Abstract
BACKGROUND AND PURPOSE: Coil embolization is safe and effective but may be followed by aneurysm recurrence. Our purpose was to explore the use of alginate as a new embolic agent that could deliver growth factors and improve results of endovascular treatment of aneurysms.
METHODS: We first assessed the potential of alginate as a vector for growth factor delivery by using in vitro binding and elution studies. Lateral wall (n = 68) and bifurcation (n = 4) aneurysms were then constructed in six pigs and 36 dogs. We explored iodine-125 transforming growth factor-β1 in vivo alginate delivery in 16 canine aneurysms. We next assessed the effects of adding alginate to gelatin sponges on angiographic and pathologic results at 3 weeks (n = 4 each) in an established model used for the study of recanalization and recurrence. We then explored techniques to control endovascular alginate delivery without protection (n = 4), with the protection of a balloon (n = 4), and with the protection of a single coil (n = 12) at the aneurysm neck in 12 porcine aneurysms, four canine lateral wall aneurysms, and four canine bifurcation aneurysms. The stability of cross-linked alginate was studied after intraoperative injections in eight aneurysms. Finally, to determine the value of the material with or without growth factor in promoting aneurysm healing, we compared angiographic results and neointima formation 3 weeks after intraoperative embolization of canine lateral wall aneurysms with alginate blocks with or without platelet-derived growth factor-BB or transforming growth factor-β1 (n = 5 each).
RESULTS: Growth factors rapidly eluted from alginate in vitro and in vivo. Alginate coating of sponges led to improved angiographic results and thick neointima formation. Intraoperative alginate block embolization did not lead to recurrence, and growth factors delivered with alginate did not show added benefits. Endovascular alginate embolization was complicated by carotid emboli, and the polymer was unstable once injected, causing delayed neurologic deficits.
CONCLUSION: Growth factor delivery can be performed with alginate, but formulation changes and improved endovascular control are necessary before contemplating its use in intracranial aneurysms.
Endovascular treatment of intracranial aneurysms is most often performed with platinum coils. This alternative to surgery has proved safe and effective during the acute phase after aneurysmal rupture (1). An important drawback of this approach is a higher incidence of recurrence as compared with surgical clipping (2–5). The search for bioactive embolic agents aims at improving long-term results by inhibiting recanalization (6) or by stimulating neointima formation at the neck of treated aneurysms (7).
Alginate is a compound that has been extensively used in cosmetic, dental, and pharmaceutical applications (8–10). More recently, it has also been proposed as an embolic agent for arteriovenous malformations, at least in experimental models (11), and for transcatheter cell grafting strategies and growth factor delivery (12, 13).
We have explored the potential use of alginate as an embolic agent that could deliver, locally, growth factors known to be involved in neointima formation, and, it was hoped, to promote aneurysmal healing after embolization (7).
Methods
Experimental Design
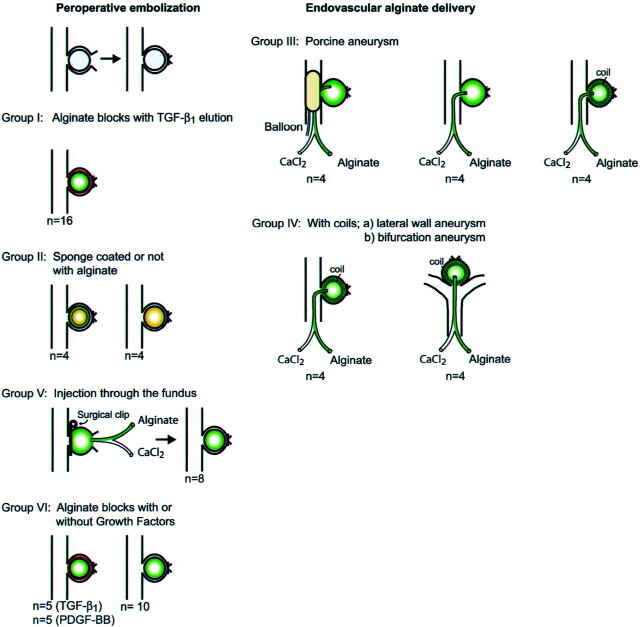
Our first goal was to study the potential of alginate as a vector for the delivery of growth factors. We first assessed growth factor binding efficiency during alginate polymerization and in vitro growth factor elution from alginate blocks after 1 hr to 7 days of incubation in phosphate-buffered saline. The various in vivo experiments that were performed are illustrated in Figure 1. We studied kinetics of iodine-125 transforming growth factor-β1 elution from alginate blocks implanted in lateral wall canine aneurysms (group I, n = 16 aneurysms). Before designing a trans-catheter delivering system, we next wanted to assess the effects of the addition of an alginate coating to gelatin sponges used for embolization on angiographic and pathologic results in a canine lateral wall aneurysm model known for its propensity for recurrences at 3 weeks (group II, n = 8 aneurysms) (14). We then explored two means of controlling trans-catheter delivery of alginate through double lumen microcatheters, first in a porcine lateral wall aneurysm model (group III, n = 12) and then in a canine lateral wall aneurysm model (group IV-A, n = 4) or bifurcation aneurysm model (group IV-B, n = 4) (total number of aneurysms, 20). Alginate delivery was achieved under the protection of a balloon occluding the aneurysm neck in four aneurysms, injection was performed after deposition of a single GDC in 12 aneurysms, and protection was not used in the remaining four aneurysms.
