Abstract
BACKGROUND AND PURPOSE: Wallerian degeneration, the secondary degeneration of axons from cortical and subcortical injury, is associated with poor neurologic outcome. Since diffusion-weighted (DW) imaging is sensitive to early changes of cytotoxic edema, DW imaging may depict the acute injury to descending white matter tracts that precedes Wallerian degeneration; this injury is not visible on conventional CT or MR images in the maturing human brain.
METHODS: Two neuroradiologists retrospectively analyzed clinical MR images in six children (aged 3 days to 5 months) with DW findings consistent with acute injury of the descending white matter tract due to territorial anterior or middle cerebral artery infarction. In five patients, images were obtained as a part of routine clinical evaluation. The remaining patient was a part of a prospective study of brain injury. Imaging findings were correlated with clinical outcomes.
RESULTS: In all six patients, DW imaging performed 2–8 days after the onset of ischemia depicted injury to the descending white matter tract ipsilateral to the territorial infarct. Conventional MR images of the ipsilateral descending white matter tracts were abnormal in three patients. In all five patients for which follow-up results were available, the presence of DW changes was correlated with persistent neurologic disability.
CONCLUSION: As shown in this retrospective analysis, DW imaging can depict acute injury to the descending white matter tract in neonates and infants, when conventional MR imaging may show normal findings. These DW findings likely precede the development of Wallerian degeneration, and they may portend a poor clinical outcome.
First described in peripheral nerves by Waller in 1850, Wallerian degeneration is the term used to describe secondary degeneration of axons and their myelin sheaths from numerous causes, including infarction, hemorrhage, neoplasm, and demyelinating disease. After cortical injury, the presence of Wallerian degeneration in the descending white matter tracts, particularly the corticospinal tracts, is associated with persistent hemiparesis and, therefore, a poor long-term neurologic outcome. The diagnosis of Wallerian degeneration with CT imaging depends on the detection of atrophy of the pyramidal tracts; hence, CT is not a sensitive test for Wallerian degeneration in the acute to subacute time period.
MR imaging is superior to CT in the diagnosis of Wallerian degeneration. In vivo experiments by Jolesz et al (1) have shown that, in rat sciatic nerves, Wallerian degeneration induces T1 and T2 changes in the distal axons of transected nerves. The histopathologic changes of Wallerian degeneration are different in the central and peripheral nervous systems. In the central nervous system of adult humans, T2-weighted images show no changes within the first 4 weeks. This time period is referred to as stage 1 Wallerian degeneration. In a study correlating MR findings with histologic findings in patients after spinal cord injury, histologic results showed early Wallerian degeneration characterized by the beginning of myelin and axon breakdown in this period when T2-weighted MR images are normal. In this stage, physical changes of degeneration occur within the axon without substantial biochemical changes in the myelin, and thus, no signal intensity abnormality is depicted on conventional MR images. Myelin sheaths break up into ellipsoids and spheres, but the fragmented sheaths retain the staining properties of myelin (2, 3). From 4 to 14 weeks, myelin protein breakdown occurs without lipid breakdown; this process alters the protein-lipid ratio and results in decreased signal intensity on T2-weighted images. This event is termed stage 2. Subsequently, the presence of increased edema and further lipid breakdown results in increased T2-weighted signal intensity; this is characterized as stage 3. Finally, after months to years, volume loss occurs due to atrophy in stage 4 (4, 5). Studies in adult patients have shown a strong correlation between Wallerian degeneration, as detected with T2-weighted MR imaging, and long-term morbidity (6). However, the identification of Wallerian degeneration in neonates and infants by using T2-weighted imaging is complicated by the high water content and the lack of myelination in immature white matter.
Cerebral palsy is a disorder of movement and posture secondary to an insult to the immature brain. Early studies with CT imaging in patients with cerebral palsy have shown a correlation between long-term hemiplegia with cortical atrophy and the presence of periventricular lesions (7). More recent work with conventional MR imaging has demonstrated that the correlation of motor outcome with Wallerian degeneration, as manifested by atrophy in the descending corticospinal tracts, is higher than the correlation with infarct size (8, 9).
Diffusion-weighted (DW) imaging has clinical utility in the early diagnosis of cytotoxic edema, such as that due to acute ischemia. MR diffusion-weighted signal intensity is related to the magnitude of water displacement in a voxel of interest. The most commonly used DW imaging technique relies on spin-echo echo-planar imaging with the application of diffusion gradients in multiple directions. Rotationally invariant measurements of the apparent diffusion coefficient (ADC) can be made with the application of diffusion gradients in at least three orthogonal directions.
DW imaging may have utility in the identification of acute white matter injury corresponding to stage 1 Wallerian degeneration, which is not detectable by means of conventional MR imaging. In this retrospective observational case series, we present examples of descending white matter tract injury in five neonates and one infant. The injuries occurred after territorial anterior ischemia or middle cerebral artery (MCA) ischemia or both, as demonstrated earlier and more clearly with DW imaging than with conventional MR imaging. These diffusion changes precede the development of Wallerian degeneration, as assessed by using conventional imaging criteria, and they may portend a poor clinical outcome.
Methods
We retrospectively analyzed MR images acquired from six patients (age range, 3 days to 5 months; three female, three male) with DW findings consistent with descending white matter tract injury due to territorial anterior artery ischemia or MCA ischemia or both (Table 1). Conventional MR imaging and DW findings (Tables 2 and 3) were correlated with the clinical outcomes (Table 1).
