Abstract
BACKGROUND AND PURPOSE: Peripheral cerebellar artery aneurysms are rare and difficult to treat surgically. We report the angiographic results and the clinical outcomes for eight patients treated by embolization for peripheral cerebellar artery aneurysms.
METHODS: Between 1994 and 2001, eight patients with peripheral cerebellar artery aneurysms were referred from the neurosurgery department for endovascular treatment. The patients consisted of four women and four men with a mean age of 43 years (range, 16–68 years). Seven patients presented with subarachnoid hemorrhage. In one patient, the aneurysm was incidental. In five cases, selective embolization of the aneurysmal sac was performed using GDCs. Two large peripheral cerebellar artery aneurysms and one small aneurysm with a wide neck were treated by parent vessel occlusion. Mean clinical and imaging follow-up duration was 18.5 months (range, 12–36 months).
RESULTS: Endovascular treatment resulted in five complete occlusions, two neck remnants, and one residual aneurysmal flow. Clinical evaluation showed that good or excellent recovery was achieved by all patients. Imaging follow-up revealed seven complete occlusions and one residual aneurysmal flow.
CONCLUSION: Our study showed that the endovascular approach to treat peripheral cerebellar artery aneurysms by selective embolization or parent vessel occlusion was feasible, safe, and effective. Imaging follow-up showed excellent anatomic results in accordance with clinical recovery.
Aneurysms of the peripheral cerebellar arteries are rare. Only a few case reports have been reported, and no large series with long-term follow-up has yet been published (1–12). No precise information is available in the literature regarding the management of peripheral cerebellar artery aneurysms or the associated long-term results. The ability to surgically treat these aneurysms depends on their location but is considered to be difficult and is associated with high morbidity and mortality rates (1, 11, 13). In an effort to provide effective treatment options for patients considered to have high risk aneurysms and unacceptable risk:benefit ratios for surgery, endovascular therapies have been developed. The aim of this study was to report the long-term results of endovascular treatment and imaging follow-up of eight patients with peripheral cerebellar artery aneurysms.
Methods
Patients
From January 1994 to December 2001, endovascular treatment was proposed for eight consecutive patients with peripheral cerebellar artery aneurysms. The patients consisted of four women and four men with a mean age of 43 years (range, 16–68 years). Locations of aneurysms were as follows: superior cerebellar artery in four patients, anterior inferior cerebellar artery in two, and posterior inferior cerebellar artery in two. Anterior inferior cerebellar artery aneurysms were associated with arteriovenous malformations.
As shown in Table 1, seven patients presented with subarachnoid hemorrhage. According to the Hunt and Hess scale (14), one patient’s condition was grade I, five were grade II, and one was grade V. In one patient, the peripheral cerebellar artery aneurysm was incidental. One 16-year-old male patient was complaining about headaches and underwent brain imaging. A large dysplastic and partially thrombosed posterior inferior cerebellar artery aneurysm was revealed by MR imaging.
TABLE 1:
Characteristics of eight patients with peripheral cerebellar artery aneurysms
| Patient No. | Age (yr)/Sex | Location | Sac | Neck | Presentation | Treatment | Initial Results | Complications | Outcome | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 43/F | SCA | S | S | SAH | Selective | Complete | Thrombus | Excellent | Unchanged |
| 2 | 68/M | AICA | S | S | SAH | Selective | Neck remnant | None | Excellent | Further thrombosis |
| 3 | 52/F | SCA | S | S | SAH | Selective | Complete | None | Excellent | Unchanged |
| 4 | 16/M | PICA | L | W | Incidental | Nonselective | Complete | None | Excellent | Unchanged |
| 5 | 57/M | PICA | L | W | SAH | Nonselective | Complete | None | Excellent | Unchanged |
| 6 | 26/F | SCA | S | S | SAH | Selective | Neck remnant | None | Excellent | Further thrombosis |
| 7 | 62/F | AICA | S | W | SAH | Nonselective | Complete | None | Nerve palsy | Unchanged |
| 8 | 19/M | SCA | S | S | SAH | Selective | Residual flow | Bleeding | Excellent | Unchanged |
Note.—F indicates female; M, male; SCA, superior cerebellar artery; AICA, anterior inferior cerebellar artery; PICA, posterior inferior cerebellar artery; S, small; L, large; W, wide; SAH, subarachnoid hemorrhage.