Fig 1.
Illustration depicts groups of animals and experimental models. TGF, transforming growth factor; CaCl2, calcium chloride; PDGF, platelet-derived growth factor.
To assess the stability of cross-linked alginate under high flow conditions, the polymer was intraoperatively injected through the fundus, under the protection of a surgical clip temporarily occluding the neck (group V, n = 8). Finally, to determine the value of local growth factor delivery in aneurysmal healing after alginate embolization, we compared angiographic results and neointima formation 3 weeks after intraoperative embolization of canine lateral wall aneurysms with alginate blocks (group VI), with or without platelet-derived growth factor-BB or transforming growth factor-β1 (n = 5 animals each).
Experimental Methods
Alginate preparation.—
Two percent by weight alginate solutions were prepared by dissolving alginate powder (Boston Scientific Corp., Natick, MA) in 0.5% sodium chloride. Calcium chloride was used as the cross-linking agent.
To determine the reproducibility of calcium chloride polymerization, amino black crystals were added to the alginate solution. Polymerization after adding 200 μL of 5% calcium chloride to 200 μL of 2% alginate solution was assessed by weighing alginate pellets and by measuring the optical attenuation of the calcium chloride excess. This test was repeated 10 times in two different experiments.
Growth factors were added to 200 μL of the alginate solution (150 ng of iodine-125 platelet-derived growth factor-BB or iodine-125 transforming growth factor-β1) (R and D Systems, Minneapolis, MN) before cross linking with 200 μL of 5% calcium chloride in wells of 96-well culture dishes. At least 10 pellets were prepared and incubated at 37°C for 1, 2, 4, and 6 hr and 1, 2, 3, and 7 days in incubation medium. Excess calcium chloride solution from each well was kept frozen for final counting. The alginate pellets were dissolved by the addition of 1 mL of 2% EDTA and then centrifugation at 37°C for 2 hr. For each time point, excess calcium chloride solution after polymerization and dissolved alginate pellets were counted by scintillation counting. Results are expressed as percent of total activity remaining in the alginate pellet. Experiments were performed in triplicate.
The blocks used for in vivo aneurysm embolization were prepared in a similar fashion to the in vitro pellets except using 10% calcium chloride and a 1.5-mL Eppendorf tube as a mold. Growth factors added to the alginate solution were 1 μg of platelet-derived growth factor-BB and 1.5 μg of transforming growth factor-β1. For in vivo elution assays, 150 ng of iodine-125 transforming growth factor-β1 was added to the growth factor alginate solution.
Surgical construction of aneurysms and intraoperative embolization.—
Protocols for animal experimentation were approved by the institutional animal committee in accordance with guidelines of the Canadian Council on Animal Care. Beagles (n = 40) weighing 10 to 15 kg each were sedated with intramuscular injection of acepromazine (0.1 mg/kg), glycopyrrolate (0.01 mg/kg), and butorphanol (0.1 mg/kg) and were anesthetized with IV administered thiopental (15 mg/kg). Six pigs weighing 20 kg each were also used to study endovascular alginate delivery. Animals were ventilated artificially and were maintained under surgical anesthesia with 2% isoflurane. Postoperative anesthesia was provided for 3 days by a 50-μg fentanyl skin patch. Lateral wall aneurysms were constructed on each common carotid artery by a technique previously described (7, 14, 15). Two segments of the same external jugular vein were harvested for construction of the venous pouches. After temporary occlusion of the common carotid artery, an oval 5-mm arteriectomy was performed and the venous pouch was sutured to the arterial wall with 7-0 Prolene. Gelfoam sponges (UpJohn, Don Mills, Ontario, Canada), coated with alginate or not coated, or alginate blocks (polymerized in vitro), with or without growth factors, were inserted from the fundus into the aneurysm to completely occlude it. To study in situ stability of cross-linked alginate delivered by the double lumen microcatheter, the material was injected perioperatively through the fundus, before closure, under the protection of a surgical clip temporarily occluding the neck. In four animals, the calcium chloride solution was labeled with 300 μCi of strontium-85 for scintigraphic studies performed at 3 hr, 1 week, and 3 weeks. The fundus of lateral wall aneurysms measured approximately 8 mm, with a 5-mm neck. Terminal bifurcation aneurysms were constructed in a similar fashion after a T-type bifurcation was created between the two common carotid arteries according to the technique presented by Graves et al (16). Bifurcation aneurysms had a 10-mm fundus and a 5-mm neck. Transfemoral angiography was undertaken immediately after surgery in all animals. During recovery, the dogs were fed with a normal diet, and their activities were not restricted.
