During the past few years, diffusion-weighted (DW) imaging of the spine has received considerable attention in the imaging literature, although its usefulness and efficacy remain controversial. The interest in spinal DW imaging stems from the fact that, with conventional MR imaging alone, it is difficult to distinguish benign from pathologic vertebral body compression fractures (1). One of the most reliable signs for this purpose is that of visualizing a fracture line in which the fracture is usually seen on T2- or postcontrast T1-weighted images as a linear hypointensity in the middle of the compressed vertebral body or adjacent to a compressed endplate (2). Other signs that favor benign compression fractures (from the most to the least specific) include the presence of intervertebral fluid, an intervertebral vacuum cleft, absence of accompanying soft tissue masses, lack of pedicle abnormalities, solitary vertebral involvement, preservation of the posterior cortical margin, and a wedge-shaped deformity (3). Unfortunately, these signs cannot be found in all patients, and assessing the cause of a fracture is difficult, particularly when only one vertebra is involved. Most vertebral compression fractures, regardless of whether they are benign or malignant, show low T1 and high T2 signal intensities and may enhance after contrast material is administered (1, 3). In the chronic stage, the bone marrow of benign vertebral compression fractures returns to its normally high T1 signal intensity (reflecting the presence of fatty bone marrow), whereas the bone marrow infiltrated by tumor remains hypointense on T1-weighted images (2). It is not acceptable to wait for these changes to occur, however, because cancer patients require treatment to avoid spinal cord compression.
The first article to describe the use of DW imaging for differentiating benign from malignant vertebral compression fractures was written by Baur et al (4) in 1998 and elicited controversy. In that article, 22 benign and 17 pathologic fractures were correctly diagnosed and showed low and high signal intensity on DW images, respectively, when compared with other vertebrae that were assumed to be normal. The authors used a steady-state free precession (SSFP) sequence with a low b value (165 s/mm2), because it resulted in optimal signal-to-noise ratio and because they believed that increasing the b value resulted in a drop of signal intensity and provided no greater diffusion-related information. The authors concluded that the tumor packing present in pathologic fractures led to restriction of water diffusion and thus high signal intensity on DW images. Criticisms of that article center on the fact that the SSFP sequence contains information from both T1 and T2 effects (particularly T2 shine-through) and thus the findings cannot be solely related to diffusion aberrations (5). In addition, because only one diffusion gradient in only one direction was applied, quantification of diffusion was not possible. In a separate article (6), the same sequence was used to evaluate spinal metastases and was compared with conventional T1- and T2-weighted images. That study has been misquoted several times (7, 8) to point out the differences between our study and the one by Baur et al (4). In our investigation, we did not set out to evaluate the utility of DW imaging in vertebral compression fractures (only two of 15 patients had compression fractures), but rather only in the identification of vertebral metastases in the absence of compression fractures. We learned from our investigation that the SSFP sequence did not offer additional benefits over noncontrast T1-weighted images and that most metastases that were hyperintense on DW images were also hyperintense on T2-weighted images. Thus, this confirmed that the findings revealed by the DW SSFP sequence are greatly influenced by T2 shine-through and are in fact no better than routinely used MR images. Byun et al (9)used an SSFG sequence with a b value of 165 s/mm2 and a single-shot stimulated-echo acquisition sequence with a b value of 650 s/mm2 to assess the response to therapy in metastatic spinal disease. They noted that a favorable response to irradiation was seen as reduced signal intensity on DW images (both sequences) 1 month after the therapy.
Different investigators have used higher b values (360 and 598 s/mm2) with a variety of sequences that included diffusion-weighted spin-echo, diffusion-weighted fat-suppressed spin-echo, and diffusion-weighted stimulated-echo sequences to differentiate between benign vertebral edema and tumor infiltration (10). Because these sequences take a relatively long time to perform and respiratory gating was not used, a navigation-echo motion correction technique had to be used. Because of the complexity of these sequences, application of diffusion-sensitizing gradients in different directions was not possible. These authors eliminated most T2 effects by obtaining baseline images without the diffusion-sensitizing gradients. The stimulated-echo sequence, which is the most promising of the three applied, uses three 90° pulses before an echo with a 180° pulse. Theoretically, this sequence should be ideal for spinal DW imaging because of its relative lack of T2 effects and the possibility of incorporating into it long diffusion sequences and using the third 90° echo to trigger either respiratory or cardiac gating. Regardless of these theoretical benefits, all three sequences evaluated were found to be equally suitable for imaging of benign versus malignant vertebral compression fractures.
Baur et al (11) revisited the topic of spinal DW imaging and evaluated a series of patients again with the SSFP sequence by using different diffusion pulse lengths (that resulted in higher b values). They found that, with increasing diffusion pulse length (which increases the b value), benign fractures became progressively hypointense, thus reducing the number of false-positive results while malignant compression fractures remained hyperintense at short and longer pulse lengths. An accompanying editorial suggests that the qualitative evaluation of the high signal intensity seen on the SSFP DW image is mostly a reflection of high T2 signal intensity and, although DW imaging has potential in spinal imaging, sequences other than the SSFP need to be used in the future (7).
