Abstract
Summary: We describe six cases of cannalicular optic nerve meningioma in which the diagnosis was missed for more than 1 year after the onset of symptoms. Clinical features led to a misdiagnosis of optic neuritis in all cases. Although atypical clinical progression led to further imaging studies, they did not provide the diagnosis because of inappropriate imaging protocols. Diagnosis was eventually made on the basis of high-spatial-resolution contrast-enhanced MR findings. Radiologists should have a high suspicion for the diagnosis of optic canal meningioma in patients with unexplained visual loss, particularly when visual loss is progressive. Investigation in these cases should include high-spatial-resolution MR imaging of the orbit before and after contrast medium administration, and fat suppression should be used in combination with contrast enhancement whenever possible.
Meningiomas are the most common intracranial tumor and involve the orbit in 0.4–1.3% of cases (1). Involvement of the optic pathways producing visual loss is not uncommon (1–3). The optic pathways can be affected within the orbit by meningiomas arising from the optic nerve sheath or within the skull by meningiomas arising from or involving the sellar and parasellar regions (2, 4). Of particular significance are meningiomas arising from and growing within the optic nerve sheath, because these tumors can cause significant optic nerve compression while small. This is particularly true when the tumor arises within the optic canal where expansion is limited by the bony canal walls, allowing very small tumors to cause significant compression of the optic nerve (1, 3). Intracanalicular spread of intracranial meningiomas can also occur, and even thin layers of en-plaque tumor extension may cause significant visual symptoms under these circumstances (1–3).
There are two specific problems with the diagnosis of optic canal meningiomas, one clinical and one radiologic. Clinically, these tumors commonly occur in young adult women, presenting with unilateral visual loss and closely mimicking the clinical presentation of optic neuritis (2, 5, 6). Radiologically, these tumors may be extremely small despite causing significant symptoms and can easily be overlooked on routine imaging protocols (2).
These case reports present a review of six patients with intracannalicular optic nerve meningioma in whom diagnosis was delayed despite extensive clinical and radiologic examination. The aims of these case reports are to raise awareness of the features of optic canal meningioma and to highlight the steps necessary for successful diagnosis.
Case Reports and Results
This study is part of a retrospective review of orbital imaging performed over the 6-year period 1996–2002. During that time, intracannalicular optic nerve meningioma was diagnosed in 18 patients. The six patients presented are the subset of those 18 in whom diagnosis was delayed by more than 1 year from initial symptoms. The diagnosis was made in all cases following referral to the Manchester Royal Infirmary and Royal Eye Hospital (a large tertiary referral center for orbital imaging) for further investigation (five cases) or review (one case). Initial investigations, presented here, were performed at a variety of other institutions.
The clinical history was extremely similar in each case. The patients were all female (age range, 24–38 years [Table]). Four patients presented with sudden visual loss in one eye, which in one case was painful. The other two patients described gradual visual deterioration with short-lived, repeated episodes of visual clouding over a period of several weeks before a more dramatic and sudden deterioration in vision on one side. On examination, all were found to have visual impairment in the affected eye. There was peripheral field restriction in five of six patients, and none demonstrated scotomal loss. Pallor of the optic disks was noted on fundoscopy in two cases, but there were no other physical symptoms or signs. Medical histories were unremarkable.
Clinical Data for Patients with Cannalicular Optic Nerve Meningloma
| Patient | Age at Presentation (years) | Time to Diagnosis (months) | Surgical Outcome | Outcome |
|---|---|---|---|---|
| 1 | 24 | 49 | No surgery | Blind |
| 2 | 28 | 22 | CR | Vision preserved |
| 3 | 28 | 32 | PR | Complete loss of vision in right eye |
| 4 | 33 | 21 | PR | Light perception in left eye only |
| 5 | 36 | 12 | CR | Vision preserved |
| 6 | 38 | 14 | CR | Vision preserved |
Note.—CR, complete resection; PR, partial resection.
Imaging was performed at presentation in three cases (CT in one and MR in two). In each case, the imaging examination consisted of a routine brain scan and no specific orbital images were performed. In case 1, “extensive high signal intensity abnormalities” were described “throughout the white matter” “in keeping with multiple sclerosis.” The imaging investigations in the other two cases were reported as normal.