Endovascular treatment of lateral wall and bifurcation aneurysms with alginate alone or under the protection of balloons or coils.—
Endovascular alginate embolization was performed immediately after aneurysm construction in porcine aneurysms and at least 2 weeks after aneurysm construction in canine aneurysms. A double lumen microcatheter (Boston Scientific) was specifically designed for the endovascular delivery of alginate and calcium chloride. The two lumens joined into a common channel at the distal end to allow polymerization into a string of alginate that would then exit the microcatheter tip. A handle designed to simultaneously inject the alginate solution and the calcium chloride, at an equal rate, was also provided. Injections were performed slowly, starting at the fundus, with periods of observation, because the inertia of the alginate string often caused an important delay between the end of the manual injection and extrusion of the alginate string. To end the embolization, the trigger on the alginate solution was removed, resulting in injection of calcium chloride only through one of the lumen and cutting of the proximal end of the alginate string still present in the distal common channel. Because injection of alginate without protection at the neck often led to stray emboli, we next explored strategies to improve the safety of endovascular alginate delivery. Lateral wall aneurysms (n = 16 in six pigs and two dogs) were used to compare stray embolization during delivery of alginate without protection (n = 4), delivery under the protection of balloons (n = 4) (Sentry; Target Therapeutics, Freemont, CA), and delivery under the protection of a single intra-aneurysmal GDC (n = 8). Canine bifurcation aneurysms (n = 4) were also used to study alginate injection under the protection of a single intra-aneurysmal coil. For alginate deposition under coil protection, a 0.010-in-caliber platinum coil (7 to 10 mm × 20–30 cm, GDC 10) (Target Therapeutics), depending on aneurysm size, was first positioned inside the aneurysm with one to three loops crossing the neck. Alginate embolization was then performed as described above.
Angiography.—
Surgical embolization of aneurysms and healing at the necks of the aneurysms were assessed in vivo by angiography immediately after embolization and before the sacrifice at 3 weeks after procedure. Results were scored according to a previously described classification (14): 0, complete obliteration; 1, “dog ears”; 2, residual or recurrent neck; and 3, residual or recurrent aneurysm.
Pathology.—
Carotid angiography was repeated in anesthetized animals, before sacrifice by barbiturate overdose, to document the degree of aneurysmal obliteration. The common carotid artery was excised. The wall of the artery was longitudinally opened to expose the luminal surface of the neck of the aneurysm. After fixation, the necks of aneurysms were photographed with an operating microscope. The aneurysms were then sectioned in the axial plane and photographed again before paraffin embedding. Healing phenomena at the necks of aneurysms, at the surface of alginate blocks, or at the surface of sponges and the presence of endothelialized clefts between the embolic agent and the aneurysmal wall were studied after formalin fixation, axial sectioning, and staining with hematoxylin-phloxine-saffron and Movat’s pentachrome stain. Neointimal thickness was measured, when possible, in five different areas of a midaxial section, as described (7). Immunohistochemistry served to characterize neointimal cells and cells inside the sponges at different time intervals after embolization, with antibodies to smooth muscle α-actin and factor VIII (7, 14).
Statistics.—
Angiographic scores were compared by using Wilcoxon’s tests. Mean neointimal thicknesses were compared with Student’s t tests.
Results
Cross Linking of Alginate
In vitro cross linking of alginate with calcium chloride was consistent and reproducible. The average weight of alginate blocks was approximately 0.2164 ± 0.0261 g, and the average optical attenuation was 0.5787 ± 0.1566 μm.
Alginate As a Vehicle for Growth Factor Delivery
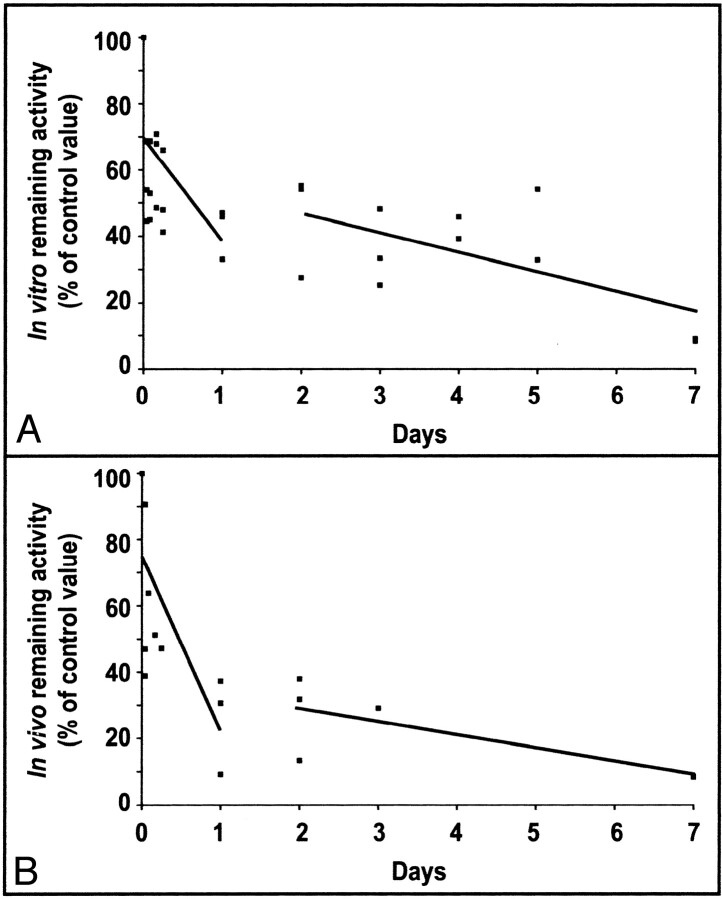
The binding efficiency of alginate for platelet-derived growth factor-BB was very variable (10–50% for a mean of 23%). Because of this wide variability, alginate pellets kept for 5 days in phosphate-buffered saline have a similar range of activities to pellets at time 0, rendering assessment of elution impossible. The binding efficiency of alginate for transforming growth factor-β1 was less variable, with a mean of 63% ± 13%; rapid elution occurred during the first hours, and then a near plateau remained at 22% for 5 days. The transforming growth factor-β1 remaining inside in vitro alginate pellets, expressed as a percent of the value at time 0, is illustrated in Figure 2A.