TABLE 1:
Clinical findings in the study group
| Patient/Sex/Age | Clinical Presentation | Initial Neurologic Findings* | Follow-up Findings |
|---|---|---|---|
| 1/M/4 d | Apnea and seizures after cesarean delivery | L upper-extremity impairment | Progressive L hemiparesis over 6 mo |
| 2/M/8 d | Skull fracture after forceps delivery | R-sided hemiparesis | Persistent R hemiparesis at 9-mo follow-up |
| 3/F/8 wk | Presented with seizures | R upper-extremity impairment | No change over 3 wk |
| 4/F/19 wk | Nonaccidental trauma | Increased tone bilateral upper and lower extremities | No change over 1 y |
| 5/M/3 d | Skull fracture after failed vacuum extraction | Mild decreased in tone, bilateral lower extremities | Delayed motor development, increased tone in all extremities (upper more than lower) at 1-y follow-up |
| 6/F/5 d | Seizures, apneic episodes | Increased tone in bilateral lower extremities, hyperreflexia | Not applicable |
TABLE 2:
Conventional MR imaging findings
| Patient | Infarct Distribution* | Projectional White Matter Pathways Involved |
|---|---|---|
| 1 | R MCA territory involving the frontal lobe (including premotor cortex, primary motor cortex), parietal lobe (including somatosensory cortex), and temporal lobe | R corticospinal, corticobulbar, corticopontine tracts, genu and splenium of the corpus callosum |
| 2 | L and R frontal lobes (prefrontal cortex), R temporal lobe | L frontopontine tract, genu of the corpus callosum |
| 3 | L MCA territory involving the L frontal lobe (primary motor cortex, premotor and supplementary motor areas, and prefrontal cortex) and parietal lobe | L corticospinal tract, L corticobulbar and L corticopontine tracts, genu and splenium of the corpus callosum |
| 4 | Bilateral MCA territory involving bilateral frontal, parietal, and temporal lobes | L corticospinal, corticobulbar, and corticopontine tracts; genu and splenium of the corpus callosum |
| 5 | Bilateral MCA territory, most prominent in the L sensorimotor cortex and in the R parieto-occipital region | Bilateral corticospinal, corticobulbar, and corticopontine tracts |
| 6 | Bilateral subcortical white matter involving the frontal, parietal, and temporal lobes | Bilateral corticospinal, corticobulbar, and corticopontine tracts and genu and splenium of the corpus callosum |
TABLE 3:
DWI findings
| Territory Involved | Infarct Age (days) | Signal Intensity |
ADC, × 10−3 mm2/s | |
|---|---|---|---|---|
| T2-Weighted Image | DWI | |||
| Patient 1 | ||||
| L internal capsule | 4 | Normal | Normal | 1.03 ± 0.06 |
| R internal capsule | 4 | Increased | Increased | 0.74 ± 0.03 |
| Patient 2 | ||||
| L cerebral peduncle | 2 | Normal | Increased | 0.8 ± 0.05 |
| R cerebral peduncle | 2 | Normal | Normal | 1.07 ± 0.05 |
| L cerebral peduncle | 4 | Increased | Increased | 0.9 ± 0.05 |
| R cerebral peduncle | 4 | Normal | Normal | 1.07 ± 0.04 |
| L cerebral peduncle | 8 | Increased | Increased | 0.95 ± 0.06 |
| R cerebral peduncle | 8 | Normal | Normal | 1.08 ± 0.04 |
| Patient 3 | ||||
| L cerebral peduncle | 3 | Normal | Increased | 0.79 ± 0.13 |
| R cerebral peduncle | 3 | Normal | Normal | 0.99 ± 0.12 |
| Patient 4 | ||||
| L internal capsule | 8 | Normal | Increased | 0.64 ± 0.02 |
| R internal capsule | 8 | Normal | Normal | 0.84 ± 0.04 |
| Patient 5 | ||||
| L internal capsule | 2 | Normal | Increased | Not applicable |
| R internal capsule | 2 | Normal | Increased | Not applicable |
| Patient 6 | ||||
| L cerebral peduncle | 6 | Increased | Increased | 0.04 ± 0.03 |
| R cerebral peduncle | 6 | Increased | Increased | 0.10 ± 0.02 |
Five patients were imaged according to clinical protocols as a part of their routine clinical care at our institution. These five patients had findings consistent with descending white matter tract injury, and the images were retrospectively analyzed. Transverse nonenhanced and contrast-enhanced images(Omniscan; Nycomed, Princeton, NJ) were obtained with a spin-echo T1-weighted imaging sequence and the following TR/TE values: 756/17 in two patients, 672/17 in one patient, 572/12 in one patient, and 500/12 in one patient. T2-weighted images were acquired with a turbo spin-echo sequence in the transverse and coronal planes, with a TR/TE of 5000/96 in all patients. All five patients underwent MR examination with DW imaging; for this, a 1.5-T imager (Magnetom Vision; Siemens, Erlangen, Germany) with a circularly polarized radio-frequency coil was used. DW images were acquired with a multisection, spin-echo echo-planar imaging sequence with the following imaging parameters: 4000/100 (TR/TE), 24 × 24-cm FOV, 96 × 128 matrix, 4- or 5-mm section thickness, and a 0- or 1-mm gap between sections. A reference T2-weighted image (b = 0 s/mm2) was obtained at each section. DW images were obtained with diffusion gradients applied in three orthogonal directions (b = 1000 s/mm2). Only one b value greater than 0 was used, because previous work had shown that the measured ADC in neonates is insensitive to b values between 300 and 1000 s/mm2 (10). DW imaging was performed in the transverse plane in all five patients, and in the coronal plane in three of five patients. An isotropic diffusion image was calculated in all five patients as the geometric mean of the three orthogonal DW images. In four of five patients, the ADC values were calculated by means of linear regression by graphically plotting the logarithm of signal intensity from the isotropic DW image and b = 0 s/mm2 images versus their respective b values. We then measured the slope of the line. Because only hard-copy DW images were available for the fifth subject, quantitative DW imaging data were not available for ADC calculation.