In each case, a presumptive diagnosis of optic neuritis was made and the patients were treated with high-dose intravenous steroids. Examination of CSF for oligoclonal bands was performed and was negative in all cases. Five patients showed some improvement in visual acuity in response to steroids; the remaining case remained stable with mild visual impairment.
In all cases, progressive visual deterioration continued following the initial steroid treatment. In two cases, the deterioration appeared to be episodic, and in the other four cases to be gradually progressive. Two cases (1 and 4) also developed visual impairment in the normal eye. Five patients received further courses of high-dose intravenous steroids (one to three courses) for subsequent episodes of visual deterioration.
In all cases, imaging investigations were performed to investigate the progressive visual loss. All patients had MR (between two and six investigations), and one patient had an orbital CT (case 2). These investigations were considered normal in patients 2 and 5. The presence of optic nerve sheath enlargement was noted in patients 3 and 6. In patient 4, the presence of a small cribriform plate meningioma was noted but was felt to be incidental in nature. In patient 1, the white matter abnormalities seen at diagnosis were not present on follow-up images and the original films were not available for review. Subsequent MR demonstrated a small (5-mm) enhancing nodule on the cribriform plate and abnormal enhancement around the anterior clinoid processes. The cribriform nodule was diagnosed as an incidental cribriform plate meningioma. The chiasmal enhancement was thought to be inflammatory, and a diagnosis of probable sarcoidosis was made. The patient continued on steroid therapy, but visual deterioration continued and she subsequently developed a third nerve palsy. Meningeal biopsy was performed but was normal; however, in the face of progressive multiple cranial nerve palsies, cytotoxic treatment with a combination of steroids and cyclophosphamide was commenced for suspected sarcoidosis. This led to an improvement in the third nerve palsy, although visual deterioration continued and the patient became bilaterally blind.
A diagnosis of optic nerve meningioma was eventually made in all six cases and was histologically confirmed in five patients who underwent surgical treatment. In case 1, histologic confirmation was not available because surgery was not considered worthwhile in the face of complete bilateral visual loss. The imaging appearances in cases 2 and 3 are illustrated in Figures 1 and 2. In all cases, final diagnosis was made on the basis of MR findings. The MR and CT examinations performed before diagnosis were reviewed retrospectively by two experienced neuroradiologists with an interest in orbital imaging (A.J., R.D.L.). Films were available from 13 of the 16 previous investigations. Abnormalities were seen in cases 1–4 and 6. The findings in cases 1, 3, 4, and 6 agreed with the reports described above. Review of the CT scan of the orbits in patient 2 demonstrated a small subtle mass in the apex of the left orbit that had not been noted on the original report (Fig 1).
Fig 1.
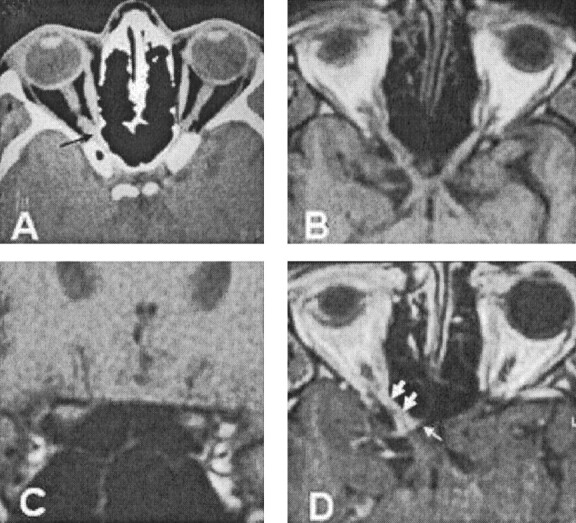
Case 2.
A, CT image from case 2, showing a small soft tissue mass at the apex of the right orbit (arrow). This image is a prediagnostic scan obtained before the patient’s referral to our center. The nerve enlargement is subtle and is difficult to appreciate because of the relatively thick sections. This abnormality was identified only on retrospective review.