Fig 2.
In vitro and in vivo clearance of transforming growth factor-β1.
A, Blocks of alginate containing transforming growth factor-β1 were kept in vitro and removed after 10 min to 7 days. The remaining activity was measured by scintillation counting, and the results are expressed as percentages of control activity.
B, Blocks of alginate containing transforming growth factor-β1 were inserted in experimental aneurysms and removed after 10 min to 7 days. The remaining activity was measured by scintillation counting, and the results are expressed as percentages of control activity of blocks kept in vitro for identical time periods. In vitro and in vivo, the clearance of transforming growth factor-β1 occurred in two phases: an initial rapid clearance (slope a) and a second slower phase (slope b).
The elution profile of transforming growth factor-β1 for alginate pellets introduced inside in vivo aneurysms is illustrated in Figure 2B. At 1 and 24 hr, 50% to 80% of delivered transforming growth factor-β1 had eluted from the alginate block. Only 6% remained inside aneurysms at 1 week.
Angiographic Results and Neointima Thickness
Median angiographic scores and neointima thickness for all groups are summarized in Table 1.
TABLE 1:
Alginate, angiographic scores, and neointima thickness
| Group | Aneurysm Type | Embolic Agent | Number of Aneurysms | Median Angiographic Scores |
Neointima Thickness (μm) | |
|---|---|---|---|---|---|---|
| T0 | T3 weeks | |||||
| I | Canine lateral wall | Alginate + 125I/TGF-β | 16 | |||
| II | Canine lateral wall | Gelfoam sponge | 4 | 3 | 3 | 94 ± 90 |
| Canine lateral wall | Sponge + alginate | 4 | 1 | 1 | >1000 | |
| III | Porcine aneurysms | Alginate | 12 | 2.5 | 1 | >1000 |
| IV | Canine lateral wall | Alginate | 4 | 2 | 2.5 | NA |
| Canine bifurcation | Alginate | 4 | 2 | 0 | NA | |
| V | Alginate | 8 | 3 | 2 | 238 ± 226 | |
| VI | Canine lateral wall | Alginate block | 10 | 3* | 1* | 255 ± 142 |
| Canine lateral wall | Alginate block + PDGF-BB | 5 | 3 | 0 | 565 ± 457 | |
| Canine lateral wall | Alginate block + TGF-β1 | 5 | 3 | 2 | 680 ± 543 | |
Note.—125 I indicates iodine-125; TGF, transforming growth factor; PDGF, platelet-derived growth factor.
P = .039
Effects of the Addition of an Alginate Coating on Intraoperative Sponge Embolization
In each animal, one lateral wall aneurysm was packed with a bare sponge and the contralateral aneurysm was packed with the same sponge coated with a layer of alginate. The addition of alginate completely changed the biology of the canine model. Angiographic scores were improved initially and at 3 weeks compared with aneurysms treated with bare sponges (Table 1). Pathologic findings at the necks of aneurysms treated with Gelfoam sponges have been described (14, 15, 17); in brief, the sponge was covered by a thin neointimal layer (mean, 94 ± 90 μm) that was contiguous with recanalizing crescents. Aneurysms treated with alginate-coated sponges no longer showed visible alginate material. Recanalizing crescents were found in one of four specimens and were absent in the others. A thick neointima, continuous with dense collagenous tissue, was found between the arterial lumen and the sponge to such an extent that neointimal thickness could no longer be measured with accuracy. Inflammatory changes were similar for both groups.
Intraoperative Alginate Embolization with Double Lumen Microcatheter
When alginate was injected intraoperatively through the fundus under the protection of a surgical clip at the aneurysm neck, angiography showed a wide residual neck and opacification of a portion of the sac postoperatively. Three of eight animals suffered embolic events 3 to 8 days after surgery. Follow-up angiograms obtained at the time of neurologic deficits documented the presence of emboli within carotid arteries. Pathology showed alginate within these emboli in all cases. Angiographic scores at 3 weeks were improved as compared with initial scores (P = .066) (Table 1). The neointima was present in all specimens and measured a mean of 238 ± 226 μm.
Four canine lateral wall aneurysms embolized with strontium-85-labeled alginate showed a localized area of high gamma activity at the level of the aneurysm that could be clearly followed until the time the animals were killed, 3 weeks after procedure. After 24 hr, no significant activity was observed at the level of the urinary tract or skeleton.
