Abstract
Summary: Alveolar soft-part sarcoma is a rare, aggressive malignancy of uncertain histologic origin with a propensity for vascular invasion and distant metastasis. This neoplasm may mimic benign vascular neoplasms or malformations but careful evaluation of the unique imaging features on CT scans, MR images, and angiograms lead to the correct diagnosis. We present a case of alveolar soft-part sarcoma of the tongue and emphasize its radiologic and clinical features.
Alveolar soft-part sarcoma (ASPS) accounts for less than 1% of soft-tissue sarcomas. This extremely vascular tumor is most commonly found in the lower extremities (44%) and arises in the head and neck in 27% of cases, with 25% of head and neck cases occurring in the tongue (1). ASPS of the tongue is a slow-growing, painless mass that typically occurs in female patients between 15 and 35 years old (1). Imaging features mimic hemangioma, but subtle features on CT scans, MR images, and angiograms may distinguish ASPS from other disease entities. Older age at presentation and continuous growth should initiate a thorough evaluation of imaging studies for features that suggest the correct diagnosis. We report a case of ASPS of the tongue and emphasize the unique imaging features that led to correct diagnosis.
Case Report
A 34-year-old woman noticed discomfort on the right side of her throat after choking on an apple and subsequently had several episodes of oral bleeding leading to anemia. Panendoscopy showed minimal lateral pharyngeal erythema and otherwise normal findings. It was thought that her bleeding was secondary to a combination of minor trauma to the lateral pharynx and uncontrolled hypertension. She was admitted for transfusion and observation.
She returned 1 month later with further oral bleeding, and flexible nasopharyngoscopy showed a small vascular lesion at the right base of tongue with extension into the vallecula. CT of the neck showed a larger heterogeneously enhancing lesion within the tongue mistakenly believed to be a hemangioma. In light of the superficial extension of the lesion, laser resection of most of the lesion was performed. She remained asymptomatic on clinical examination at 1 month follow-up.
The patient returned 3 months after surgery with oral bleeding, tongue swelling, dysphonia, and progressive dysphagia. MR imaging redemonstrated a large tongue lesion of high signal intensity on T1- and T2-weighted images and heterogeneous enhancement following intravenous contrast material administration. A central region of low signal intensity was seen on pre- and postcontrast T1-weighted images, with corresponding high signal intensity on T2-weighted images, suggesting necrosis. Several flow voids also were seen.
Preoperative angiography and embolization were performed. Angiography showed a large hypervascular mass predominately supplied from the right lingual artery with a small contribution from the left lingual artery. A mild arteriovenous shunt was noted, and there was a dense persistent tumor blush. Supraselective catheterization of the right lingual artery was performed, and tumor embolization was accomplished by using 250–355-μ and 350–500-μ polyvinyl alcohol particles. The lesion was noted to be well circumscribed and encapsulated at surgical resection performed 4 days after embolization. She had an uneventful postoperative course with improved speech and swallowing.
Histopathologic findings showed nests of large granular cells separated by fibrovascular stroma. The cells were PAS positive with diastase-resistant intracytoplasmic inclusions. Immunohistochemical stains for HMW, LMW, and S-100 were negative. The neoplasm showed vascular invasion and extension to the margin of the specimen. A final diagnosis of ASPS was made. Chest and abdominal CT examinations were performed for tumor staging and showed both lung and liver metastasis.
Radiation therapy of the tumor bed was performed, because the surgical margins were positive, but the patient refused chemotherapy. She presented with hemoptysis 7 months later, and CT examination of the chest showed increase in the size and number of pulmonary metastases. She agreed to chemotherapy and was given five cycles of Ifosfamide and Adriamycin over the next 5 months without response. She subsequently was lost to follow-up and returned 7 months later with stable metastatic disease by CT and was asymptomatic.
Discussion
ASPS typically presents in young women as a slowly enlarging, well-circumscribed, and often encapsulated mass. Vascular invasion is frequent, and metastatic disease is frequently present at diagnosis. Lung metastases are seen in 42–65% of patients at presentation (2). Brain and bone are the next most common sites of metastasis, with lymph node involvement seen in only 10% of patients (2). ASPS has a close clinical and imaging resemblance to common benign vascular tumors such as hemangioma, which may lead to misdiagnosis and inadequate or delayed treatment.
Benign vascular soft-tissue vascular lesions are best classified into two categories, hemangioma and vascular malformation, according to the system of Mulliken and Glowaski (3). The term “hemangioma” is restricted to the classic involuting vascular lesion of infancy. Vascular malformations are divided into low-flow and high-flow subtypes, characterized by the predominant vessel involved; namely, capillary, venous, or lymphatic involvement. Vascular malformations are present at birth, grow in proportion to patient growth, and do not involute. Rarely, they may spontaneously enlarge through the development of arteriovenous fistulas, thrombosis, or ectasia.