B, T1-weighted MR image, reconstructed along the optic pathways, showing slight widening of the soft tissue of the optic nerve–optic nerve sheath complex at the orbital apex. The abnormality is still subtle and could easily be overlooked.
C, Coronal T1-weighted MR image also showing subtle increase in the size of the soft tissue signal intensity within the right optic canal.
D, Postcontrast T1-weighted MR image, reconstructed along the optic pathways, showing enhancement surrounding the optic nerve within the optic canal (large arrows). There is en-plaque growth along the walls of the sulcus chiasmaticus, giving a “rose thorn” appearance (small arrows).
Fig 2.
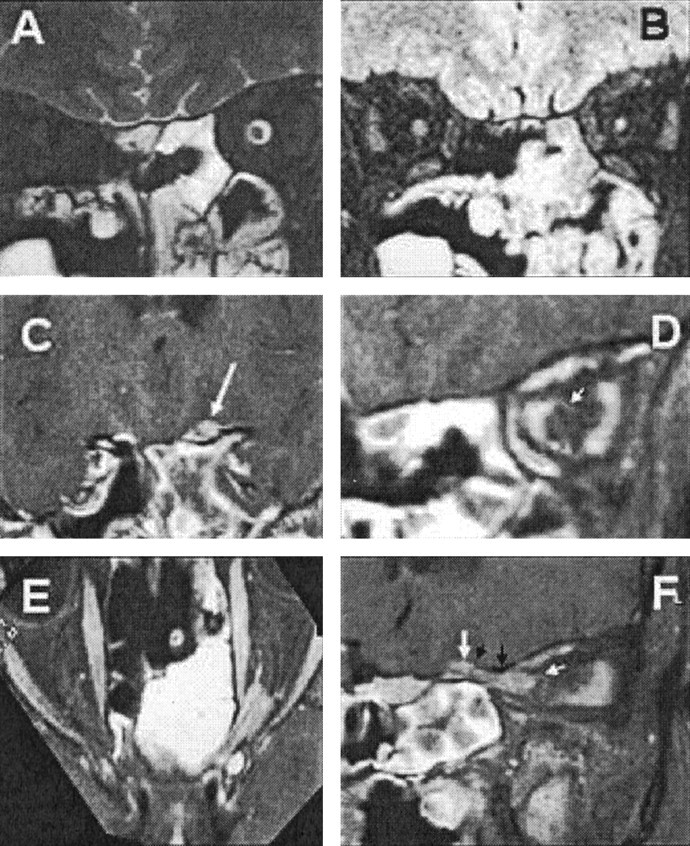
Case 3.
A, T2-weighted coronal MR image, showing dilatation of the left optic nerve sheath.
B, Heavily T2-weighted image with fat and water suppression (SPIR/FLAIR), showing increased T2 signal intensity and atrophy of the optic nerve.
C, Coronal images through the sulcus chiasmaticus, demonstrating a small tumor nodule arising from the cranial end of the left optic canal (arrow).
D, Coronal image from a contrast-enhanced T1-weighted volume-rendered MR image, acquired with fat suppression through the optic nerve in the midorbit, showing en-plaque growth of tumor within the left optic nerve sheath (arrow).
E, Axial oblique reconstruction from a contrast-enhanced T1-weighted volume-rendered MR image, acquired with fat suppression along the optic canals, showing the extent of the tumor. Note the optic nerve emerging from the tumor anteriorly and the en-plaque growth of tumor along the anterior optic nerve sheath.
F, Sagittal oblique reconstruction from a contrast-enhanced T1-weighted volume-rendered MR image, acquired with fat suppression along the course of the left optic canal, showing the extent of the tumor. Note the tram track sign due to the central nonenhancing optic nerve, the mural nodule (large white arrow) arising behind the roof of the optic canal (black arrows), and the en-plaque growth of the tumor along the anterior part of the orbital optic nerve sheath (small white arrow).