Intraoperative Alginate Block Embolization with or without Growth Factors
When alginate blocks, with or without growth factors, were used for intraoperative embolization of lateral wall canine aneurysms, progressive occlusion of residual lesions occurred with time (see Table 1 and Fig 2). Angiographic scores were significantly better at 3 weeks than initially (P = .039). The alginate block was shown as a well-encapsulated material, unmixed with clot, surrounded by thick fibrotic tissue (Fig 3). The collagenous tissue was again continuous with the neointima that sealed the neck, so that neointimal measurements were sometimes difficult to estimate with accuracy.
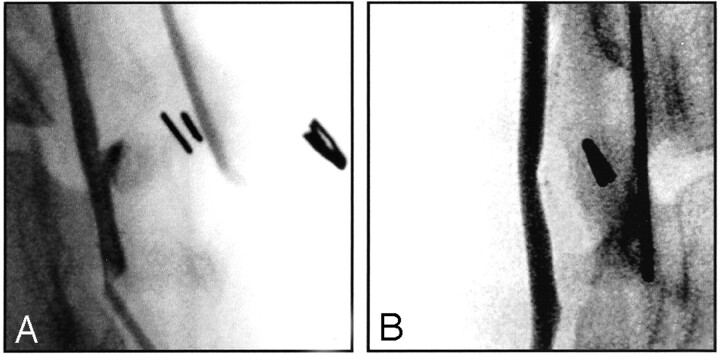
Fig 3.
Angiographic evolution after alginate embolization. Selected view carotid angiograms are shown.
A, Obtained immediately after intraoperative Gelfoam block embolization without growth factors.
B, Obtained 3 weeks after intraoperative Gelfoam block embolization without growth factors. Note progressive obliteration of residual lesion with time.
No significant difference was noted in the angiographic evolution of lesions treated with alginate containing platelet-derived growth factor-BB or transforming growth factor-β1 or with alginate alone (Table 1). The neointimal thickness at the surface of alginate blocks did not show any significant difference between alginate that contained or did not containplatelet-derived growth factor-BB or transforming growth factor-β1.
Endovascular Alginate Embolization of Aneurysms
The injection of alginate with the double lumen microcatheter without any protection at the neck led to carotid emboli in three of four lateral wall porcine aneurysms in which it was attempted, with carotid occlusion occurring in one case. The use of a single coil before alginate injection improved control of alginate embolization in seven of 10 lateral wall aneurysms (eight porcine, two canine). Carotid emboli still occurred in three of 10 when the material reached the necks of the aneurysms (Fig 4). In the more difficult canine bifurcation aneurysm model, the use of a single coil to control alginate delivery failed in two of four aneurysms, leading to neurologic deficits and early sacrifice in two animals.
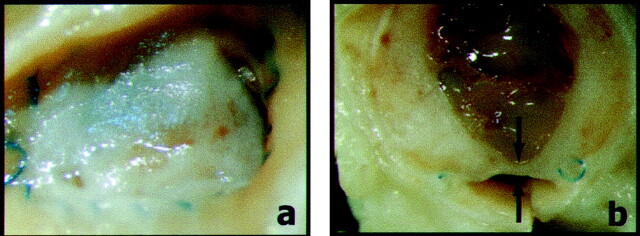
Fig 4.
Neointima formation after alginate embolization. Photographs of neointimal coverage of the neck of the aneurysm are shown.
A, Obtained 3 weeks after intraoperative alginate block embolization, “en face.”
B, Obtained after axial section. Note thick neointima between arrows.
When a balloon was temporarily occluding the neck of lateral wall aneurysms during alginate deposition, embolization was more controlled. However, aneurysm filling was incomplete in two aneurysms. Difficulties were encountered at properly cutting the alginate string at the end of embolization in two cases, leading to protrusion of embolic material inside the carotid artery (Fig 4). Alginate emboli also occurred in one dog 48 hr after successful embolization, leading to a neurologic deficit and early sacrifice. Pathologic studies after endovascular injection of alginate were reminiscent of coil embolization, with fibrous tissue replacement of spaces located between “alginate coils” (Fig 4). Neointima formation at the neck was of variable thickness, contiguous with recanalizing corners, and was thus impossible to measure with accuracy.
No neurologic deficit was observed in any of the pigs after embolization. Control angiography performed 2 weeks after embolization did not show any change in the position of the embolic material that seemed stable once properly deposited inside porcine aneurysms. Pathologic studies revealed the formation of a thick neointima at the surface of the embolic agent (Fig 5). Fibrous tissue was observed between alginate coils. No recanalizing corners were observed.
Fig 5.