One patient was originally registered as part of a prospective study of brain injury (11). This patient underwent T2-weighted imaging with a fast spin-echo sequence (5000/96) in the transverse plane and spin-echo T1-weighted imaging (500/12) in the transverse plane. This patient also underwent diffusion tensor (DT) imaging performed by using a 1.5-T imager (Magnetom Vision; Siemens) with circularly polarized radio-frequency coils. DT imaging was performed with a single-shot, multisection, spin-echo echo-planar sequence and these parameters: 3000/106; FOV, 240 mm; and a 1.9 × 1.9-mm in-plane resolution interpolated to a 256 × 256 matrix for display and analysis. Available were four tetrahedrally oriented DW images (b = 800 s/mm2), three orthogonally oriented DW images (b = 340 s/mm2), and a reference T2-weighted image (b = 0 s/mm2) for each section. Eighteen sections were obtained as two separate stacks of nine sections, which were manually interleaved. The section thickness was 5 mm. All raw diffusion images were realigned in two dimensions by using a combination of intra- and cross-technique realignment procedures to correct for image displacements and eddy current–induced linear stretch or shear. For each pixel, the elements of the diffusion tensor were derived from the combination of tetrahedral and perpendicular diffusion measurements. The ADC (in millimeters squared per second) was calculated as one-third the trace of the diffusion tensor (10). Two experienced neuroradiologists (R.C.M., P.M.) retrospectively reviewed all images. Diffusion data were assessed both qualitatively (on DW images) and quantitatively (on ADC maps) for changes in the descending white matter pathways on the side of the territorial infarct, as well as on the contralateral side.
Clinical Presentation and Course
The following information is summarized in Table 1.
Patient 1.—
A full-term 4-day-old male neonate was born by means of emergency cesarean section at an outside hospital. His course was complicated by two apnea-associated seizures. After intubation, the patient was transferred to our medical center for further care. Results of initial cultures and lumbar puncture were normal. The patient’s physical examination findings were notable for caput succedaneum in the occipitoparietal region, as well as decreased tone in the left arm. The patient’s neurologic examination was limited because of the intubation, but the results were reported to be otherwise normal. Head CT scans obtained at the outside hospital showed cytotoxic edema in a right MCA distribution consistent with infarction. MR examination was performed for further evaluation. The MR findings are summarized in Tables 2 and 3. Follow-up examination at 6 months showed that the patient had increased tone in the left upper and lower extremities. He had persistent clenching of the left hand, which he could open intermittently. He had decreased use of his left leg. These findings were consistent with evolving left hemiparesis from an infarct of the right MCA.
Patient 2.—
A full-term 8-day-old male neonate who had birth trauma as a result of forceps delivery. A CT scan showed left frontal, left temporal, and right frontal skull fractures; bilateral frontal subdural hematomas; and multiple contusions in the left frontal lobe, right frontal lobe, and right cerebellum. The patient was enrolled in a prospective brain injury MR protocol. Results of initial neurologic examination were remarkable for decreased tone in the right upper and lower extremities; this progressed to spastic right hemiparesis at 9-month follow-up.
Patient 3.—
A 2-month-old female infant presented to an outside hospital emergency department with decreased oral intake, decreased urine output, and emesis. She became hypotensive and went into respiratory arrest. She was intubated and transferred to our medical center. Her hospital course was complicated by a seizure. An MR study was ordered for further evaluation. The patient had decreased tone in her right upper extremity, which developed after her seizure; this did not improve during her 3-week hospital stay. This patient was not followed up at our institution, and no further follow-up information was available.
Patient 4.—
A 19-week-old female infant had nonaccidental trauma. She was brought into the emergency department at our institution because of generalized seizures. On initial presentation, the patient had increased tone and reflexes in all extremities. Her cranial nerves were intact. CT scans showed left frontal and left temporoparietal skull fractures, as well as a 5-mm left subdural hematoma. Gray matter–white matter differentiation was lost throughout both hemispheres. MR examination was performed to further delineate the extent of her injury. One-year follow-up examination showed no substantial improvement.
Patient 5.—
A full-term 3-day-old male neonate delivered by means of emergency cesarean section at an outside hospital after two failed attempts at vacuum delivery. The Apgar score was 1 at 1, 5, and 10 minutes. The patient was resuscitated and transported to our institution. Initial head CT showed a large left frontal epidural hematoma, a left parietal skull fracture, and a right frontal subgaleal hemorrhage. Initial neurologic examination showed decreased tone in all extremities. On follow-up examination 1 year after the initial insult, the patient had increased tone in all extremities, greater in the upper extremities than in the lower extremities and greater on the right than on the left. Though he demonstrated delayed motor development, this patient had motor function in all extremities. The child could push a toy in the standing position, though he had some difficulty transitioning from a supine position to a sitting position.
Patient 6.—
A 5-day-old female neonate who presented with neonatal seizures. Head CT showed intraventricular hemorrhage and diffuse edema. MR imaging was ordered for further evaluation. Initial neurologic examination after resolution of status epilepticus showed hyperreflexia in all extremities and bilaterally increased tone. Follow-up findings were not available for this patient.
Results
Table 1 provides a summary of the age, clinical presentation, and clinical sequelae of each patient, when this information was available. Table 2 lists the involved descending white matter tracts in each case. Table 3 summarizes the T2-weighted, DW, and ADC findings in the descending white matter tracts of the six subjects, as well as the time interval between initial injury and MR imaging.
Initial MR imaging was performed 2–8 days after the initial injury. In all six cases, T2-weighted images showed increased signal intensity in the cortical gray matter and subcortical white matter of the territorial injury. In three cases, no T2 hyperintensity was present in the descending white matter tracts at the level of the internal capsules and cerebral peduncles. In the other three cases, T2-weighted images showed hyperintensity in the ipsilateral descending white matter tract (Figs 1 and 2); this was more clearly apparent on the DW images.