The diagnosis of opticnerve meningioma rested on the successful identification of tumor within the optic canal (six of six cases), orbital apex (six of six cases), and along the wall of the sulcus chiasmaticus (three of six cases). Typical radiologic features were also noted that support the diagnosis of optic canal meningioma, these were: 1) Visualization of the optic nerve within the enhancing meningioma in the optic canal (tram-track sign; five of six cases); 2) En-plaque growth along the optic nerve sheath in the orbit (three of six cases); 3) En-plaque growth of the tumor along the wall of the sulcus chiasmaticus giving a “rose thorn” appearance on axial images (two of six cases); 4) Growth of a nodule of tumor extending ventrally from the cranial end of the optic canal (three of six cases); and 5) Evidence of en-plaque connection between the optic canal mass and a cribriform plate tumor (two of six cases).
None of these radiologic signs were observed on prediagnostic images, even on review after the diagnosis had been made. Comparison of the imaging protocols of diagnostic and nondiagnostic images showed that the use of high-spatial-resolution (<3-mm through-plane) postcontrast images of the orbit is common to all diagnostic images, and in all cases the tumor was best demonstrated on a contrast-enhanced T1-weighted volume acquisition with a through-plane spatial resolution of 0.5–1.5 mm. The use of multiplanar imaging or image reconstruction was also common to all diagnostic images. Although it was not essential for the demonstration of disease, it was helpful in appreciating the topography and extent of individual lesions. Demonstration of the orbital extent of the tumor relied on a combination of high spatial resolution, contrast enhancement, and fat suppression and in case 2, where fat suppression was not available; the orbital extent of the tumor was difficult to appreciate.
Discussion
The diagnosis of optic canal meningioma is a crucial one, because the natural progress of the disease is an inexorable visual deterioration and the results of surgical decompression in the late stages are poor (2, 7–9). Surgical and modern conformal and stereotaxic approaches at an early stage may allow total resection and improve the chances for preservation of vision (10–15). We have presented six cases in which the diagnosis was missed for an initial period ranging from 1 to just over 4 years from the onset of symptoms, resulting in severe unilateral or bilateral visual loss in three of six cases.
These case reports contain several important lessons for the radiologists dealing with the imaging investigation of visual symptoms. First, it should be appreciated that this condition is rare (1, 3). The Manchester Royal Infirmary is a large tertiary referral center dealing with a referral population of more than seven million people. Despite this fact, we have identified only 18 intracanalicular meningiomas over a 6-year period, including the six presented here.
Second, in each of the cases seen here, a diagnosis of optic neuritis was made and the patients were treated with high-dose steroids with some resulting improvement in visual symptoms. In light of the incidence of true optic neuritis, it is clear that identification of patients with optic canal meningioma may be extremely difficult (3, 6). The selection of patients for detailed MR investigation must rely on the suspicions of the individual clinician; however, the presence of a progressive visual loss despite treatment in the absence of confirmatory indicators of demyelination should raise suspicions (2, 5, 7, 16). It is interesting that all the patients described here were referred for at least one additional imaging procedure during their follow-up period, because of clinical suspicions based on the atypical clinical course. Despite this fact, diagnosis of optic canal meningioma was not made in any case.
Examination of the imaging strategies adopted in these cases indicates that the principle reason the diagnosis was missed was the use of an inappropriate imaging protocol. In particular, none of these investigations included high-spatial-resolution MR of the orbit. In addition only nine of 11 follow-up MR images used contrast enhancement, only two (in one patient) used specific orbital images, and neither of them used fat suppression. In contrast, diagnostic images employed high-spatial-resolution pre- and postcontrast T1-weighted images of the orbit in all cases. Five cases used fat suppression following contrast medium administration; the other examination was performed before fat suppression was available at our center. These findings are in keeping with the observations of previous authors (1, 2, 17, 18). Finally, all diagnostic images used multiplanar imaging or multiplanar reconstructions from volume acquisitions. Although this did not affect the diagnostic process, it appears to improve appreciation of the anatomy and extent of tumor.
The radiologic appearances of the cases presented here reflect the known growth patterns of cannalicular meningioma (1, 2, 19). In two of six cases, en-plaque meningioma extended posteriorly from a cribriform plate meningioma along the cribriform plate to the sulcus chiasmaticus, where it extended into the optic canal, through the canal, and into the orbital apex. In the remaining four cases, the tumor was limited to the canal and the immediately adjacent walls of the sulcus chiasmaticus and orbital apex. The en-plaque growth of the tumors could be clearly seen along the walls of the optic nerve sheath (in two of six cases), along the sulcus chiasmaticus (in three of six cases), or along the cribriform plate (in two of six cases). At the cranial end of the optic canal, distinctive appearances were observed in five cases, with a discrete tumor nodule in three and en-plaque growth with a “rose thorn” appearance in two (Figs 1D).