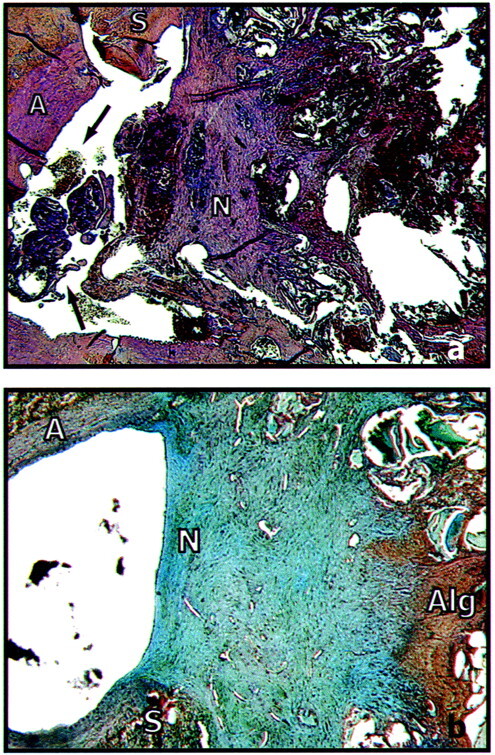
Pathologic examination after alginate embolization.
A, Photomicrophotograp of axial section of canine lateral wall aneurysm obtained 3 weeks after endovascular alginate embolization. Alginate is seen as black material. The neck is covered by neointima of variable thickness. Some alginate covered with neointimal tissue protrudes inside the carotid artery (arrows) (hematoxylin and eosin; original magnification, ×20). S, suture between carotid and aneurysm; a, carotid arterial wall; N, region of the neck of the aneurysm.
B, Photomicrograph of axial section of lateral wall porcine aneurysm obtained 3 weeks after endovascular alginate embolization. Note very thick neointima contiguous with fibrous replacement of clot surrounding alginate coils (Movat’s pentachrome stain; original magnification, ×20). a, carotid arterial wall; N, region of the neck of the aneurysm; Alg, alginate within partially organized clot; S, suture between carotid and aneurysm.
Discussion
Experimental Models
We have used various models to address specific issues of this research program. The search for a sophisticated delivery system may be futile if the candidate embolic agent shows no potential benefits regarding aneurysmal healing after endovascular treatment. We thus think it is useful to explore the angiographic evolution and the pathologic changes after an intraoperative technique that does not necessitate the development of such a delivery system and in which results do not vary with its safety or efficacy. Each model has advantages and drawbacks. The reliability of alginate cross linking, the growth factor binding capacity, and elution profiles of the alginate’s formulation were first tested in vitro. However, growth factor elution from intra-aneurysmal alginate may significantly differ from in vitro data. Because growth factor-containing alginate blocks were prepared in vitro and were intraoperatively introduced into aneurysms, the amount of growth factor introduced inside aneurysms at the beginning of the experiment was precisely known and the radioactivity recovered at various time points did not depend on the success of alginate delivery, which would have been the case had the study been performed by using an endovascular technique.
We extensively studied a perioperative Gelfoam sponge embolization model, which has a propensity to recur in dogs. We had previously studied the effects of growth factors on neointima formation with this model (7). Coating the sponge with the test substance is an elegant way to assess its potential benefits without having to design a specific delivery system (18). Results are difficult to interpret, however, because of the confounding presence of the sponge. An intraoperative embolization strategy using alginate blocks was also used to assess the effects of the addition of growth factors on angiographic results and neointima formation, with minimal variability between embolized aneurysms as compared with endovascular techniques. Certain characteristics of the embolic agents, such as concentration, strength, adhesiveness, and viscosity, can be modified to improve transcatheter delivery but may affect the stability of the agent once it is delivered. Intraoperative injections with a dedicated delivery catheter through the fundus, under the protection of a surgical clip at the neck, are useful to test the stability of the embolic agent once it is reliably polymerized inside the aneurysm.
These models, however, cannot accurately predict the behavior of the embolic agent during transcatheter delivery. To assess the safety and efficacy of the delivery system, lateral wall porcine aneurysm models have frequently been used. These aneurysms have a strong propensity to heal, even when embolization is incomplete (15, 19). Lateral wall canine aneurysms are therefore more appropriate in that respect and permit assessment of protection of the aneurysm necks with balloons. Canine bifurcation aneurysms provide a better evaluation of the embolic agent and delivery system, because they more closely mimic human aneurysms and have a stronger tendency to recur (20).
Alginate for Endovascular Embolization
Endovascular alginate delivery using a double lumen microcatheter was possible, and an alginate “string” was consistently formed and deposited within the aneurysmal lumen. However, because of lack of adhesive properties and because of its softness, the alginate string could be carried by blood flow into the parent artery, a clearly dangerous event in clinical practice. Balloon occlusion of the parent artery and of the aneurysm necks could prevent emboli from straying into the carotid artery at the time of deposition. Complete alginate replacement of the blood trapped inside the aneurysm was difficult to achieve, however, and resulted in only partial embolization of the aneurysms; when more complete embolization was attempted, alginate could still escape into the carotid artery, probably because sealing of the neck by the balloon was imperfect. A perfect and “watertight” seal, probably difficult to achieve in clinical practice, may not be desirable to prevent excessive intra-aneurysmal pressures during injections. The placement of a single coil inside the aneurysm to retain alginate after deposition led to improved control, but because of its softness, the alginate string was able to find its way through the coil loops to escape into the parent vessel in some cases, particularly in high flow bifurcation aneurysms. These difficulties in controlling polymer deposition were also encountered when we attempted to use cyanoacrylate for aneurysmal embolization and may be shared by many polymeric substances (21, 22). Once it protruded or escaped to the parent vessel, the alginate string could sometimes be reaspirated, but the security afforded by the retrievable nature of detachable coils will be difficult to achieve with polymers, in our opinion.