To avoid the problems described above, other investigators have used DW imaging techniques that allow for the calculation of apparent diffusion coefficient (ADC) values. In one article, qualitative ADC mapping showed that pathologic compression fractures had lower values (1.9 ± 0.3 × 10−4 mm2/s versus 3.2 ± 0.5 × 10−4 mm2/s) than benign fractures (12). The b values used in that investigation were low (0, 150, and 250 s/mm2), and the qualitative evaluation of their images resulted in nearly 50% false-positive results. Conversely, quantitative evaluation demonstrated a statistically significant separation between pathologic and benign compression fractures. Their results were also presented in the form of ADC histograms, and an accompanying editorial notes that an overlap for both groups of fractures exists (13). Other investigators have achieved similar results by using b values as high as 880–1000 s/mm2 (8, 14). Most of the images shown in these articles have low signal-to-noise ratios because of the high b value used and despite the fact that a spine phase-array coil was used. Using a phase array-dedicated spine coil with an SSFP sequence negates this problem and results in images of good quality. Images of adequate quality by using the spinal phase-array coil may be obtained with line-scan diffusion-weighted imaging. In one recent article, this technique resulted in good-quality images, and, despite its relatively long acquisition time (6 minutes 30 seconds), motion artifacts were absent (15). The authors used two b values (5 and 3005 s/mm2), allowing them to obtain mean diffusion coefficients. Imaging with such a high b value is possible because the short echo time used with this sequence allows it to maintain a high signal-to-noise ratio. High diffusion coefficients were found in benign compression fractures when compared with the normal vertebral bodies. Because that work is preliminary, the usefulness of line-scan diffusion imaging in differentiating benign from malignant compression fractures was not evaluated, but I believe it is promising.
To illustrate some of the problems encountered with the SSFP sequence, images of the spine of a 51-year-old man with hepatocellular carcinoma involving the caudate lobe of the liver are shown (Figs 1 and 2). The tumor was successfully resected. Three months later, however, the patient developed acute midthoracic pain. An MR imaging study of that area showed that the T7 vertebral body was slightly wedged anteriorly and that its superior endplate was depressed. On T1-weighted images (500/15 [TR/TE]) this vertebral body was slightly hypointense (Fig 1A). On T2-weighted (3500/90) and short Taub inversion recovery (3600/60 [TR/TE]; TI = 150 ms) images, the vertebral body was hyperintense (Figs 1B and C). After intravenous contrast material injection, the vertebral body demonstrated mild enhancement and no accompanying soft tissue abnormalities. A radionuclide bone scan demonstrated increased tracer activity in T7. Because of the concern for metastatic disease, we performed DW imaging of the spine. Three 4-mm-thick (one midline, two parasagittal) sections by using a diffusion-sensitized SSFP sequence with a b value of 600 s/mm2 were used. On this sequence, the T7 vertebral body was significantly more hyperintense than the adjacent normal vertebrae, suggesting the possibility of an underlying malignancy (Fig 1D). A CT-guided needle biopsy was performed and yielded normal bone findings. Because of this finding, therapy was postponed, and 6 months later MR imaging of the thoracic spine was repeated. At the time of this last study, the patient was asymptomatic, and the T1-weighted images showed that the normal bone marrow signal intensity abnormality had returned and that the involved vertebral body was more hyperintense than the adjacent vertebrae (Fig 2), implying the presence of fatty marrow and no tumor. Approximately 3 months after the last MR imaging study, the patient continues to be without back pain.
Fig 1.
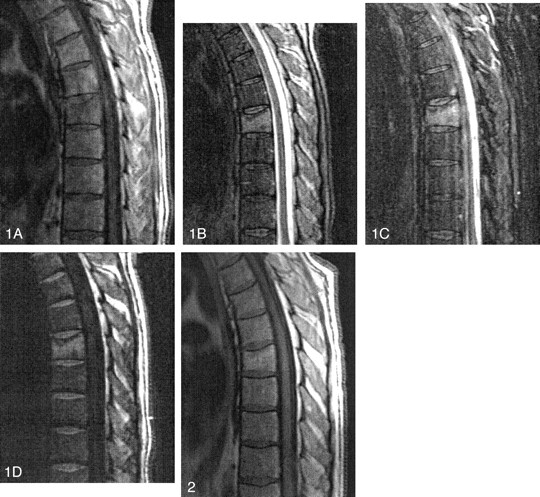
Initial MR study.
A, Midsagittal T1-weighted image, showing a mild compression deformity of the T7 vertebral body. The bone marrow is slightly hypointense, particularly along the superior endplate.
B, T2-weighted image obtained at the same location. Vertebra T7 is bright.
C, Corresponding short Taub inversion recovery image shows high signal intensity throughout the T7 vertebral body.
D, Diffusion-weighted image, showing that T7 has much higher signal intensity than the adjacent vertebrae.
Fig 2.

Follow-up study, 6 months after initial one. Midsagittal T1-weighted image shows that the signal intensity of the T7 vertebral body is now high compatible with fatty bone marrow.