All patients underwent conventional angiography of both carotid arteries and vertebral arteries. Peripheral cerebellar artery aneurysms were classified into four groups according to their size and their neck width. Five aneurysmal sacs were small (diameter < 11 mm) and had small necks (neck < 4 mm), two were large (diameter = 11–25 mm), and one was small with a wide neck (neck > 4 mm). Of the two large aneurysms, one was dysplastic.
Therapeutic alternatives were discussed by neurosurgical and neurointerventional teams. Indications for endovascular therapy in our patient group mainly concerned the anticipated surgical difficulties due to aneurysm size or location.
Endovascular Procedure
In all cases, the endovascular treatment was performed with the patient under general anesthesia and systemic heparinization. The adequacy of systemic anticoagulation was monitored by frequent measurements of the activated clotting time. A baseline activated clotting time was obtained before the bolus infusion of heparin (50–75 IU/kg body weight) and hourly thereafter. After the bolus infusion of heparin, a continuous drip was administered (1000–1500 IU/hr) for the purpose of doubling the baseline activated clotting time. In most patients, systemic heparinization was reversed with protamine sulfate after the procedure. In some cases, with periprocedural complications or coil herniation into the parent artery, systemic heparinization was prolonged for 24 to 48 hr. Five small aneurysms with small necks were treated by selective embolization with GDCs. The technique for the GDC procedure has been published in the literature (15–17). Two patients with large aneurysms and one patient with a small aneurysm with a wide neck were treated by parent artery occlusion with GDCs detached next to the aneurysm neck. No test occlusion was performed in patients treated by nonselective embolization. After endovascular treatment, patients were transferred to the intensive care unit where fluid balance, neurologic status, and blood pressure were carefully monitored.
Immediate Outcome
Patients were evaluated by angiography to document aneurysm obliteration. Angiographic results were classified as follows: complete occlusion, no contrast material filling the aneurysmal sac; neck remnant, residual contrast material filling the aneurysmal neck; and residual flow, residual contrast material filling the aneurysmal body. A senior neurosurgeon recorded the clinical course, including worsening of symptoms and death. Clinical outcome was graded according to a modified Glasgow Outcome Scale (18) as follows: excellent, neurologically intact; good, mild hemiparesis, cranial nerve palsy, or other deficit that does not interfere with daily functioning or work; fair, significant hemiparesis, aphasia, confusion, or other deficit that interferes with daily activities or prevents return to work; and poor, coma or severe neurologic deficit rendering the patient dependent on family or nursing staff.
Patient Follow-up
Imaging follow-up included MR imaging performed 6, 12, 24, and 36 months after treatment and conventional angiography performed at 12 months. We assessed brain tissue by using an axial view spin-echo T1-weighted sequence (700/14 [TR/TE]; 19 sections; section thickness, 5 mm; intersection gap, 0.7 mm; one average; matrix, 144 × 256), a T2-weighted sequence (3800/22 and 90; 19 sections; section thickness, 5 mm; intersection gap, 0.7 mm; two averages; matrix, 190 × 256), and a fluid-attenuated inversion recovery sequence (119/9000; 19 sections; section thickness, 5 mm; intersection gap, 0.7 mm; one average; matrix, 182 × 256). We assessed intracranial vessels by using time-of-flight angiography (34/5.4; flip angle, 50 degrees; field of view, 25 cm; acquisition volume, 55 mm; matrix, 200 × 512) and contrast-enhanced angiography (6.8/2.3; flip angle, 35 degrees; field of view, 25 cm; acquisition volume, 55 mm; matrix, 150 × 512). Clinical examination by a senior neurosurgeon was obtained simultaneously with the imaging control. Five patients were lost to follow-up after 12 months so that the mean duration of follow-up was 18.5 months (range, 12–36 months). Follow-up angiograms were compared with immediate postembolization angiograms and were then assigned to one of three categories: category 1, further thrombosis, when the amount of contrast material filling the aneurysm decreased; category 2, unchanged, when a similar degree of aneurysm occlusion in multiple projections was found; and category 3, recanalization, when an increase of the amount of contrast material filling in the aneurysm was observed. Conventional angiography, MR angiography, and MR imaging were reviewed for all patients by two senior neuroradiologists together (B.L., X.L.).