In contrast, ASPS is not present at birth, grows rapidly, and occurs in an older age group. Patients with ASPS of the tongue experience dysphagia, dysphonia, or mild discomfort. Clinical examination often reveals pulsation with a thrill. Although there usually is no overlying ulceration or bleeding, our patient had significant hemorrhage as the tumor grossly extended through the overlying mucosa (4). Bone erosion is also rare (4). ASPS has a blue coloration on gross examination, which suggests a vascular nature, and may mimic venous malformations.
ASPS derives its name from its histologic appearance and suspected origin. As a result of formalin fixation, granular cells that are normally arranged in well-defined nests separated by a rich vascular network lose cohesion and create a pseudoalveolar pattern. Although the histogenesis remains controversial, immunohistochemical stains indicate a myogenic origin. The tumor’s common occurrence in skeletal muscle and desmin reactivity support a myogenic origin. Although recent studies have been unable to confirm myoD1 expression or myogenin reactivity (5), this may be attributed to its derivation from a myoid precursor with lack of differentiation. ASPS are deceptively benign histologically with no atypical mitotic figures or nuclear pleomorphism. Demonstration of PAS-positive diastase-resistant crystals with the appropriate clinical history confirms the diagnosis (6).
Several imaging features help distinguish ASPS from benign hemangiomas and venous malformations (cavernous hemangioma). Unenhanced CT imaging shows a low-attenuation lesion similar to muscle with peripheral enhancement and central areas of low attenuation on enhanced images consistent with necrosis (Fig 1; 2, 7). CT imaging of hemangioma in contrast typically shows a slightly hyperattenuated lesion on unenhanced images, with intense homogeneous enhancement after contrast material administration. Hemangiomas and venous malformations may also contain phleboliths seen as punctate calcifications on unenhanced CT images. T1-weighted MR images typically show hyperintense signal as compared with muscle that is not as intense as that seen with hemangiomas but more intense than that of other soft-tissue sarcomas that are typically isointense relative to muscle (Fig 2) (7). Similar to hemangioma, ASPS has very high signal intensity on T2-weighted images, but flow voids are also seen, suggesting the correct diagnosis (2, 7). Contrast-enhanced MR images show intense, heterogeneous enhancement and central necrosis as seen on CT images. Angiography shows a hypervascular mass with multiple, enlarged tortuous vessels, arteriovenous shunts, and an intense prolonged tumor blush (Fig 3) (7). This is in contrast to a high-flow vascular malformation such as an arteriovenous malformation that has an arteriovenous shunt but no persistent tumor blush and a low-flow vascular malformation that has prolonged contrast blush but no arteriovenous shunt.
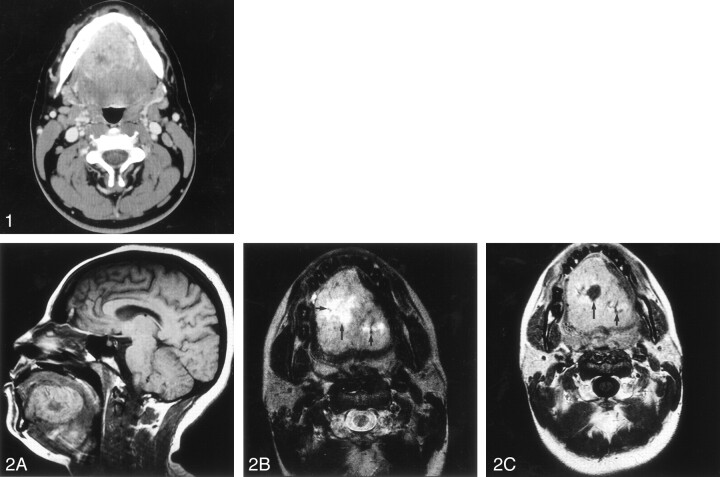
Fig 1.
Axial contrast-enhanced CT shows a relatively well-circumscribed tongue mass with slightly greater peripheral enhancement and central areas of low attenuation due to tumor necrosis.
Fig 2.
A, Sagittal T1-weighted unenhanced image shows a hyperintense signal intensity lesion in tongue with central low signal intensity representing an area of tumor necrosis.
B, Axial T2-weighted image shows a large hyperintense lesion in the tongue with a central region of greater signal intensity (long arrow) due to necrosis. A few scattered flow voids (short arrows) are seen within the lesion consistent with the diagnosis of ASPS.
