social life, and work productivity.8,25 The 10-item Myeloproliferative Neoplasm Self-Assessment Form Total Symptom Score (MPN-SAF TSS) is a valid and clinically relevant tool for assessing MPN symptom burden, including for patients with PV.5 In the first study of the MPN-SAF TSS, of the 10 symptoms assessed, 8 were reported by more than 50% of patients with PV; this analysis did not include longitudinal evaluation of symptoms.
The durable resolution of PV-related symptoms and peripheral blood count reduction are established primary clinical objectives for the optimal management of PV as outlined by the European Leukemia Net and National Comprehensive Cancer Network (NCCN).1,26–29 The NCCN MPN guidelines recommend symptom monitoring with the MPN-SAF TSS during the treatment course of patients with MPNs, including PV. To our knowledge, to date, no analysis of the relationship between blood count control and symptom control has been performed.
The ongoing Prospective Observational Study of Patients with Polycythemia Vera in US Clinical Practices (REVEAL) study is an observational study being conducted to describe burden of disease, clinical management, patient-reported outcomes, and health care resource use among patients with PV during an approximate 60-month observation period in the United States. This analysis examines the effect of blood count control on symptom burden measured using the MPN-SAF TSS in these patients.
Patients and Methods
REVEAL (ClinicalTrials.gov, NCT02252159) is an ongoing multicenter, noninterventional, nonrandomized, prospective, observational study of patients with PV in the US community and academic study sites. The data cutoff for the current analyses was May 18,2017.
Patients
Patients had to be ≥ 18 years old, have a clinical diagnosis of PV, be under the care of a physician, willing and able to provide informed consent, and able to complete patient assessments and questionnaires, either alone or with minimal assistance. Patients with a life expectancy of <6 months, a diagnosis of primary MF, post-PV MF, post essential thrombocythemia MF, secondary AML, or myelodysplastic syndrome were excluded. The study protocol was approved by each investigative site’s institutional review board, and written informed consent was obtained from each participant.
Study Design
Patients were enrolled in the study during the course of usual care at each investigative site; patients could be enrolled at any point in their PV disease course. Management of PV was per the discretion of the treating physician; all decisions regarding patient care were made by the treating physician. Patients completed the MPN-SAF TSS survey at the time of enrollment and at approximately 3-month intervals thereafter. Patients included in the current analysis were required to have a complete blood count result within 30 days before completing the MPN-SAF TSS at enrollment. Only at-enrollment MPN-SAF TSS survey results and near-enrollment blood counts were included in the current analysis.
Data Collection
Symptom severity was quantified using the MPN-SAF TSS. MPN-SAF TSS items included worst fatigue, early satiety, abdominal discomfort, inactivity, problems with concentration, night sweats, itching (pruritus), bone pain, fever, and weight loss. Items were scored on a linear analogue self-assessment scale ranging from 0 (absent) to 10 (worst imaginable).5 Individual symptom scores >7 were considered “severe,” scores of 4 to 6 were considered “moderate,” scores of 1 to 3 were considered “mild,” and a score of 0 was considered “none.”5 For each patient, the 10 individual symptom scores were added together to calculate a total symptom score (TSS); the maximum score was 100, and the minimum score was 0. Complete hematologic response (CHR) was defined as patients who satisfied all 3 blood count criteria, per the International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) Consensus Project, including hematocrit (HCT) <45%, white blood cell count (WBC) <10 × 109/L, and platelet count (PLT) ≤400 × 109/L; these same criteria were used to determine if HCT, WBC, and PLT were controlled.26,30
Statistical Analysis
Patient demographic and clinical characteristics were summarized using descriptive statistics. The proportions of patients in each symptom severity group were summarized for patients with and without different blood count control. Significance of the association between MPN-SAF TSS and blood count control was assessed using a regression model adjusting for age, sex, risk category, and disease duration. HCT, WBC, and PLT were the individual laboratory results included in the assessments of blood count control. The degree of blood count control (0, ≥1, ≥2, or 3 counts controlled) was also evaluated by determining the number of cell lines (HCT, WBC, PLT) that met the levels specified in the IWG-MRT criteria. A sensitivity analysis was conducted to assess the association between the absolute number of blood counts controlled (0, 1, 2, or 3) and TSS. The interaction between sex and blood count control was used to assess the sex-associated differences in MPN-SAF TSS among blood count control groups, using a regression model controlling for age, risk category, and disease duration. P values were not corrected for multiple testing.