Results
Immediate Anatomic Outcome
Selective embolization (Fig 1) was performed in five patients who each had a small aneurysm with a small neck. Complete occlusion was achieved in two patients, a neck remnant was present in each of two, and residual flow was observed in one. Parent artery occlusion (Fig 2) performed in large peripheral cerebellar artery aneurysms (two patients) or in a small aneurysm with a wide neck (one patient) led to complete aneurysmal thrombosis.
Fig 1.
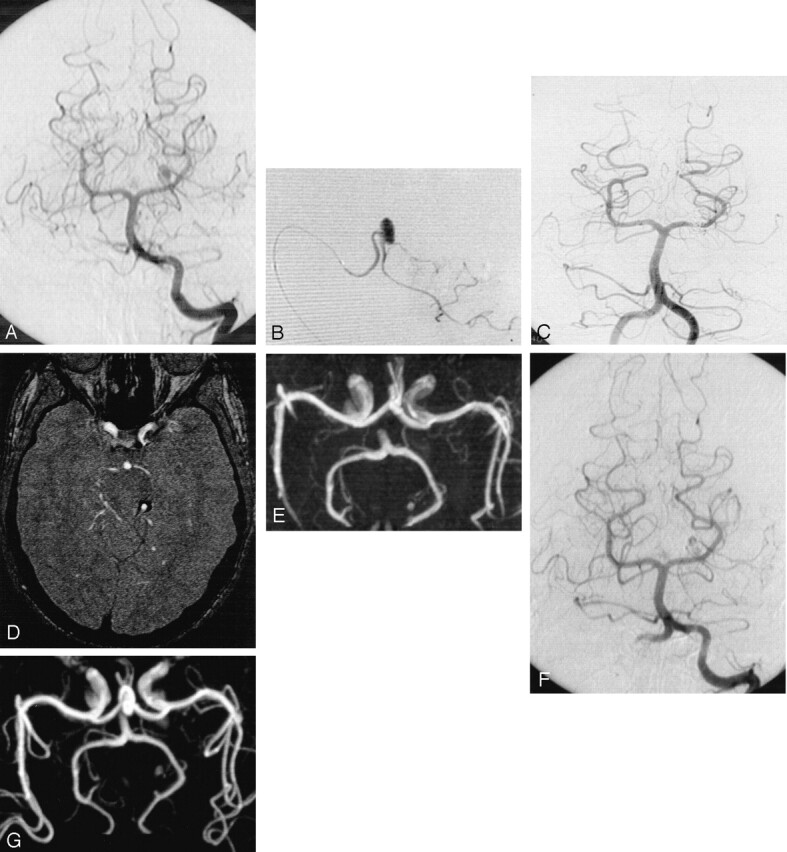
Subarachnoid hemorrhage (Hunt and Hess grade V) in a 19-year-old male patient.
A, Cerebral angiogram of the left vertebral artery shows distal aneurysm of the left superior cerebellar artery.
B, Selective opacification of the left superior cerebellar artery shows small aneurysm with favorable neck:body ratio.
C, Control angiogram obtained immediately after treatment shows complete aneurysmal occlusion.
D, MR image obtained at 6 months shows residual flow in the central part of the aneurysm without neck opacification.
E, MR angiogram obtained at 6 months shows residual flow in the central part of the aneurysm without neck opacification.
F, Follow-up conventional angiogram obtained at 12 months shows unchanged residual flow.
G, Follow-up MR angiogram obtained at 36 months shows unchanged residual flow.
Fig 2.