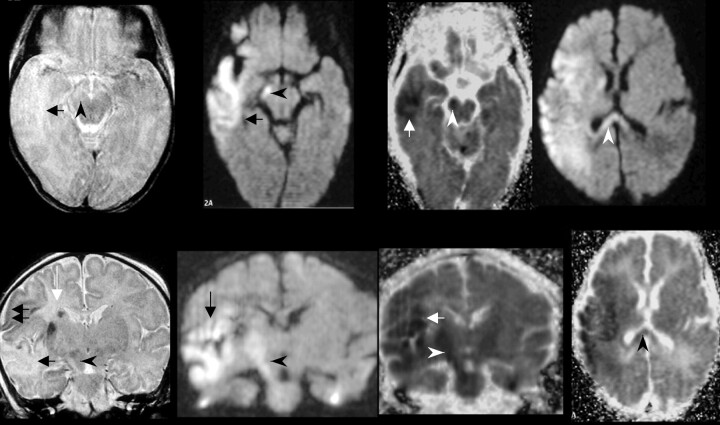
Fig 1.
Patient 1 was a 4-day-old neonate with a right MCA infarct, which occurred 4 days before MR imaging was performed. At 3- and 6-month clinical follow-up, the patient had clinical progression of the left hemiparesis, consistent with the evolution of Wallerian degeneration.
Far left, Transverse (top) and coronal (bottom) fast spin-echo T2-weighted images (5000/96 [TR/TE]) show abnormal signal intensity in the right corticospinal tract (arrowhead). Increased signal intensity is present in the region of the right temporal lobe subcortical white matter (black arrow, top), along with areas of decreased signal intensity in the cortical gray matter (black arrows, bottom). A focus of hemorrhage (white arrow) is seen in the corona radiata.
Middle left, Transverse (top) and coronal (bottom) DW imagess (4000/100) acquired with a spin-echo echo-planar imaging technique show increased signal intensity in the right cerebral peduncle (arrowhead) and subcortical white matter in the territorial infarct (arrow).
Middle right, Corresponding transverse (top) and coronal (bottom) ADC maps and reduced ADC in the right cerebral peduncle (arrowhead) and subcortical white matter in the territorial infarct (arrow).
Far right, Transverse DW image (top) and corresponding ADC map (bottom) show involvement of the splenium of the corpus callosum (arrowhead).
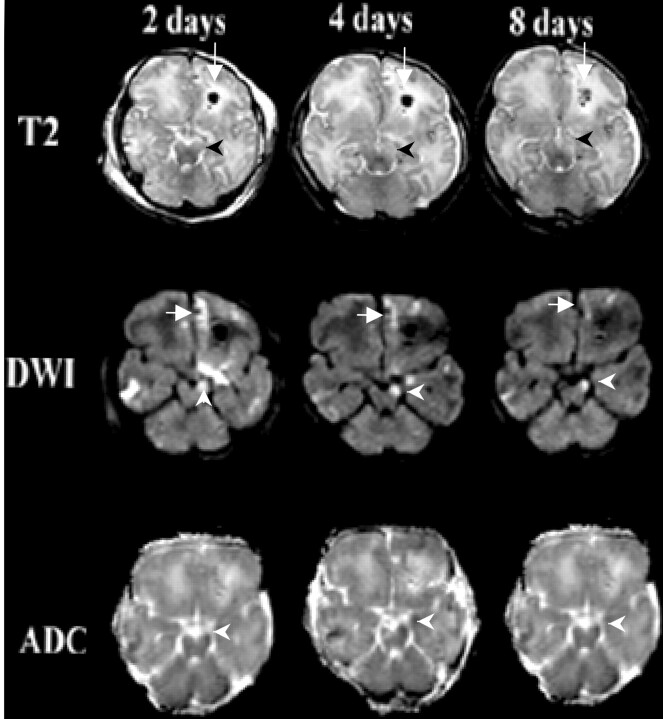
Fig 2.
Patient 2 was an 8-day-old neonate with a skull fracture after forceps delivery. Transverse images obtained at days 2, 4, and 8.
Top row, T2-weighted images acquired with a fast spin-echo technique (5000/96) show an area of hemorrhage with surrounding edema in the left frontal lobe (arrow). At 4 and 8 days, progressive development of the increased signal intensity in the left cerebral peduncle is seen (arrowhead).
Middle row, DW images obtained by using a spin-echo echo-planar imaging sequence (3000/106) show areas of increased signal intensity in the right frontal, right temporal, left frontal lobes, as well as in the left frontopontine tract (arrowhead). Also shown is the area of hemorrhage with surrounding edema in the left frontal lobe (arrow).
Bottom row, ADC maps confirm truly reduced diffusion in the left cerebral peduncle (arrowhead). Interestingly the diffusion changes in the left frontal lobe subcortical white matter become less apparent on days 4 and 8, consistent with ADC pseudonormalization.
In all six patients, DW imaging showed increased signal intensity in the descending white matter tract ipsilateral to the territorial infarct at the level of the internal capsule or cerebral peduncle. In the five patients for which ADC data were available, the ADC values were decreased in the involved white matter tract compared with those in normal white matter. We used the contralateral cerebral peduncle in each patient, as well as data from healthy neonates and infants (12), as reference ADC values. Within the internal capsule, the average ADC for a normal-term neonate was 1.1 × 10−3 mm2/s, whereas the average ADC value for a healthy 5-month-old infant was 0.86 × 10−3 mm2/s. Five of six patients had diffusion changes in the corpus callosum, as well as in the cerebral peduncle (Table 2 and Fig 1).
Patient 2 was enrolled as a part of a prospective study of brain injury at our institution (11). This patient underwent serial examinations at 2, 4, and 8 days after the injury (Fig 2). The initial study on day 2 showed DW signal intensity abnormality in the descending white matter tracts, including the left frontopontine tract as well some fibers of the corticospinal and corticopontine tracts, along with reduced diffusion on the ADC map. Subsequent MR images on days 4 and 8 demonstrated the interval development of a T2-weighted signal intensity abnormality in the left cerebral peduncle. The DW signal intensity changes in the left cerebral peduncle persisted, although a quantitative increase in the ADC value was observed between days 2 and 8.