Results
A total of 2510 patients were enrolled over an approximate 2-year period (July 2014 to August 2016), with 2307 patients having completed the MPN-SAF TSS at enrollment (Table 1). Of these, 1813 patients (72.2%) had a complete blood count within 30 days before completion of the at-enrollment MPN-SAF TSS and were evaluable. Among evaluable patients, the median age was 67 years (range, 22-95 years), 985 (54.3%) were male, and 1642 (90.6%) were white. The median age at PV diagnosis was 61 years, and the median time from PV diagnosis to enrollment was 4.1 years (range, 0.0-39.2 years). A large proportion (57.0%) of patients had a PV duration of <5 years, and approximately one-fifth (18.8%) of patients had a PV duration of ≥10 years.
Table 1.
Patient Demographic Characteristics, Disease Management, and Blood Count Control at Time of Enrollment
| Variable | Evaluable Patients (n = 1813) |
|---|---|
| Median Age (Range), Y | 67 (22-95) |
| Sex, n (%) | |
| Male | 985 (54.3) |
| Female | 828 (45.7) |
| Race, n (%) | |
| White | 1642 (90.6) |
| African American | 93 (5.1) |
| Asian | 21 (1.2) |
| Other | 57 (3.1) |
| Median Time From PV Diagnosis to Enrollment (Range), Y | 4.1 (0.0-39.2) |
| Median Age at PV Diagnosis (Range), Y | 61 (16-93) |
| Male | 60 (18-89) |
| Female | 63 (16-93) |
| Duration of PV at Enrollment, % | |
| <1 | 21.6 |
| 1 to <5 | 35.4 |
| 5 to <10 | 23.5 |
| 10 to <15 | 11.2 |
| ≥15 | 7.6 |
| Missing | 0.7 |
| PV Risk Category, n (%)a | |
| High | 1404 (77.4) |
| Low | 409 (22.6) |
| Disease Management, n (%)b | |
| Watchful waiting only | 99 (5.5) |
| PBT only | 640 (35.3) |
| HU only | 447 (24.7) |
| PBT and HU | 494 (27.2) |
| All otherc | 133 (7.3) |
| Anagrelide | 37 (2.0) |
| Interferons | 15 (0.8) |
| Ruxolitinib | 69 (3.8) |
| Other | 15 (0.8) |
| Blood Count Control, n (%) | |
| HCT <45% | 934 (51.5) |
| WBC <10 × 109/L | 1118 (61.7) |
| PLT ≤400 × 109/L | 1152 (63.5) |
| HCT <45% and WBC <10 × 109/L | 627 (34.6) |
| ≥1 Controlled valued | 1614 (89.0) |
| ≥2 Controlled valuesd | 1122 (61.9) |
| CHRd | 468 (25.8) |
Abbreviations: CHR = complete hematologic remission; HCT = hematocrit; HU = hydroxyurea; PBT = phlebotomy; PLT = platelet count; PV = polycythemia vera; WBC = white blood cell count.
High risk is age ≥60 years and/or history of thromboembolism; low risk is age <60 years and no history of thromboembolism.
With or without aspirin use throughout.
Subcategories are not mutually exclusive.
Groups on the basis of degree of blood count control are not mutually exclusive.
At the time of enrollment, most patients (n = 1714; 94.5%) were being managed with cytoreductive therapy; 1581 patients (87.2%) were managed with phlebotomy, hydroxyurea, or a combination thereof (Table 1). Patients may have used over-the-counter aspirin as part of treatment regimens12; however, because of the method of data collection for this study, its use might not have always been captured.
Approximately one-quarter of evaluable patients (n = 468; 25.8%) had CHR at the time of enrollment; 1614 patients (89.0%) had at least 1 controlled blood count, and 1122 patients (61.9%) had at least 2 controlled blood counts (Table 1).
Mean TSS and Blood Count Control
Mean TSS was calculated and compared in patients grouped according to number of controlled blood counts (uncontrolled counts, ≥1 controlled count, ≥2 controlled counts, and CHR). Mean scores were similar among the blood count control groups and differed by ≤1.5 (Figure 1A). Mean scores were 20.2, 18.7, 18.7, and 19.1 in patients with uncontrolled counts, ≥1 count controlled, ≥2 counts controlled, and CHR, respectively (P > .05 for each control group compared with the uncontrolled group). The sensitivity analysis did not show a significant association between TSS and the exact number of blood counts controlled (ie, 1, 2, 3, or no counts controlled; P = .64). Mean scores were also similar among patients regardless of which specific blood counts were controlled, except for WBC (Figure 1B). Scores for patients with WBC control were 10% (20.2 vs. 18.0) lower than scores for patients without WBC control (P = .0036); mean controlled and uncontrolled WBC counts were 6.83 × 109/L and 16.33 × 109/L, respectively. Female patients reported a higher mean TSS than male patients (21.5 vs. 16.6); however, there were no sex-associated differences in mean scores for controlled versus uncontrolled blood counts.