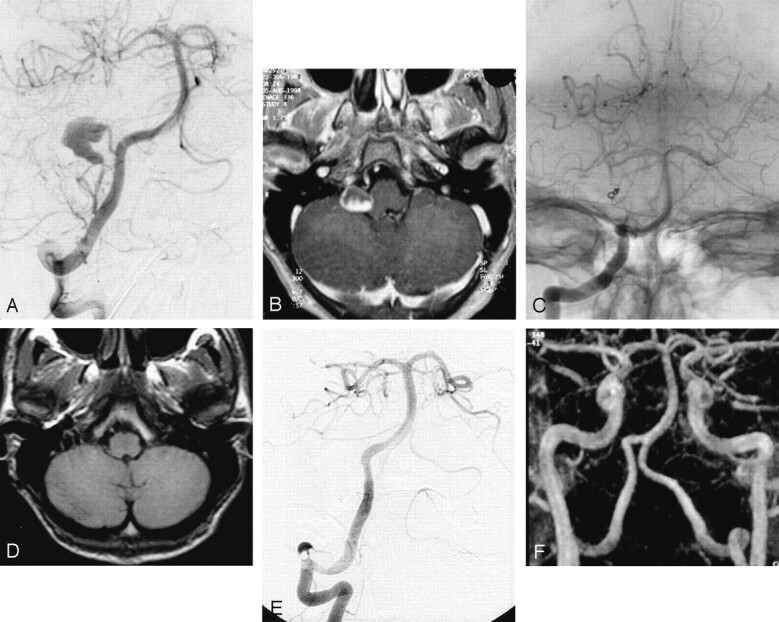
Incidental aneurysm revealed by MR image of a 16-year-old male patient who complained of headaches.
A, Cerebral angiogram of the right vertebral artery shows large dysplastic aneurysm located 2 cm distal to the origin of the posterior inferior cerebellar artery.
B, Contrast-enhanced axial view T1-weighted image shows a partially enhanced mass close to the right part of the medulla.
C, Nonsubstracted control angiogram obtained immediately after occlusion by coils of the posterior inferior cerebellar artery at its origin shows complete occlusion of both the aneurysm and the parent vessel.
D, Axial view T1-weighted image obtained at 6 months shows complete disappearance of mass effect.
E, Follow-up conventional angiogram obtained at 12 months reveals no aneurysm recurrence.
F, Follow-up MR angiogram obtained at 36 months reveals no aneurysm recurrence.
Immediate Clinical Outcome
Based on a modified Glasgow Outcome Scale (18), all patients treated by selective coiling (n = 5) achieved excellent clinical outcomes. One patient treated by parent vessel occlusion for an anterior inferior cerebellar artery aneurysm associated with an arteriovenous malformation (patient 7), developed VIIth cranial nerve palsy 1 day after treatment. The other two patients treated by parent artery occlusion achieved excellent clinical recoveries.
Procedural Complications
Two complications occurred during selective embolization. One 43-year-old woman (patient 1) with a superior cerebellar artery aneurysm experienced a periprocedural transient ischemic attack. Conventional angiography revealed a small thrombus in the parent artery. Systemic heparinization was prolonged for 48 hr, and the patient achieved an excellent recovery. One 19-year-old male patient (patient 8) with a superior cerebellar artery aneurysm experienced aneurysm rupture during embolization, as revealed by slight extravasation of contrast material shown by control angiography. Systemic heparinization was immediately reversed with protamine sulfate, and the second control angiography performed 5 min later showed no extravasation of contrast material and complete occlusion of the aneurysm. The patient woke up with a moderate increase of headaches without neurologic deficit revealed by clinical examination. CT performed immediately after the procedure revealed a small hematoma of the cerebellar vermis, and a 36-month follow-up examination showed complete resolution of the symptoms.
Imaging and Clinical Follow-up
Follow-up conventional angiograms and MR angiograms were obtained for all patients. Mean duration of follow-up was 18.5 months. MR imaging and MR angiography performed at 6 months after treatment were in accordance with the results of the conventional angiography performed at 12 months. All five aneurysms that were completely occluded after endovascular treatment remained unchanged. Control angiography and control MR imaging performed at 1 year for patients with neck remnants shown by postprocedural angiography (two patients) showed further thrombosis with complete occlusion of the malformation (patients 2 and 6). In one patient with a superior cerebellar artery aneurysm and residual flow, the degree of occlusion remained unchanged 36 months after treatment (Fig 1). This patient’s follow-up included MR angiography, and no further treatment was planned. All patients remained clinically unchanged during the follow-up period; one patient (patient 7), therefore, still had VIIth cranial nerve palsy.
