Abstract
BACKGROUND AND PURPOSE: Whether human rabies of different forms, encephalitic (furious) and paralytic (dumb), share similar MR imaging patterns is unknown. We assessed the diagnostic value of MR imaging in both forms of the disease and compared the clinical and neuroimaging findings.
METHODS: Three patients with paralytic and two with encephalitic rabies were examined during preserved or deteriorated levels of consciousness. Six MR examinations of the brain, three of the spinal cord, and one of the brachial plexus were performed with a 1.5-T superconducting magnet.
RESULTS: No difference was noted between the MR findings in both clinical forms of human rabies. Nonenhancing, ill-defined, mild hyperintensity changes in the brain stem, hippocampi, hypothalami, deep and subcortical white matter, and deep and cortical gray matter were demonstrated on T2-weighted images in the noncomatose patients with rabies. Enhancement along the brachial plexus of the bitten arm was noted in one patient with encephalitic rabies who at that time had only local neuropathic pain symptoms. Enhancement with gadolinium-based contrast material was seen at the hypothalami, brain stem nuclei, spinal cord gray matter, and intradural cervical nerve roots only when the patients became comatose.
CONCLUSION: Both forms of human rabies share a similar MR imaging pattern. Such pattern and the lack of enhancement in a noncomatose patient with suspected encephalitis may differentiate rabies from other viral encephalitides.
Rabies is a uniformly fatal disease caused by neurotropic RNA viruses in the family Rhabdoviridae, genus Lyssavirus (1). Classically, human rabies manifests in either encephalitic (furious) or paralytic (dumb) form (2). Diagnosis is extremely difficult in the case of paralytic rabies and when the patient lapses into coma. Moreover, atypical presentations have been described in patients with bat and dog exposures (2).
There have been only anecdotal reports of neuroimaging in human rabies (3–9). This may be due to the difficulty in performing the study, especially in the case of encephalitic rabies, as well as to a lack of treatment. Preferential involvement of the brain stem, thalamus, and spinal cord has been previously reported in some bat- and dog-associated cases (3, 8). This does not explain the clinical diversity of the encephalitic and paralytic forms or the mental dysfunctions in patients with encephalitic rabies. Moreover, whether such structures are involved at similar stages of consciousness levels in both clinical forms is unknown. We performed MR imaging examinations of the brain and cervical cord and brachial plexus in patients with both forms of rabies. Our purpose was to determine whether there were differences in rabies-related lesions as depicted with MR imaging in patients with either clinical form during the time when they remained alert or arousable as compared with those when they were in a coma and to determine the value of MR imaging in the diagnosis of rabies.
Methods
Five patients with rabies, three paralytic and two encephalitic, were included in this study. All had history of dog bites in Bangkok, Thailand, or neighboring provinces. The incubation period ranged from 3 weeks to 3 months (Table). Partial autopsy was performed in two cases (patients 1 and 5) with only the brain and spinal cord removed, whereas only necropsy via transorbital approach was allowed in the other patients.
Patient data and study details
| Patient No./Age (y)/Sex | Bite Site | Incubation Period | Survival Period (days) | Date of MR Study after Clinical Onset | Consciousness Level | MR Studies Performed |
|---|---|---|---|---|---|---|
| Encephalitic rabies | ||||||
| 1/50/M | L wrist | 7 wk | 7 | Day 3 | Fully conscious | Brain, spinal cord, nerve roots, and brachial plexus, with contrast material (Figs 1, 3) |
| Day 7 | Comatose | Brain and spinal cord, without contrast material (Fig 1) | ||||
| 2/26/F | R leg | 2 mo | 15 | Day 2 | Fully conscious | Brain, with contrast material (Fig 2) |
| Paralytic rabies | ||||||
| 3/43/F | L hand | 3 mo | 9 | Day 4 | Arousable | Brain, with contrast material (no figure) |
| 4/72/F | L leg | 3 mo | 13 | Day 12 | Comatose | Brain and upper cervical cord, with contrast material (Fig 4) |
| 5/70/F | L face | 3 wk | 21 | Day 20 | Comatose | Brain, cervical cord, and nerve roots, with contrast material (Fig 5) |
Diagnosis of rabies was confirmed by means of fluorescent antibody test on brain impression smear and mouse inoculation test and nucleic acid sequence based amplification or reverse-transcriptase polymerase chain reaction technique.
Six MR imaging exmainations of the brain, three of the cervical spinal cord, and one of the brachial plexus and two MR spectroscopic examinations of the brain were performed. Images were obtained in two patients with encephalitic rabies while they were fully conscious, in one patient with paralytic rabies who was still arousable, and in one patient with encephalitic and two with paralytic rabies while they were comatose (Table). The findings in two patients with paralytic rabies were reported previously (3). All MR imaging studies were performed with a 1.5-T Signa MR imager (GE Medical Systems, Milwaukee, WI) at Ramathibodi Hospital, except for patient 3 whose study was performed with a 1.5-T Horizon MR imager (GE Medical Systems) at Chulalongkorn Hospital. Standard T1-weighted (480/24/2 [TR/TE/excitations]), fast spin-echo proton density- or T2-weighted (3500/16-96/2 [effective TE]), and gradient-echo T2-weighted (480–780/20/2, 25° flip angle) sequences were performed with intravenous administration of 0.1 mmol/kg gadolinium (Magnevist; Schering AG, Berlin, Germany). One MR imaging examination in the comatose patient with encephalitic rabies was performed without administration of contrast material. Diffusion-weighted imaging was not performed.