Figure 1. Mean Total Symptom Score (TSS) Among Blood Count Control Groups. (A) Mean TSS Is Listed for Patients According to Blood Count Control Status. (B) Mean TSS Is Listed for Patients According to Blood Count, Noting With or Without Control of the Indicated Blood Count Category. Groups on the Basis of Degree of Blood Count Control (ie, ≥1, ≥2, CHR) Are Not Mutually Exclusive.
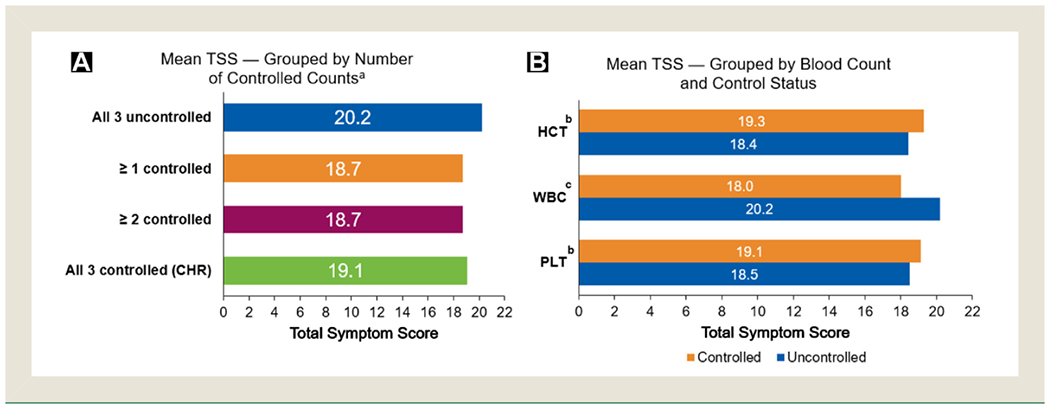
Abbreviations: CHR = complete hematologic remission; HCT = hematocrit; PLT = platelet count; WBC = white blood cell count.
a Groups “all 3 controlled,” “≥ 1 controlled,” and “≥ 2 controlled” are not statistically different from the “all 3 uncontrolled (CHR)” group; P > .05. b Not statistically different between controlled versus uncontrolled groups; P > .05.c Statistically significant; P = .0036.
Symptoms and Blood Count Control
The 5 most common patient-reported symptoms were fatigue, early satiety, inactivity, pruritus, and problems with concentration (Figure 2). Fatigue was the most frequently reported symptom, with approximately 80% of patients in each blood count control group experiencing at least mild severity. Additional symptoms experienced by patients at lower frequencies, other than those already mentioned, included night sweats, abdominal discomfort, bone pain, unintentional weight loss, and fever (see Supplemental Figure 1 in the online version). The severity of individual symptoms, except that of pruritus and night sweats, was not affected by CHR or an increasing number of controlled blood counts. A similar proportion of patients who achieved CHR, ≥2 controlled counts, or ≥1 controlled count reported severe pruritus (11.0%, 11.3%, and 13.1%, respectively), whereas 17.7% of patients with no controlled counts reported severe pruritus. Furthermore, a similar proportion of patients who achieved CHR, ≥2, or ≥1 controlled count reported severe night sweats (11.6%, 9.8%, 9.8%, respectively), whereas 15.1% of patients with no controlled counts reported severe night sweats.
Figure 2. Symptom Severity According to Degree of Blood Count Control. Symptom Severity (Severe, Moderate, Mild, None) Percentages for (A) Fatigue, (B) Early Satiety, (C) Inactivity, (D) Itching (Pruritus), and (E) Problems With Concentration Are Listed for Patients According to Blood Count Control Status. Groups on the Basis of Degree of Blood Count Control (ie, ≥1, ≥2, CHR) Are Not Mutually Exclusive.