In all five patients for which follow-up data were available, the presence of DW changes that preceded Wallerian degeneration was correlated with persistent neurologic impairment on follow-up examination (Table 1). In three patients, evidence of unilateral injury was found during DW imaging and clinical examination (Figs 1–3). In these patients, persistent unilateral neurologic deficit was noted at 6 months, 9 months, and 3 weeks. Patient 4 had unilateral corticospinal tract injury on DW images but bilateral neurologic findings, presumably from bilateral cortical and subcortical injury that included the side without the DW abnormality in the corticospinal tract. This patient showed no improvement at 12-month follow-up. Two patients had evidence of bilateral corticospinal tract injury at clinical examination and DW imaging at initial presentation, with persistent neurologic deficits documented in patient 5. Follow-up data were not available for patient 6 (Fig 4).
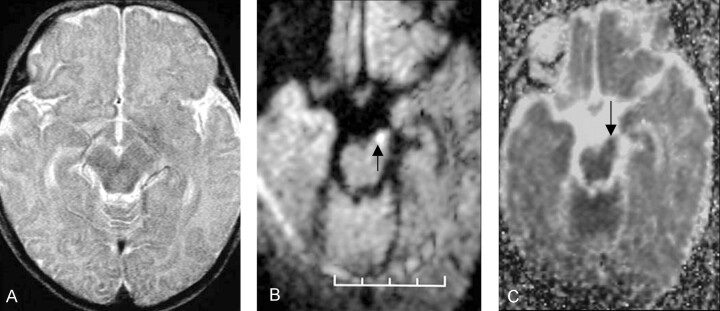
Fig 3.
Patient 3 was an 8-week-old female infant with a history of seizures that started 3 days before MR imaging was performed. DW imaging had shown a left MCA distribution infarct (not shown). The patient had decreased tone in the right arm at the time of her discharge from the hospital 3 weeks after initial injury; the patient’s initial clinical presentation did not change.
A, Normal transverse fast spin-echo T2-weighted image (5000/96) shows the left cerebral peduncle.
B, Transverse DW image acquired with a spin-echo echo-planar imaging technique (4000/100) shows increased signal intensity in the left cerebral peduncle (arrow).
C, Corresponding ADC map demonstrates that the ADC value in the left cerebral peduncle (arrow) is lower that that in the normal right cerebral peduncle.
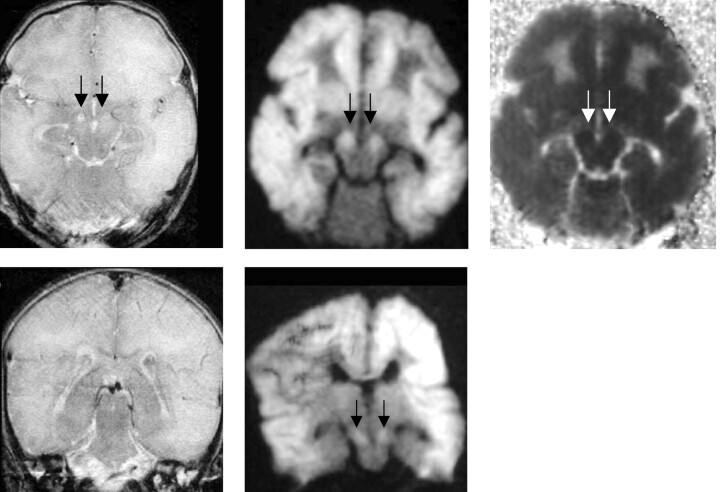
Fig 4.
Patient 6 was a 5-day-old neonate with hypoxic encephalopathy.
A, Transverse turbo spin-echo T2-weighted image (5000/96) shows diffusely increased signal intensity in the subcortical white matter in both cerebral hemispheres, with increased signal intensity in both cerebral peduncles (arrows).
B, Transverse DW image acquired with a spin-echo echo-planar technique (4000/100) shows diffusely increased signal intensity in the subcortical white matter and both cerebral peduncles (arrows).
C, Transverse ADC map confirms the finding shown in B (arrows).
D, Coronal T2-weighted image acquired with a turbo spin-echo technique shows no evidence of corticospinal tract injury.
E, Coronal DW image acquired with a spin-echo echo-planar technique (4000/100) shows bilateral corticospinal tract injury (arrows).
Discussion
We present examples of altered water diffusion in descending white matter tracts within 8 days of acute territorial ischemia, in five neonates and one infant. This preliminary evidence suggests that DW imaging can depict early injury to the descending motor pathways of the maturing human brain. In all six cases, qualitative evaluation of DW images showed increased signal intensity in the projectional white matter, including the internal capsule or the cerebral peduncle or both, which occurred after a neurologic insult to the ipsilateral cerebral hemisphere. In the five cases, quantitative data from the affected cerebral peduncle (when available) showed ADC values lower than those of the contralateral normal-appearing cerebral peduncle or the cerebral peduncle in healthy neonates and infants (12). In the three cases for which 1-year follow-up data were available, a persistent neurologic deficit was present in the distribution of DW changes. In two other cases, a progressive deficit was documented at 9 months in one subject, and at 3 weeks in the other subject. Follow-up clinical information was not available for the remaining case.
Interestingly, patient 4 had conventional MR findings of bilateral subcortical white matter injury that was greater on the left than the right. At follow-up examination, she had persistent bilateral neurologic dysfunction but only left-sided descending white matter tract injury on DW images. These findings suggest that the extent and severity of territorial MCA ischemia are related to the development of descending white matter tract injury detectable by DW imaging. Furthermore, this possibility implies that the correlation between DW findings and long-term neurologic outcomes may not be absolute. A larger prospective study is needed to better define this relationship.