C, Axial contrast-enhanced T1-weighted image shows intense heterogeneous enhancement with an internal area of low signal intensity due to necrosis (long arrow). Flow voids are once again seen (short arrow).
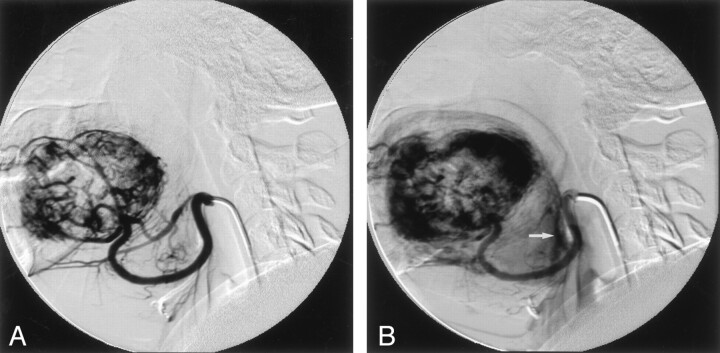
Fig 3.
Lateral-projection angiograms acquired during mid- (A) and late (B) arterial phases show a hypervascular mass with irregular, tortuous tumor vessels and persistent tumor blush. An early draining vein is seen at the base of tongue (arrow).
In addition to hemangioma and vascular malformation, other less likely differential diagnoses include hyperplastic lingual thyroid, dermoid, and squamous cell carcinoma. These neoplasms are easily differentiated from ASPS on the basis of CT and MR findings. Lingual thyroid typically occurs in the posterior oral tongue and does not have areas of necrosis or flow voids. Although rare, dermoids of the tongue appear as hypoattenuated and well-circumscribed masses on CT images that may contain fat and typically show peripheral enhancement of the cystic wall. Squamous cell carcinoma of the tongue accounts for nearly one quarter of oral cavity cancer but typically does not have well-circumscribed borders, flow voids, intense enhancement, and hyperintense signal on T2-weighted images as seen in ASPS.
Massive bleeding during surgical resection of ASPS has been reported and when located posteriorly in the tongue may be difficult to control at surgery (1, 6). Preoperative embolization for ASPS is useful to control blood loss and the need for transfusion therapy (4). Surgical blood loss in our patient was limited to 100 mL, and she did not require transfusion during the perioperative period. Aggressive surgical excision is recommended, because local recurrence is common. Five-year and 20-year survival rates of 59% and 15%, respectively, have been reported, with local recurrence and distant metastasis primarily responsible for the poor prognosis of this neoplasm (2).
Conclusion
Enlarging hypervascular masses of the tongue in young adult patients should raise suspicion of a malignant neoplasm and deserve careful scrutiny of imaging studies. CT and MR imaging features of ASPS suggest the correct diagnosis and, if recognized, will help the surgeon in performing a wide surgical resection to reduce the risk of local recurrence. Preoperative staging in this setting is also recommended in light of the frequency of metastasis at presentation and the relatively poor prognosis of ASPS. Angiography adds useful diagnostic information in excluding low- and high-flow vascular malformations, and preoperative embolization is suggested to control blood loss.
References
- 1.Ordóñez NG, Mackay B. Alveolar soft-part sarcoma: a review of the pathology and histogenesis. Ultrastruct Pathol 1998;22:275–292 [DOI] [PubMed] [Google Scholar]
- 2.Lorigan JG, O’Keeffe FN, Evans HL, Wallace S. The radiologic manifestations of alveolar soft-part sarcoma. AJR Am J Roentgenol 1989;153:335–339 [DOI] [PubMed] [Google Scholar]
- 3.Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg 1982;69:412–420 [DOI] [PubMed] [Google Scholar]
- 4.Donald PJ. Alveolar soft part sarcoma of the tongue. Head Neck Surg 1987;9:172–178 [DOI] [PubMed] [Google Scholar]
- 5.Hunter BC, Ferlito A, Devaney KO, Rinaldo A. Clinicopathological consultation: alveolar soft part sarcoma of the head and neck region. Ann Otol Rhinol Laryngol 1998;107:810–814 [DOI] [PubMed] [Google Scholar]
- 6.Ordóñez NG. Alveolar soft part sarcoma: a review and update. Adv Anat Pathol 1999;6:125–139 [DOI] [PubMed] [Google Scholar]
- 7.Iwamoto Y, Morimoto N, Chuman H, et al. The role of MR imaging in the diagnosis of alveolar soft part sarcoma: a report of 10 cases. Skeletal Radiol 1995;24:267–270 [DOI] [PubMed] [Google Scholar]