Routine imaging of patients with suspected optic neuritis is performed in some centers; however, management of these patients without imaging is far more common practice, because isolated optic neuritis is extremely common. Furthermore, imaging examinations are often considered unnecessary, because clinical symptoms are usually typical and fixed dose steroid therapy is a standard treatment. Imaging should certainly be performed in any case in which the clinical presentation is atypical or where progressive visual loss continues despite treatment, and MR is clearly superior to CT in this situation. In many cases, imaging will identify evidence of widespread demyelination, which will support a presumptive diagnosis of multiple sclerosis. It is also essential, however, to adequately exclude optic nerve compression. This requires coverage of the entire optic nerve from the globe to the optic tracts and must employ adequate spatial resolution to clearly delineate the nerve from adjacent structures. Delineation of the optic nerve will require fat suppression in the orbit, and this should be combined with contrast enhancement to demonstrate blood vessels and any pathologic process. In many cases, the original diagnosis of optic neuritis will be correct. Differentiation of optic neuritis for optic nerve sheath and cannalicular meningiomas can be difficult. Optic neuritis in the acute inflammatory stage will result in expansion of the nerve and of the nerve sheath complex associated with neural enhancement. At this stage, the appearances of a limited segmental enlargement with enhancement may cause confusion. Some of the features described here, particularly evidence of extracanalicular-intracranial tumor extension, distal en-plaque extension along the optic nerve sheath, and absence of the tram-track sign on enhanced images will indicate the presence of meningioma rather than neuritis. The use of high-resolution MR techniques—such as the spectral inversion recovery/fluid-attenuated inversion recovery (FLAIR) sequence—that identify the high T2 signal intensity in the neuritic nerve, free from confounding high signal intensity due to fat and CSF, may also be helpful (20). The neuritic nerve will be well seen on these images (Fig 2B), and high signal intensity will persevere into the atrophic phase of the neuritic process. It must be appreciated that the demonstration of a high T2 signal intensity within the nerve with or without associated atrophy may arise from proximal nerve compression (case 3, Fig 2) or vascular insult. Identification of a high-signal-intensity “neuritic” segment on T2-weighted images is therefore not diagnostic of primary demyelination and must prompt review of the examination to exclude proximal compressive lesions.
Conclusion
We have described six cases of optic canal meningioma in which the diagnosis was missed for a period in excess of 1 year from presentation. Clinical features led to a misdiagnosis of optic neuritis in all cases. Although atypical clinical progression led to further imaging studies, they did not provide the diagnosis, because of inappropriate imaging protocols. Diagnosis was eventually made on the basis of high-spatial-resolution MR combined with contrast enhancement. Radiologists should have a high suspicion for the diagnosis of optic canal meningioma in patients with unexplained visual loss, particularly when visual loss is progressive. Investigation in these cases should include high-spatial-resolution MR of the orbit before and after contrast enhancement, and fat suppression should be used in combination with contrast enhancement whenever possible.