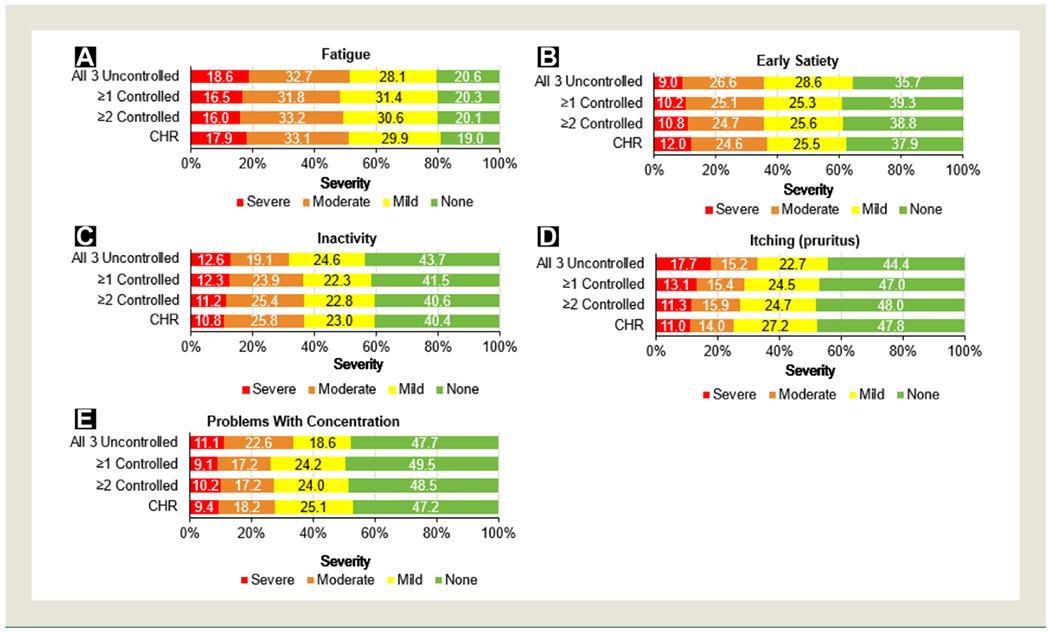
Abbreviation: CHR = complete hematologic remission.
Discussion
In this analysis, mean TSSs were similar in patients with varying degrees of blood count control, with scores differing by ≤ 1.5 among categories. Mean TSSs were also similar regardless of which specific blood counts were controlled, with the exception of WBC. Although female patients had higher mean TSSs, in male and female patients, mean TSS did not vary with controlled or uncontrolled blood counts. Among the 10 items included on the MPN-SAF TSS, the most frequently reported symptom was fatigue, which is consistent with results of previous studies.5,8,31 The next most frequent symptoms were early satiety, inactivity, pruritus, and problems with concentration. Severity was similar among blood count control groups for all symptoms, with the exception of pruritus and night sweats.
The pivotal Cytoreductive Therapy in Polycythemia Vera (CYTO-PV) study showed that patients with a target HCT <45% had significantly lower risks of cardiovascular death and major thrombosis.6 In a post hoc subanalysis of CYTO-PV, the risk of thrombosis was elevated with increasing WBC counts, reaching statistical significance for WBC >11 × 109/L.32 These results lend support to the importance of controlling HCT and WBC counts, which have been central in the management of patients with PV.
The data presented in this analysis suggest that patients might experience symptom burden irrespective of the degree of blood count control. Mean TSSs were similar across all blood count control groups. With the exception of pruritus and night sweats, patients reported similar symptom burden regardless of blood count control. These data also highlight that symptom burden might not be fully addressed by controlling blood counts. It is notable that a higher percentage of patients reported severe night sweats and pruritus in the context of uncontrolled blood counts (0 blood counts controlled). In MPNs, there is recognition that the Janus kinase cascade plays a central role, not only in myeloproliferation, but also in the signaling of inflammatory cytokines.33 Furthermore, emerging data demonstrate clear relationships between specific MPN symptoms and specific cytokines. Additional research might well be warranted to determine whether severe night sweats or pruritus, in the context of uncontrolled blood counts, have unique cytokine signatures. With respect to limitations, this analysis was designed to assess the association between symptom control and blood count control at a single time point, a median of 4.1 years into the course of the disease. In this population, the TSS scoring scheme (0-100) has a rather narrow dynamic range with 75% of subjects having scores <3234; most of the scores of the patients presented herein (18-20) reflected an “intermediate” symptom burden. Also, the correlations between mean TSS and blood count control might have been affected by patients who had overlapping symptoms from comorbidities.