Extensive literature describes the utility of both DW and DT imaging for delineating white matter tracts and for detecting white matter injury in the adult population (13–18). However, compared with the mature brain, the developing brain presents unique diagnostic challenges. Neonatal white matter is either unmyelinated (eg, in the anterior limb of the internal capsule) or only partially myelinated (eg, in the posterior limb of the internal capsule) (19). Hence, myelin breakdown is not as useful a marker for acute white matter injury in the immature brain. Also, the high water content of the immature brain contributes to the very high T2-weighted signal intensity of normal white matter, confounding the detection of disease. Diffusion imaging shows the development of anisotropy in white matter tracts before the demonstration of myelination by means of inversion recovery sequences or histologic analysis (15, 20, 21).
To our knowledge, only seven cases of altered water diffusion in the descending white matter tracts due to acute MCA injury are reported (16–18), and no such reports are specific to the pediatric population. In a series of three patients, Abdullah et al (16) showed that hyperintense DW signal intensity and decreased ADC values are both present within the territorial MCA infarct, as well as the ipsilateral corticospinal tract. The DW signal intensity abnormality in the corticospinal tract is not a part of the infarct itself because it is outside of the involved vascular territory. Castillo and Mukherji (17) conducted a retrospective study of 11 patients aged 1 month to 79 years who underwent DW imaging within 72 hours of acute ischemia. The investigators found that two patients had increased DW signal intensity in the ipsilateral corticospinal tracts. This study was limited in that ADC maps were not generated, and long-term follow-up information was not available to determine who eventually developed conventional MR changes of Wallerian degeneration or long-term neurologic dysfunction. These results suggest that DW changes of acute corticospinal tract injury may occur in only a small minority of territorial infarcts.
Kang et al (18) reported two patients with increased DW signal intensity in the corticospinal tract and elevated ADC values 12 days after a stroke. If the time courses of DW and ADC changes in the corticospinal tracts are similar to their time courses in territorial ischemia, this finding may reflect expected elevation of the ADC in the subacute phase of injury, with T2 shine-through on DW images. However, one of the two patients had increased DW signal intensity in a right MCA infarct, with normal DW findings in the ipsilateral corticospinal tract within 24 hours of the onset of ischemia. Follow-up imaging at 12 days showed the interval development of hyperintense DW signal intensity in the ipsilateral corticospinal tract. This finding indicates that the DW and ADC time courses in the region of territorial injury and the corticospinal tract injury may be different, with relatively delayed development of diffusion abnormality in the descending white matter tracts. Therefore, in their study in which DW imaging was performed within 72 hours of ischemia, Castillo and Mukherji (17) may have underestimated the proportion of patients who eventually develop corticospinal tract injury, as demonstrable with DW imaging.
On the basis of these prior reports, as well as the present study, the finding of high DW signal intensity and reduced ADC in the descending white matter tracts ipsilateral to an MCA infarct should not be mistaken for a second area of infarction. The altered water diffusion in these projectional white matter tracts is presumably the sequelae of MCA ischemia, as the axons of the descending motor pathways have their cell bodies in layer V of primary motor cortex, within the ischemic vascular zone. Results of experimental investigations suggest that decreases in the volume of the extracellular space and a reduction in intracellular water diffusivity are responsible for the decrease in ADC observed in territorial infarction. Studies in neonatal and juvenile rats show that neuronal swelling is correlated with a decrease in the size of the extracellular compartment and with the ischemic ADC reduction accompanying carotid artery occlusion (22, 23). Research in mature rats with [18F]-2-fluoro-2-deoxyglucose-6-phosphate as both an extracellular and intracellular marker has shown approximately equal decreases in extracellular ADC and intracellular ADC due to ischemia (24). These mechanisms that diminish apparent diffusion may also operated within the descending white matter tracts outside of the ischemic vascular distribution. Energy depletion occurring in layer V neurons located in the ischemic zone may eventually lead to failure of ion channel activity in their axolemma, resulting in axonal swelling in the descending white matter tract that reduces the size of the extracellular compartment. However, histologic analysis of ischemic white matter in rats (25) and cats (26) has revealed both intracellular and extracellular accumulation of water in myelinated white matter. Whether this edema also occurs in white matter fibers outside the ischemic territory is unknown. A more important factor in explaining the observed decrease in ADC of the descending white matter pathways may be the cessation of energy-dependent axoplasmic transport processes, resulting in decreased intracellular ADC. One prediction of this hypothesis is that there in diffusion anisotropy of the involved projectional white matter would also be reduced, as measured by means of DT imaging.
The magnitude and time course of the ADC reduction has been quantitatively characterized in adults with territorial infarcts (27) and in term neonates with perinatal brain injury, including territorial infarction (11). However, the ADC response to ischemia may differ among tissue types. In the subacute period after MCA infarction in adults, similar to the 2–8-day interval examined in this study, the ADC reduction in white matter was found to be greater than that in gray matter within MCA infarcts (28). Also, the mechanism of ischemia may influence the changes in water diffusion of the affected tissue. For example, in adults, the ADC reduction in arterial borderzone infarction has been observed to be much more prolonged than that of arterial territorial infarction (29). In the case presented by Kang et al (18), the onset of altered water diffusion in the corticospinal tract was delayed more than 24 hours after the associated MCA infarct; this case indicates that the time course of the DW and ADC abnormalities of this phenomenon may also differ from that of territorial infarction. This observation is supported by our findings in patient 2 (Fig 2), in whom the DW signal intensity abnormality in the cerebral peduncle persisted, even as the DW hyperintensity in the ipsilateral cerebral hemisphere faded.