Discussion
Frequency of Cerebellar Artery Aneurysms
Aneurysms of the cerebellar arteries are rare and represented 0.8% of 2349 single bleeding aneurysms in the series presented by Locksley (19). Among them, posterior inferior cerebellar artery aneurysms are the most frequent but account for <1% of all intracranial aneurysms in large series: McDonald and Korb (20) reported a 0.8% incidence, whereas the 1966 Cooperative Study (19) reported a 0.49% incidence. Most of them are located at the origin of the posterior inferior cerebellar artery (21–23). Aneurysms located distal to the posterior inferior cerebellar artery origin are very rare and represent less than a fifth of posterior inferior cerebellar artery aneurysms (22, 24, 25). Approximately 0.1% and 0.2% of cerebral aneurysms are located on the anterior inferior cerebellar artery (26, 27) and superior cerebellar artery, respectively (19). Only 60 cases of anterior inferior cerebellar artery aneurysms have been reported in the literature. Locksley (19) reported six aneurysms arising from the superior cerebellar artery in a series of 2349 aneurysms, and Gacs et al (28) reported six peripheral superior cerebellar artery aneurysms in a surgical series of 910 vertebrobasilar aneurysms. At our institution, 1450 aneurysms were seen between 1994 and 2001; eight of them (0.55%) were located on peripheral cerebellar arteries.
Endovascular Treatment
Surgical treatment of cerebellar artery aneurysms is considered difficult and is associated with high morbidity and mortality rates (1, 11, 29). Many complications are related to lower cranial nerve dysfunction (1, 11, 13) because of the intimate relationship between these aneurysms and the cranial nerves. Endovascular coiling with GDCs has become the method of reference for the management of unclippable intracranial aneurysms. This treatment has shown its effectiveness in patients with ruptured aneurysms of the posterior circulation (30). In our series, selective embolization was successfully performed in the five patients with small aneurysmal sacs with small necks. The main limitations of this technique concern intracranial aneurysms with wide necks because of the risk of coil migration from the aneurysmal lumen to the parent vessel and the presence of a narrowed parent artery, which may prevent the progression of the microcatheter. In these cases, parent artery occlusion has been suggested as an alternative treatment of peripheral aneurysms. This technique has been previously reported for the treatment of mycotic aneurysms (31), and excellent results have been reported in most recent studies evaluating the efficacy of parent vessel occlusion with coils or glue in the treatment of peripheral aneurysms (2–6). In our series, we performed parent artery occlusion as the primary treatment in three patients because of the large aneurysmal sac (patients 4 and 5) or because of the wide neck (patient 7). We used short GDCs for vessel occlusion because of the small diameter of cerebellar arteries. The potential risk of this technique is represented by ischemic brain damages distal to the coils in the parent vessel territory. However, this complication never occurred in the present series.
Clinical Outcome
Patients with ruptured intracranial aneurysms of the posterior circulation have a poor prognosis: in the series presented by Schievink et al (32), the 48-hr survival rate was 32% for aneurysms of the posterior circulation and 77% for aneurysms of the anterior circulation; the 30-day survival rates were 11% and 53%, respectively. Several authors have reported outcomes after surgery in cases of peripheral cerebellar artery aneurysm. In the series presented by Andoh et al (1), clinical outcomes were good for 10 of 15 patients and fair for two. Three patients died. Nishizaki et al (10), in a surgical review, reported good results in 82% of the cases and fair results in 8%. Ten percent of the patients died as a result of the procedure.
Our five patients treated by selective embolization achieved excellent clinical recoveries. Only one patient treated by parent artery occlusion (patient 7) developed VIIth cranial nerve palsy despite appropriate collateral circulation shown by conventional angiography. Eckard et al (6) monitored the neurologic statuses of eight of their nine patients by Amytal injection and concluded that Amytal testing brings an idea of the expected deficit for the patient but is overly predictive of deficits. We think, as do many authors (2–5, 30), that this test occlusion is not helpful because collateral circulation is generally efficient (2) in a case of cerebellar artery aneurysm, preventing significant infarction after parent vessel occlusion. Furthermore, the treatment remains the same even if the test is not tolerated.
Anatomic Outcome
Conventional angiography performed immediately after treatment showed five complete occlusions, two neck remnants, and one residual aneurysmal flow. As expected, all aneurysms treated by parent vessel occlusion were completely thrombosed at the end of the procedure. In cases of selective embolization, 18.5-month imaging follow-up revealed four complete occlusions and one residual aneurysmal flow. In two peripheral cerebellar artery aneurysms, further thrombosis occurred during long-term follow-up whereas postprocedural angiography had shown neck remnants. This might be explained by the absence of a “water-hammer effect” (33) on the aneurysmal neck in this peripheral part of the posterior circulation.