Conclusion
This analysis suggests that achieving blood count control does not necessarily result in symptom control, with the notable exception that patients with uncontrolled WBC counts experienced higher symptom burden; further analyses might be required to confirm this association. Consequently, physicians should regularly monitor symptom burden in their patients with PV and not rely exclusively on blood counts as a measurement of disease control.
Supplementary Material
Clinical Practice Points.
Approximately half of patients with PV experience substantial symptom burden.
We analyzed data from the ongoing REVEAL study to evaluate the relationship between blood count control and symptoms.
The severity of individual symptoms, except for pruritus and night sweats, was not affected by blood count control.
Consequently, regular monitoring of symptom burden should be factored into disease control assessment.
Acknowledgments
The authors thank the patients and their families, the investigators, and the site personnel who participated in this study. This study was sponsored by Incyte Corporation (Wilmington, DE). Medical writing assistance was provided by Michael R. Convente, PhD, of Scientific Pathways, Inc (Hamilton, NJ), and funded by Incyte Corporation.
Disclosure
Michael R. Grunwald has provided consultancy to Incyte, Abbvie, Amgen, Cardinal Health, Celgene, Pfizer, Agios, Merck, and Daiichi Sankyo, and has received research funding from Incyte, Janssen, Forma Therapeutics, and Genentech/Roche. John M. Burke has provided consultancy to Incyte, Celgene, Bayor, Genentech, and Gilead. David J. Kuter has provided consultancy to Syntimmune, Rigel, Protalex, BMS, Incyte, 3SBIO, Pfizer, Zafgen, Fujifilm, ONO, Merck, Genzyme, Argenx, Shire, Alexion, Amgen, Novartis, and Dova, and has received research funding from Syntimmune, Rigel, Protalex, BMS, Incyte, Shire, and Alexion, and royalties from Up-To-Date. Dr Kuter has also served on an advisory committee for Dova. Aaron T. Gerds has provided consultancy to Incyte, CTI Biopharma, Apexx Oncology, and Celgene. Brady Stein has provided consultancy to Incyte and Apexx Oncology. Mark A. Walshauser has no conflicts of interest to disclose. Shreekant Parasuraman, Philomena Colucci, and Dilan Paranagama are employees and shareholders of Incyte Corporation. Michael R. Savona receives licensing fees from Boehringer-Ingelheim, has served as advisory for Astex, Celgene, Incyte, Karyopharm, and TG Therapeutics, had received research funding from Astex, Incyte Corporation, Sunesis, Takeda, and TG Therapeutics, and is a shareholder of Karyopharm. Ruben Mesa has provided consultancy to Novartis and received research funding from Incyte, CTI, Genentech, and Celgene.
Footnotes
Supplemental Data
Supplemental figure accompanying this article can be found in the online version at https://doi.org/10.10l6/j.clml.2019.06.001
References
- 1.Stein BL, Oh ST, Berenzon D, et al. Polycythemia vera: an appraisal of the biology and management 10 years after the discovery of JAK2 V617F. J Clin Oncol 2015; 33:3953–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma 2014; 55:595–600. [DOI] [PubMed] [Google Scholar]
- 3.Johansson P Epidemiology of the myeloproliferative disorders polycythemia vera and essential thrombocythemia. Semin Thromb Hemost 2006; 32:171–3. [DOI] [PubMed] [Google Scholar]
- 4.Tefferi A Essential thrombocythemia, polycythemia vera, and myelofibrosis: current management and the prospect of targeted therapy. Am J Hematol 2008; 83: 491–7. [DOI] [PubMed] [Google Scholar]
- 5.Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol 2012; 30:4098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med 2013; 368:22–33. [DOI] [PubMed] [Google Scholar]
- 7.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127:2391–405. [DOI] [PubMed] [Google Scholar]
- 8.Mesa R, Miller CB, Thyne M, et al. Myeloproliferative neoplasms (MPNs) have a significant impact on patients’ overall health and productivity: the MPN Landmark survey. BMC Cancer 2016; 16:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finazzi G, Caruso V, Marchioli R, et al. Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood 2005; 105:2664–70. [DOI] [PubMed] [Google Scholar]
- 10.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005; 365:1054–61. [DOI] [PubMed] [Google Scholar]