This case is interesting because it confirms that spinal DW imaging is a controversial technique and should serve as a warning regarding overinterpretation of this type of study. Certainly, other authors have shown that benign compression fractures may be falsely hyperintense on DW images but for the most part if only an SSFP sequence with a low b value is used (3). In our patient, despite the fact that a relatively high b value (600 s/mm2) was used, the affected vertebral body was deceptively hyperintense. I am not able to comment on the ADC of the lesion presented here, because an SSFP sequence was used to study the patient. This sequence uses only a unidirectional diffusion-sensitizing gradient and a baseline image without it cannot be acquired; thus, ADC values cannot be calculated. The false-positive result of spinal DW imaging in our patient is probably due to a combination of factors, such as limited application of a diffusion gradient in only one direction and T2 shine-through. Therefore, it is obvious that, despite the 100% accuracy reported by some authors by using the SSFP sequence with high b values (11), discrimination of pathologic versus benign compression fractures is not always clear. During the past few months, we have seen another case identical to the one here presented. Much work remains to be done with respect to spinal DW imaging, but for now caution is recommended when interpreting DW findings of the spine, especially if the SSFP sequence is being used.
References
- 1.Yuh WT, Zachar CK, Barloon TJ, et al. Vertebral compression fractures: distinction between benign and malignant causes with MR imaging. Radiology 1989;172:215–218 [DOI] [PubMed] [Google Scholar]
- 2.Palmer WE, Suri R, Kattapuram SV. Benign versus malignant vertebral collapse: value of a fracture line on MR images. Radiology (S)1999;213–293
- 3.Baur A, Stabler A, Arbogast S, et al. Acute osteoporotic and neoplastic vertebral compression fractures: fluid sign at MR imaging. Radiology 2002;225:730–735 [DOI] [PubMed] [Google Scholar]
- 4.Baur A, Stabler A, Brunning R, et al. Diffusion-weighted MR imaging of bone marrow: differentiation of benign versus pathologic compression fractures. Radiology 1998;207:349–356 [DOI] [PubMed] [Google Scholar]
- 5.LeBihan DJ. Differentiation of benign versus pathologic compression fractures with diffusion-weighted MR imaging: a closer step toward the “holy grail” of tissue characterization. Radiology 1998;207:305–307 [DOI] [PubMed] [Google Scholar]
- 6.Castillo M, Arbelaez A, Smith JK, Fisher LL. Diffusion-weighted MR imaging offers no advantage over routine noncontrast MR imaging in the detection of vertebral metastases. AJNR Am J Neuroradiol 2000;21:948–953 [PMC free article] [PubMed] [Google Scholar]
- 7.Finelli DA. Diffusion-weighted imaging of acute vertebral compressions: specific diagnosis of benign versus malignant pathologic fractures. AJNR Am J Neuroradio 2001;22:241–242 [PMC free article] [PubMed] [Google Scholar]
- 8.Chan JHM, Peh WCG, Tsui EYK, et al. Acute vertebra body compression fractures: discrimination between benign and malignant causes using apparent diffusion coefficients. Br J Radiol 2002;75:207–214 [DOI] [PubMed] [Google Scholar]
- 9.Byun WM, Shin SO, Chang Y, et al. Diffusion-weighted MR imaging of metastatic disease to the spine: assessment of response to therapy. AJNR Am J Neuroradiol 2002;23:906–912 [PMC free article] [PubMed] [Google Scholar]
- 10.Spuentrup E, Buecker A, Adam G, van Vaals JJ, Guenther RW. Diffusion-weighted MR imaging for differentiation of benign fracture edema and tumor infiltration of the vertebral body. Am J Radiol 2001;176:351–358 [DOI] [PubMed] [Google Scholar]
- 11.Baur A, Huber A, Ertl-Wagner B, et al. Diagnostic value of increased diffusion weighting of a steady-state free precession sequence for differentiating acute benign osteoporotic fractures from pathologic vertebral compression fractures. AJNR Am J Neuroradiol 2001;22:366–372 [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou X, Leeds NE, McKinnon GC, Kumar AJ. Characterization of benign and metastatic vertebral compression fractures with quantitative diffusion MR imaging. AJNR Am J Neuroradiol 2002;23:165–170 [PMC free article] [PubMed] [Google Scholar]
- 13.Falcone S. Diffusion-weighted imaging in the distinction of benign from metastatic vertebral compression fractures: is this a numbers game. AJNR Am J Neuroradiol 2002;23:5–6 [PMC free article] [PubMed] [Google Scholar]
- 14.Herneth AM, Philipp MO, Naude J, et al. Vertebral metastases: assessment with apparent diffusion coefficient. Radiology 2002;225:889–894 [DOI] [PubMed] [Google Scholar]
- 15.Bammer R, Herneth AM, Maier SE, et al. Line scan diffusion imaging of the spine. AJNR Am J Neuroradiol 2003;24:5–12 [PMC free article] [PubMed] [Google Scholar]