References
- 1.Ortiz O, Schochet SS, Kotzan JM, Kostick D. Radiologic-pathologic correlation: meningioma of the optic nerve sheath. AJNR Am J Neuroradiol 1996;17:901–906 [PMC free article] [PubMed] [Google Scholar]
- 2.Mafee MF, Goodwin J, Dorodi S. Optic nerve sheath meningiomas: role of MR imaging. Radiol Clin North Am 1999;37:37–58 [DOI] [PubMed] [Google Scholar]
- 3.Castel A, Boschi A, Renard L, De Potter P. Optic nerve sheath meningiomas: clinical features, functional prognosis and controversial treatment. Bull Soc Belge Ophtalmol 2000;275:73–78 [PubMed] [Google Scholar]
- 4.Kinjo T, Mukawa J, Koga H, Shingaki T. An extensive cranial base meningioma extending bilaterally into Meckel’s cave: case report. Neurosurgery 1997;40:615–617; discussion 617–618 [DOI] [PubMed] [Google Scholar]
- 5.Jakobiec FA, Depot MJ, Kennerdell JS, et al. Combined clinical and computed tomographic diagnosis of orbital glioma and meningioma. Ophthalmology 1984;91:137–155 [DOI] [PubMed] [Google Scholar]
- 6.Vaphiades MS. Disk edema and cranial MRI optic nerve enhancement: how long is too long? Surv Ophthalmol 2001;46:56–58 [DOI] [PubMed] [Google Scholar]
- 7.Delfini R, Missori P, Tarantino R, et al. Primary benign tumors of the orbital cavity: comparative data in a series of patients with optic nerve glioma, sheath meningioma, or neurinoma. Surg Neurol 1996;45:147–153; discussion 153–154 [DOI] [PubMed] [Google Scholar]
- 8.Hodes JE, Sanders M, Patel P, Patchell RA. Radiosurgical management of meningiomas. Stereotact Funct Neurosurg 1996;66:15–18 [DOI] [PubMed] [Google Scholar]
- 9.Verheggen R, Markakis E, Muhlendyck H, Finkenstaedt M. Symptomatology, surgical therapy and postoperative results of sphenoorbital, intraorbital-intracanalicular and optic sheath meningiomas. Acta Neurochir Suppl (Wien) 1996;65:95–98 [DOI] [PubMed] [Google Scholar]
- 10.Moyer PD, Golnik KC, Breneman J. Treatment of optic nerve sheath meningioma with three-dimensional conformal radiation. Am J Ophthalmol 2000;129:694–696 [DOI] [PubMed] [Google Scholar]
- 11.Roche PH, Regis J, Dufour H, et al. Gamma knife radiosurgery in the management of cavernous sinus meningiomas. J Neurosurg 2000;93 (Suppl 3):68–73 [DOI] [PubMed] [Google Scholar]
- 12.Shimano H, Nagasawa S, Kawabata S, et al. Surgical strategy for meningioma extension into the optic canal. Neurol Med Chir (Tokyo) 2000;40:447–451; discussion 451–452 [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Jeun SS, Evans J, Kosmorsky G. Surgical management of clinoidal meningiomas. Neurosurgery 2001;48:1012–1019; discussion 1019–1021 [DOI] [PubMed] [Google Scholar]
- 14.Ohta K, Yasuo K, Morikawa M, et al. Treatment of tuberculum sellae meningiomas:a long-term follow-up study. J Clin Neurosci 2001;8 (Suppl 1):26–31 [DOI] [PubMed] [Google Scholar]
- 15.Miralbell R, Cella L, Weber D, Lomax A. Optimizing radiotherapy of orbital and paraorbital tumors: intensity-modulated X-ray beams vs. intensity-modulated proton beams. Int J Radiat Oncol Biol Phys 2000;47:1111–1119 [DOI] [PubMed] [Google Scholar]
- 16.Boschetti NV, Smith JL, Osher RH, et al. Fluorescein angiography of optociliary shunt vessels. J Clin Neuroophthalmol 1981;1:9–30 [PubMed] [Google Scholar]
- 17.Hendrix LE, Kneeland JB, Haughton VM, et al. MR imaging of optic nerve lesions: value of gadopentetate dimeglumine and fat-suppression technique. AJR Am J Roentgenol 1990;155:849–854 [DOI] [PubMed] [Google Scholar]
- 18.Jackson A, Sheppard S, Johnson AC, et al. Combined fat- and water-suppressed MR imaging of orbital tumors. AJNR Am J Neuroradiol 1999;20:1963–1969 [PMC free article] [PubMed] [Google Scholar]
- 19.Dutton JJ. Optic nerve sheath meningiomas. Surv Ophthalmol 1992;37:167–183 [DOI] [PubMed] [Google Scholar]
- 20.Jackson A, Sheppard S, Laitt RD, et al. Optic neuritis: MR imaging with combined fat- and water-suppression techniques. Radiology 1998;206:57–63 [DOI] [PubMed] [Google Scholar]