- 11.Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia 2013; 27: 1874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol 2017; 92: 94–108. [DOI] [PubMed] [Google Scholar]
- 13.Schafer AI. Molecular basis of the diagnosis and treatment of polycythemia vera and essential thrombocythemia. Blood 2006; 107:4214–22. [DOI] [PubMed] [Google Scholar]
- 14.Spivak JL. Narrative review: thrombocytosis, polycythemia vera, and JAK2 mutations: the phenotypic mimicry of chronic myeloproliferation. Ann Intern Med 2010; 152:300–6. [DOI] [PubMed] [Google Scholar]
- 15.Pourcelot E, Trocme C, Mondet J, Bailly S, Toussaint B, Mossuz P. Cytokine profiles in polycythemia vera and essential thrombocythemia patients: clinical implications. Exp Hematol 2014; 42:360–8. [DOI] [PubMed] [Google Scholar]
- 16.Ahn BY, Choi KD, Choi YJ, Jea SY, Lee JE. Isolated monocular visual loss as an initial manifestation of polycythemia vera. J Neurol Sci 2007; 258:151–3. [DOI] [PubMed] [Google Scholar]
- 17.Gangat N, Strand JJ, Lasho TL, Li CY, Pardanani A, Tefferi A. Pruritus in polycythemia vera is associated with a lower risk of arterial thrombosis. Am J Hematol 2008; 83:451–3. [DOI] [PubMed] [Google Scholar]
- 18.Parija S, Mohapatra MM, Pattnaik BK. Polycythemia vera presenting with bilateral papilledema: a rare case report. Indian J Ophthalmol 2008; 56:327–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rai R, Pieters T. An unusual psychiatric presentation of polycythaemia ‘Difficulties lie in our habits of thought rather than in the nature of things’ Andre Tardieu. BMJ Case Rep 2013; 2013, bcr2012008215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang HS, Joe SG, Kim JG, Park SH, Ko HS. Delayed choroidal and retinal blood flow in polycythaemia vera patients with transient ocular blindness: a preliminary study with fluorescein angiography. Br J Haematol 2013; 161: 745–7. [DOI] [PubMed] [Google Scholar]
- 21.Passamonti F, Brusamolino E, Lazzarino M, et al. Efficacy of pipobroman in the treatment of polycythemia vera: long-term results in 163 patients. Haematologica 2000; 85:1011–8. [PubMed] [Google Scholar]
- 22.Spivak JL. Polycythemia vera: myths, mechanisms, and management. Blood 2002; 100:4272–90. [DOI] [PubMed] [Google Scholar]
- 23.Raedler LA. Diagnosis and management of polycythemia vera: proceedings from a multidisciplinary roundtable. Am Health Drug Benefits 2014; 7(7 suppl 3): S36–47. [PMC free article] [PubMed] [Google Scholar]
- 24.Reiter A, Harrison C. How we identify and manage patients with inadequately controlled polycythemia vera. Curr Hematol Malig Rep 2016; 11:356–67. [DOI] [PubMed] [Google Scholar]
- 25.Harrison CN, Koschmieder S, Foltz L, et al. The impact of myeloproliferative neoplasms (MPNs) on patient quality of life and productivity: results from the international MPN Landmark survey. Ann Hematol 2017; 96:1653–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood 2013; 121:4778–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NCCN Clinical Practice Guidelines in Oncology. Myeloproliferative Neoplasms. V2.2019, Available at: https://www.nccn.org/professionals/physician_gLs/pdf/mpn.pdf. Accessed: March 13, 2019.
- 28.Patel AB, Vellore NA, Deininger MW. New strategies in myeloproliferative neoplasms: the evolving genetic and therapeutic landscape. Clin Cancer Res 2016; 22: 1037–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbui T, Tefferi A, Vannucchi AM, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European Leukemia Net. Leukemia 2018; 32:1057–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barosi G, Birgegard G, Finazzi G, et al. Response criteria for essential thrombocythemia and polycythemia vera: result of a European Leukemia Net consensus conference. Blood 2009; 113:4829–33. [DOI] [PubMed] [Google Scholar]
- 31.Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood 2011; 118:401–8. [DOI] [PubMed] [Google Scholar]
- 32.Barbui T, Masciulli A, Marfisi MR, et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood 2015; 126:560–1. [DOI] [PubMed] [Google Scholar]
- 33.Geyer H, Scherber R, Kosiorek H, et al. Impact of inflammation on myeloproliferative neoplasm symptom development. Mediators Inflamm 2015; 2015: 284706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geyer H, Scherber R, Kosiorek H, et al. Symptomatic profiles of patients with polycythemia vera: implications of inadequately controlled disease. J Clin Oncol 2016; 34:151–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