Axonal injury in the descending motor pathways is a major determinant of the magnitude of the motor deficit in patients with stroke. DW imaging has the potential to depict changes in the descending motor pathways earlier than conventional MR imaging, and thus, DW imaging may aid in the treatment of patients with stroke or head trauma. In a prospective study of 18 patients, Sawlani et al (6) showed that signal intensity changes of Wallerian degeneration, as detected with conventional MR imaging, was correlated with subsequent neurologic disability. Karibe et al (30) examined 28 patients who presented with acute hemiparesis and who were found to have CT findings of intracerebral hemorrhage on admission. Within 24 hours of presentation, conventional MR imaging and DW imaging were performed. DW imaging of the corticospinal tracts was acquired by using two contiguous coronal acquisitions. In this study, DW changes in the corticospinal tracts at the time of initial admission was not correlated with motor function on admission, but a highly significant correlation with upper and lower extremity motor impairment was noted 1 month after the acute injury. This study is limited in that trace DW images were not calculated. Rather, DW images were evaluated separately, with diffusion gradients applied in three orthogonal directions. Also, ADC values were not calculated.
Larger prospective clinical trials are necessary to establish the correlation between diffusion changes in the descending motor pathways and clinical outcomes; this information can serve as a prognostic marker in the setting of pediatric and adult neurologic insults. Although data available in the adult population show that DW changes in the descending motor pathways at presentation are correlated with long-term neurologic function, this relationship has not yet been established for the pediatric population. DW imaging is not sensitive to the full extent of perinatal brain injury in term neonates during the first 24 hours (11), unlike its high sensitivity for hyperacute territorial infarction in adults. Also, the developing brain is more plastic than the mature brain, and therefore, neonates may recover more neurologic function than adults with a comparable insult. Developing brains have less myelin and greater water content, and these characteristics may limit the usefulness of conventional MR imaging techniques in the detection of acute corticospinal tract injury. A TE longer than that used in this study might possibly improve the detection of acute disease in the neonatal brain with T2-weighted imaging, given the increased water content of the neonatal brain. A prospective comparison of DW imaging with conventional MR imaging for the detection of acute projectional white matter injury in neonates should include a T2-weighted sequence with a very long TE.
In this article, we presented six cases in which DW imaging showed acute injury of the descending white matter tract. In three of these cases, conventional MR findings were normal. However, larger prospective studies are required to establish a correlation between DW changes and clinical outcomes in the pediatric population. Other factors may also be correlated with long-term neurologic outcome; these include the mechanism and magnitude of initial injury, the initial clinical neurologic status, the initial extent of cortical damage on CT and MR images, and the presence of damage in eloquent or non-eloquent regions. A larger prospective trial controlling for these factors is necessary to determine if DW imaging is an independent prognostic factor for the long-term neurologic outcome.
Potential avenues of future research include the use of DT imaging. The application of a minimum of six separate diffusion gradients allows the computation of the full DT. From the DT, measures of the magnitude and anisotropy of water diffusion can be derived. DT imaging can provide more complete diffusion information than can traditional DW imaging techniques. Werring et al (31) performed a study in five adult volunteers and five adult patients with MCA infarcts. They found that diffusion anisotropy was reduced in the distal corticospinal tracts 2–6 months after infarction, with normal ADC values. Within the area of territorial MCA infarction, reduced diffusion anisotropy is observed, as is increased ADC. In adult stroke patients more than 1 year after infarction, Pierpaoli et al (32) found that diffusion anisotropy is more sensitive than ADC and T2-weighted signal intensity for detecting changes related to chronic Wallerian degeneration. However, to our knowledge, DT studies of the descending motor pathways in the setting of acute injury have been published, and none specific to the developing brain are reported.
Conclusion
We present six examples of acute injury of the descending white matter tract in the maturing brain of neonates and infants associated with territorial arterial infarction. In all six cases, abnormal diffusion findings were present in the ipsilateral descending white matter tracts, without corresponding T2-weighted signal intensity abnormality in three cases. These DW signal intensity changes likely represent descending white matter tract injury that precedes the development of Wallerian degeneration, as has been reported in the adult population. DW evidence of descending white matter tract injury at initial presentation has been correlated with long-term neurologic disability in adults. If this is also true of the developing brain, DW results could serve as valuable prognostic indicators in neonates and infants with intracranial injury, and they could aid in the selection of patients for early therapeutic intervention or rehabilitation or both.
Acknowledgments
The authors wish to thank Jeffrey J. Neil, MD, PhD, for providing one of the cases described in this article.