Conclusion
Peripheral cerebellar artery aneurysms are rare, and little information is available regarding their management, treatment, and follow-up. With this study, we show that endovascular treatment of these aneurysms is feasible, safe, and effective. Selective embolization can be achieved in small aneurysms with small necks. In large aneurysms or in aneurysms with wide necks, parent artery occlusion may be proposed as a therapeutic alternative. By using both techniques, our series revealed excellent anatomic results and excellent clinical outcomes.
References
- 1.Andoh T, Itoh T, Yoshimura S, et al. Peripheral aneurysms of the posterior inferior cerebellar artery: analysis of 15 cases [in Japanese]. No Shinkei Geka 1992;20:683–690 [PubMed] [Google Scholar]
- 2.Chaloupka J, Putman C, Awad I. Endovascular therapeutic approach to peripheral aneurysms of the superior cerebellar artery. AJNR Am J Neuroradiol 1996;17:1338–1442 [PMC free article] [PubMed] [Google Scholar]
- 3.Cloft H, Kallmes D, Jensen M, Lanzino G, Dion J. Endovascular treatment of ruptured, peripheral cerebral aneurysms: parent artery occlusion with short Guglielmi detachable coils. AJNR Am J Neuroradiol 1999;20:308–310 [PMC free article] [PubMed] [Google Scholar]
- 4.Cognard C, Weill A, Tovi M, Castaings L, Rey A, Moret J. Treatment of distal aneurysms of the cerebellar arteries by intraaneurysmal injection of glue. AJNR Am J Neuroradiol 1999;20:780–784 [PMC free article] [PubMed] [Google Scholar]
- 5.Danet M, Raymond J, Roy D. Distal superior cerebellar artery aneurysm presenting with cerebellar infarction: report of two cases. AJNR Am J Neuroradiol 2001;22:717–720 [PMC free article] [PubMed] [Google Scholar]
- 6.Eckard D, O’Boynick P, McPherson C, et al. Coil occlusion of the parent artery for treatment of symptomatic peripheral intracranial aneurysms. AJNR Am J Neuroradiol 2000;21:137–142 [PMC free article] [PubMed] [Google Scholar]
- 7.Kamiya K, Nagai H, Koide K, Yamashita N, Shimazu N. Peripheral anterior inferior cerebellar artery aneurysms. Surg Neurol 1994;42:46–51 [DOI] [PubMed] [Google Scholar]
- 8.Mabuchi S, Kamiyama H, Abe H. Distal aneurysms of the superior cerebellar artery and posterior inferior cerebellar artery feeding an associated arteriovenous malformation: case report. Neurosurgery 1992;30:284–287 [DOI] [PubMed] [Google Scholar]
- 9.Mizushima H, Kobayashi N, Yoshiharu S, et al. Aneurysm of the distal anterior inferior cerebellar artery at the medial branch: a case report and review of the literature. Surg Neurol 1999;52:137–142 [DOI] [PubMed] [Google Scholar]
- 10.Nishizaki T, Tamaki N, Nishida Y, Fujita K, Matsumoto S. Aneurysms of the distal posterior inferior cerebellar artery: experience with three cases and review of the literature. Neurosurgery 1985;16:829–832 [DOI] [PubMed] [Google Scholar]
- 11.Spallone A, De Santis S, Giuffre R. Peripheral aneurysms of the anterior inferior cerebellar artery: case report and review of the literature. Br J Neurosurg 1995;9:537–541 [DOI] [PubMed] [Google Scholar]
- 12.Suzuki K, Meguro K, Wada M, Fujita K, Nose T. Embolization of a ruptured aneurysm of the distal anterior inferior cerebellar artery: case report and review of the literature. Surg Neurol 1999;51:509–512 [DOI] [PubMed] [Google Scholar]
- 13.Batjer H, Samson D. Causes of morbidity and mortality from surgery of aneurysms of the distal basilar artery. Neurosurgery 1989;25:904–915 [DOI] [PubMed] [Google Scholar]