Even if we could deposit alginate inside aneurysms with full control, the stability of the embolic agent was insufficient for safe application. This instability was shown by the experiment using a surgical clip to ensure complete control of alginate deposition (group IV). Once the carotid artery was reopened, blood flow at the aneurysm neck caused fractures in the alginate mass, with delayed embolic complication occurring in three of eight dogs. Clearly, the formulation should be modified to add strength and/or adhesiveness of the polymer (23).
This experiment also emphasized the need to use appropriate animal models to test the safety of embolic materials. None of the porcine aneurysms have shown delayed embolic complication after alginate embolization, an observation that is probably linked to the propensity for extensive thrombosis of this species. The thick thrombus formed at the neck of porcine aneurysms probably prevented blood flow from breaking and carrying the polymeric material. This was not the case in canine aneurysms, and this weakness of the polymer became obvious with the use of this model. We conclude that neither safety nor efficacy of embolic materials can be tested reliably in porcine models.
Healing after Alginate Embolization
We did not study a sufficient number of animals, and the observation period was too short to assess long-term results of alginate embolization or incidence of recurrence. However, alginate coating of gelatin sponges seemed to change the biology of the model in favor of healing at 3 weeks. When alginate was injected intraoperatively or a block was directly introduced, angiographic results improved with time and recanalizing crescents were absent at pathologic examination.
However, endovascular alginate embolization led to the formation of a mass of alginate strings mixed with entrapped clot (Fig 4). Basically, this conformation was similar to coil embolization, with which the clot can be subjected to the recanalization process (24). The endovascular technique used for alginate deposition should be designed to fill as completely as possible the aneurysmal cavity, to prevent recanalization of the clot that could be trapped within or at the surface of the polymeric mass. Again, this could be a difficult goal to achieve with any transcatheter polymeric embolization (20).
Potential Applications for Growth Factor Delivery and in Situ β-Radiation
Growth factor binding and intra-aneurysmal delivery were possible with alginate, but growth factor elution from the polymer was rapid. Kinetics of growth factor delivery with alginate was not significantly different from experiments performed using a Gelfoam sponge as the vehicle (7). Although optimal doses and dose rates of growth factors necessary to improve healing after endovascular treatment remain to be defined, we had hoped for a more sustained delivery. We have previously shown the formation of a thicker neointima with the use of platelet-derived growth factor-BB or transforming growth factor-β1 in porcine aneurysms but no effect on canine aneurysms, which showed very thin neointima and recurrences at 3 weeks (7). Because the tendency for recurrences in our intraoperative model was lost when the Gelfoam sponge was replaced with an alginate block and because of exuberant neointima formation, the potential added value of growth factors could not be shown.
Replacement of the calcium ions used to polymerize alginate by radioactive strontium can easily be performed. We were able to show the feasibility of this strategy with only diagnostic quantities of strontium-85 to dope the calcium chloride solution used for alginate polymerization. Retention of radioactivity within treated aneurysms could be shown up to the time the animals were killed at 3 weeks, indicating the feasibility of alginate embolization as a technique for in situ β-radiation to prevent recanalization (6).
Conclusion
Alginate embolization of aneurysms is currently unsafe, and formulation changes are necessary to increase stability of the polymer. Control of alginate deposition also needs to be refined before clinical application can be considered. Alginate could offer a means to deliver growth factors that could encourage healing phenomenon after embolization, but this potential could not be verified in our animal model. Local in situ β-radiation could be performed using strontium-85 in the calcium chloride solution.
Acknowledgments
We thank Guylaine Gevry for assistance with preparation of the manuscript and artwork.
Footnotes
This work was supported by a grant from Boston Scientific Corporation and was also partially supported by Fonds de la Recherche en Santé du Québec, the Heart and Stroke Foundation of Canada, and Canadian Institutes for Health Research.