References
- 1.Jolesz F, Polak JF, Ruenzel P, Adams D. Wallerian degeneration demonstrated by magnetic resonance: spectroscopic measurements on peripheral nerves. Radiology 1984;152:85–87 [DOI] [PubMed] [Google Scholar]
- 2.Becerra JL, Puckett WR, Hiester ED, et al. MR-pathologic comparisons of Wallerian degeneration in spinal cord injury. AJNR Am J Neuroradiol 1995;16:125–133 [PMC free article] [PubMed] [Google Scholar]
- 3.Daniel PM, Strich S. Histological observations on Wallerian degeneration in the spinal cord of the baboon, Papio papio. Acta neuropath 1969;12:314–328 [DOI] [PubMed] [Google Scholar]
- 4.Pennock JM, Rutherford MA, Cowan FM, Bydder GM. MRI: early onset of changes in Wallerian degeneration. Clin Radiol 1993;47:311–314 [DOI] [PubMed] [Google Scholar]
- 5.Kuhn MJ, Mikulis DJ, Ayoub DM, Kosofsky BE, Davis K, Taveras J. Wallerian degeneration after cerebral infarction: evaluation with sequential MR imaging. Radiology 1989;172:179–182 [DOI] [PubMed] [Google Scholar]
- 6.Sawlani V, Gupta RK, Singh MJ, Kohli A. MRI demonstration of Wallerian degeneration in various intracranial lesions and its clinical implications. J Neurol Sci 1997;146:103–108 [DOI] [PubMed] [Google Scholar]
- 7.Wiklund LM. Hemiplegic cerebral palsy: correlation between CT morphology and clinical findings. Dev Med Child Neurol 1991;33:512–533 [DOI] [PubMed] [Google Scholar]
- 8.Staudt M, Niemann G, Grodd W, Krageloh-Mann I. The pyramidal tract in congenital hemiparesis: relationship between morphology and function in periventricular lesions. Neuropediatrics 2000;31:257–264 [DOI] [PubMed] [Google Scholar]
- 9.Bouza H, Dubowitz L, Rutherford M, Pennock J Prediction of outcome in children with congenital hemiplegia: a magnetic resonance imaging study. Neuropediatrics 1994;25 ;60–66 [DOI] [PubMed] [Google Scholar]
- 10.Neil JJ, Shiran SI, McKinstry RC, et al. Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured Using diffusion tensor MR imaging. Radiology 1998;209:57–66 [DOI] [PubMed] [Google Scholar]
- 11.McKinstry RC, Miller JH, Snyder AZ, et al. A prospective, longitudinal diffusion tensor imaging study of brain injury in newborns. Neurology 2002;59:824–833 [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee P, Miller JH, Shimony JS, et al. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology 2001;221:349–358 [DOI] [PubMed] [Google Scholar]
- 13.Holodny A, Ollenschleger M, Liu WC, Schilder M, Kalnin A. Identification of the corticospinal tracts achieved using blood oxygen level dependent and diffusion functional MR imaging in patients with brain tumors. AJNR Am J Neuroradiol 2001;22:83–88 [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue T, Shimzu H, Yoshimoto T. Imaging the pyramidal tract in patients with brain tumors. Clin Neurol Neurosurg 1999;101:4–10 [DOI] [PubMed] [Google Scholar]
- 15.Rutherford MA, Cowan F, Manzur AY, et al. MR imaging of anisotropically restricted diffusion in the brain of neonates and infants. J Comput Assist Tomogr 1991;15:188–198 [DOI] [PubMed] [Google Scholar]
- 16.Abdullah ND, Phillips MD, Matthews VP, Lowe MJ. Findings of descending motor fiber injury by diffusion-weighted imaging in patients with middle cerebral artery infarction. AJR Am J Roentgenol 1999;172:66 [Google Scholar]
- 17.Castillo M, Mukherji SK. Early abnormalities related to postinfarction wallerian degeneration: evaluation with MR diffusion-weighted imaging. J Comput Assist Tomogr 1999;23:1004–1007 [DOI] [PubMed] [Google Scholar]
- 18.Kang DW, Chu K, Yoon BW, Song IC, Chang KH, Roh JK. Diffusion-weighted imaging in Wallerian degeneration. J Neurol Sci 2000;178:167–169 [DOI] [PubMed] [Google Scholar]
- 19.Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, ed. Regional Development of the Brain in Early Life. Oxford: Blackwell Scientific;1967. :3–70
- 20.Wimberger DM, Roberts TP, Barkovich AJ, et al. Identification of “premyelination” by diffusion weighted MRI. J Comput Assist Tomogr 1995;19:28–33 [DOI] [PubMed] [Google Scholar]
- 21.Prayer D, Barkovich AJ, Kirschner DA, et al. Visualization of nonstructural changes in early white matter development on diffusion weighted MR images: evidence supports premyelination anisotropy. AJNR Am J Neuroradiol 2001;22:1572–1576 [PMC free article] [PubMed] [Google Scholar]
- 22.Miyasaka N, Kuroiwa T, Zhao FY, et al. Cerebral ischemic hypoxia: discrepancy between apparent diffusion coefficients and histologic changes in rats. Radiology 2000;215:199–204 [DOI] [PubMed] [Google Scholar]
- 23.Qiao M, Malisza KL, Del Biggio MR, Tuor UI. Transient hypoxia-ischemia in rats: changes in diffusion-sensitive MR imaging findings, extracellular space, and Na. Radiology 2002;223:65–75 [DOI] [PubMed] [Google Scholar]
- 24.Duong TQ, Sehy JV, Yablonsky DA, Snider BJ, Ackerman JJ, Neil JJ. Extracellular apparent diffusion in rat brain. Magn Reson Med 2001;45:801–810 [DOI] [PubMed] [Google Scholar]
- 25.Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke 1996;13:1641 1646 [DOI] [PubMed] [Google Scholar]
- 26.Kuroiwa T, Nagaoka T, Ueki M, Yamada I, Miyasaka N, Akimoto H. Different apparent diffusion coefficient: water content correlations of gray and white matter during early ischemia. Stroke 1998;29:859–865 [DOI] [PubMed] [Google Scholar]
- 27.Schlaug G, Siewart B, Benfield A, Edelman RR, Warach S. Time Course of the apparent diffusion coefficient (ADC) abnormality in acute stroke. Neurology 1997;49:113–119 [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee P, Bahn MM, McKinstry RC, et al. Differences between gray and white matter water diffusion in stroke: diffusion-tensor MR imaging in 12 patients. Radiology 2000;215:211–220 [DOI] [PubMed] [Google Scholar]
- 29.Huang IJ, Chen CY, Chung HW, et al. Time course of cerebral infarction in the middle cerebral arterial territory: deep watershed versus territorial subtypes on diffusion-weighted MR images. Radiology 2001;221:35–42 [DOI] [PubMed] [Google Scholar]
- 30.Karibe H, Shimizu H, Tominaga T, Koshu K, Yoshimoto T. Diffusion-weighted magnetic resonance imaging in the early evaluation of corticospinal tract injury to predict functional motor outcome in patients with deep intracerebral hemorrhage. J Neurosurg 2000;92:58–63 [DOI] [PubMed] [Google Scholar]
- 31.Werring DJ, Toosy AT, Clark CA, et al. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Psychiatry 2000;69:269–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierpaoli C, Barnett A, Pajevic S, et al. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. NeuroImage 2001;13:1174–1185 [DOI] [PubMed] [Google Scholar]