- 14.Hunt W, Hess R. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 1968;28:14–20 [DOI] [PubMed] [Google Scholar]
- 15.Gulielmi G, Viñuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach: part 2. preliminary clinical experience. J Neurosurg 1991;75:8–14 [DOI] [PubMed] [Google Scholar]
- 16.Guglielmi G, Viñuela F, Duckwiler G, et al. Endovascular treatment of posterior circulation aneurysms by electrothrombosis using electrically detachable coils. J Neurosurg 1992;77:515–524 [DOI] [PubMed] [Google Scholar]
- 17.Viñuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475–482 [DOI] [PubMed] [Google Scholar]
- 18.Jennett B, Bond M. Assessment of outcome after severe brain damage. A practical scale. Lancet 1975;1:480–484 [DOI] [PubMed] [Google Scholar]
- 19.Locksley HB. Report on the Cooperative Study of Intracranial Aneurysms and Subarachnoid Hemorrhage: section V part 1. natural history of subarachnoid hemorrhage intracranial aneurysms and arteriovenous malformations: based on 6368 cases in the cooperative study. J Neurosurg 1966;25:215–239 [DOI] [PubMed] [Google Scholar]
- 20.McDonald C, Korb M. Intracranial aneurysms. Arch Neurol Psychiatry 1939;42:298–328 [Google Scholar]
- 21.Horowitz M, Kopitnik T, Landreneau F, et al. Posteroinferior cerebellar artery aneurysms: surgical results for 38 patients. Neurosurgery 1998;43:1026–1031 [DOI] [PubMed] [Google Scholar]
- 22.Salcman M, Rigamonti D, Numaguchi Y, Sadato N. Aneurysms of posterior inferior cerebellar artery-vertebral artery complex: variations on a theme. Neurosurgery 1990;27:12–20 [DOI] [PubMed] [Google Scholar]
- 23.Yamaura A. Diagnosis and treatment of vertebral aneurysms. J Neurosurg 1988;69:345–349 [DOI] [PubMed] [Google Scholar]
- 24.Hudgins R, Day A, Quisling R, et al. Aneurysms of the posterior inferior cerebellar artery: a clinical and anatomical analysis. J Neurosurg 1983;58:381–387 [DOI] [PubMed] [Google Scholar]
- 25.Yoshimoto T, Kodama N, Fujita K. Distribution of intracranial aneurysms. In: Suzuki J, ed. Cerebral Aneurysms. Tokyo: Neuron;1979. :14–19
- 26.Kamano S, Kirino T, Mizuno S, et al. Intrameatal aneurysm. Neurochirurgia (Stuttg) 1986;29:28–30 [DOI] [PubMed] [Google Scholar]
- 27.Suzuki J, Hori S, Sakurai Y. Intracranial aneurysms in the neurosurgical clinics in Japan. J Neurosurg 1971;35:34–39 [DOI] [PubMed] [Google Scholar]
- 28.Gacs G, Viñuela F, Fox A, Drake C. Peripheral aneurysms of the cerebellar arteries: review of 16 cases. J Neurosurg 1983;58:63–68 [DOI] [PubMed] [Google Scholar]
- 29.Drake C. The treatment of aneurysms of the posterior circulation. Clin Neurosurg 1979;26:96–144 [DOI] [PubMed] [Google Scholar]
- 30.Lempert T, Malek A, Van Halbach V, et al. Endovascular treatment of ruptured posterior circulation cerebral aneurysms. Stroke 2000;31:100–111 [DOI] [PubMed] [Google Scholar]
- 31.Khayata M, Aymard A, Casaco A, et al. Selective endovascular techniques in the treatment of cerebral mycotic aneurysms. J Neurosurg 1993;78:661–665 [DOI] [PubMed] [Google Scholar]
- 32.Schievink W, Wijdicks E, Piepgras D, et al. The poor prognosis of ruptured intracranial aneurysms of the posterior circulation. J Neurosurg 1995;82:791–795 [DOI] [PubMed] [Google Scholar]
- 33.Kwan ESK, Heilman CB, Shucart WA, Kulcznik RP. Enlargement of basilar artery aneurysms following balloon occlusion: “water hammer effect”: report of two cases. J Neurosurg 1991;75:963–968 [DOI] [PubMed] [Google Scholar]