References
- 1.Raymond J, Roy D. Safety and efficacy of endovascular treatment of acutely ruptured aneurysms. Neurosurgery 1997;41:1235–1246 [DOI] [PubMed] [Google Scholar]
- 2.Murayama Y, Viñuela F, Suzuki Y, et al. Ion implantation and protein coating of detachable coils for endovascular treatment of cerebral aneurysms: concepts and preliminary results in swine models. Neurosurgery 1997;40:1233–1244 [DOI] [PubMed] [Google Scholar]
- 3.Raymond J, Roy D, Bojanowski M, Moumdjian R, L’Espérance G. Endovascular treatment of acutely ruptured and unruptured aneurysms of the basilar bifurcation. J Neurosurg 1997;86:211–219 [DOI] [PubMed] [Google Scholar]
- 4.Feuerberg I, Lindquist C, Lindquist M, Steiner L. Natural history of postoperative aneurysm rests. J Neurosurg 1987;66:30–34 [DOI] [PubMed] [Google Scholar]
- 5.Lin T, Fox AJ, Drake CG. Regrowth of aneurysm sacs from residual neck following aneurysm clipping. J Neurosurg 1989;70:556–560 [DOI] [PubMed] [Google Scholar]
- 6.Raymond J, Leblanc P, Desfaits AC, et al. In situ beta radiation to prevent recanalization after coil embolization of cerebral aneurysms. Stroke 2002;33:421–427 [DOI] [PubMed] [Google Scholar]
- 7.Desfaits AC, Raymond J. Growth factors stimulate neointimal cells in vitro and increase the thickness of the neointima formed at the neck of porcine aneurysms treated by embolization. Stroke 2000;31:498–507 [DOI] [PubMed] [Google Scholar]
- 8.Roeck-Holtzhauer YG. Uses of seaweeds in cosmetics. In: Guiry MD, Blunden G, eds. Seaweed Resources in Europe: Uses and Potential. Sussex: John Wiley & Sons;1991. :83–93
- 9.Doubleday B. Impression materials. Br J Orthod 1998;25:133–140 [DOI] [PubMed] [Google Scholar]
- 10.Read TA, Farhadi M, Bjerkvig R, et al. Intravital microscopy reveals novel antivascular and antitumor effects of endostatin delivered locally by alginate-encapsulated cells. Cancer Res 2001;61:6830–6837 [PubMed] [Google Scholar]
- 11.Becker TA, Kipke DR, Preul MC, Bichard WD, McDougall CG. In vivo assessment of calcium alginate gel for endovascular embolization of a cerebral arteriovenous malformation model using the swine rete mirabile. Neurosurgery 2002;51 ;453–459 [PubMed] [Google Scholar]
- 12.Abruzzo T, Cloft HJ, Shengelaia GG, et al. In vitro effects of transcatheter injection on structure, cell viability, and cell metabolism in fibroblast-impregnated alginate microspheres. Radiology 2001;220:428–435 [DOI] [PubMed] [Google Scholar]
- 13.Laham RJ, Sellke FW, Edelman ER, et al. Local perivascular delivery of basic fibroblast growth factor in patients undergoing coronary bypass surgery: results of a phase I randomized, double-blind, placebo-controlled trial. Circulation 1999;100:1865–1871 [DOI] [PubMed] [Google Scholar]
- 14.Raymond J, Desfaits AC, Roy D. Fibrinogen and vascular smooth muscle cell grafts promote healing of experimental aneurysms treated by embolization. Stroke 1999;30:1657–1664 [DOI] [PubMed] [Google Scholar]
- 15.Raymond J, Venne D, Allas S, et al. Healing mechanisms in experimental aneurysms: I. vascular smooth muscle cells and neointima formation. J Neuroradiol 1999;26:7–20 [PubMed] [Google Scholar]
- 16.Graves VB, Partington CR, Rufenacht DA, Rappe AH, Strother CM. Treatment of carotid artery aneurysms with platinum coils: an experimental study in dogs. AJNR Am J Neuroradiol 1990;11:249–252 [PMC free article] [PubMed] [Google Scholar]
- 17.Venne D, Raymond J, Allas S, et al. Healing of experimental aneurysms: II. platelet extracts can increase the thickness of the neointima at the neck of treated aneurysms. J Neuroradiol 1999;26:92–100 [PubMed] [Google Scholar]
- 18.Metcalfe A, Desfaits AC, Salazkin I, Yahia L, Sokolowski WM, Raymond J. Cold hibernated elastic memory foams for endovascular interventions. Biomaterials 2003;24:491–497 [DOI] [PubMed] [Google Scholar]
- 19.Byrne JV, Hope JK, Hubbard N, Morris JH. The nature of thrombosis induced by platinum and tungsten coils in saccular aneurysms. AJNR Am J Neuroradiol 1997;18:29–33 [PMC free article] [PubMed] [Google Scholar]
- 20.Raymond J, Berthelet F, Desfaits AC, Salazkin I, Roy D. Cyanoacrylate embolization of experimental aneurysms. AJNR Am J Neuroradiol 2002;23:129–138 [PMC free article] [PubMed] [Google Scholar]
- 21.Macdonald RL, Mojtahedi S, Johns L, Kowalczuk A. Randomized comparison of Guglielmi detachable coils and cellulose acetate polymer for treatment of aneurysms in dogs. Stroke 1998;29:478–486 [DOI] [PubMed] [Google Scholar]
- 22.Murayama Y, Viñuela F, Ulhoa A, et al. Nonadhesive liquid embolic agent for cerebral arteriovenous malformations: preliminary histopathological studies in swine rete mirabile. Neurosurgery 1998;43:1164–1175 [DOI] [PubMed] [Google Scholar]
- 23.Kuo CK, Ma PX. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. structure, gelation rate and mechanical properties. Biomaterials 2001;22:511–521 [DOI] [PubMed] [Google Scholar]
- 24.Raymond J, Sauvageau E, Salazkin I, Ribourtout E, Gevry G, Desfaits AC. Role of the endothelial lining in persistence of residual lesions and growth of recurrences after endovascular treatment of experimental aneurysms. Stroke 2002;33:850–855 [DOI] [PubMed] [Google Scholar]