Results
Encephalitic Rabies
Serial MR studies were performed in one patient (patient 1) while he was fully conscious and again while comatose, days 3 and 7 after clinical onset, respectively (Table). In patient 2, MR imaging was performed on day 2 while he was alert. She received 25 g of intravenous human rabies immune globulin (HRIG) 16 hours before this MR imaging study and developed flaccid paralysis of all limbs with total ophthalmoplegia, while remaining alert and rational 36 hours after intravenous HRIG treatment.
Nonenhancing, ill-defined, mild hyperintensity changes involving the brain and spinal cord were demonstrated on the T2-weighted images in these two patients (Figs 1 and 2). Ill-defined enhancement along the left brachial plexus and left intrathecal cervical nerve roots was observed in patient 1 (day 3), who had been bitten at the left wrist (Fig 3). Mild progression of subcortical deep white matter and spinal cord hyperintensity changes on T2-weighted images was seen when comparing the findings from day 3 with those from day 7 in patient 1 (Fig 1).
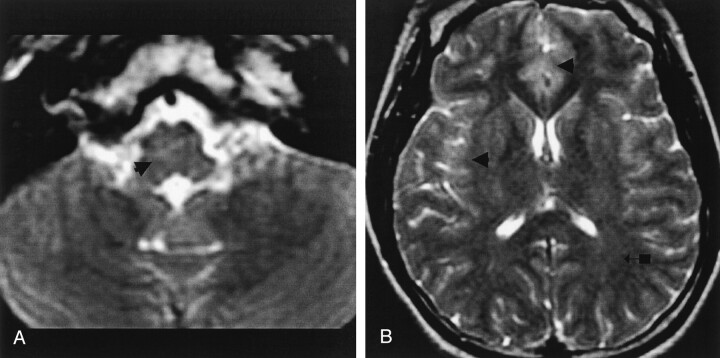
Fig 1.
Patient 1. Encephalitic rabies.
A and B, Coronal fast spin-echo T2-weighted MR images of the brain, and C and D, axial gradient-echo T2-weighted MR images of the cervical cord. A and C were obtained on day 3 and B and D on day 7 after clinical onset. Note slight progression of an ill-defined mild hyperintensity change involving the deep and subcortical white matter (arrow with block in A and double arrows in B), hippocampal gyri (black arrow with circle in A and B), brain stem (arrowhead in A and B), and cervical cord (arrow in C and D).
Fig 2.
Patient 2. Encephalitic rabies.
A and B, Axial fast spin-echo T2-weighted MR images of the brain, after the patient received intravenous HRIG, demonstrate ill-defined hyperintensity changes involving the brain stem (arrow in A), both deep and cortical gray matter (arrowheads in B), and deep and subcortical white matter (arrow with block in B).
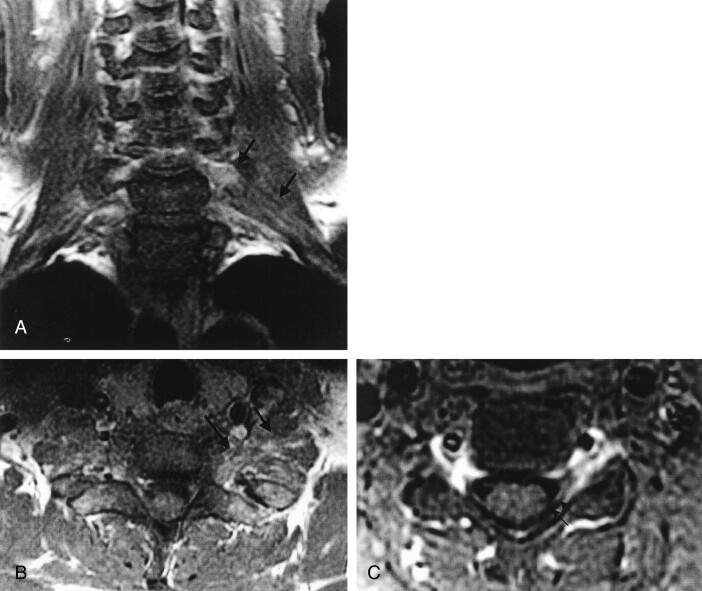
Fig 3.
Patient 1. Encephalitis rabies.
A, Coronal and B, axial gadolinium-enhanced T1-weighted fat-suppressed MR images of the brachial plexi, and C, axial gadolinium-enhanced T1-weighted image of the cervical spine reveal ill-defined, mild to moderate enhancement along the left brachial plexus (arrows in A and B) and intradural left dorsal cervical nerve root (arrows in C).
Paralytic Rabies
Of the three patients with paralytic rabies (Table), patient 3 underwent MR imaging on day 4 after clinical onset. She was drowsy but still communicable at the time of examination. Patients 4 and 5 were imaged while they were comatose (on days 12 and 20, respectively). Nonenhancing, ill-defined, mild to moderate hyperintensity changes involving the deep white matter of the brain including the temporal lobe and brain stem were seen on T2-weighted images (Figs 4A and B, and 5A and B). A prominent anteromedial temporal lobe atrophy with dilated temporal horn and ventricles was seen in patient 5. Mild to moderate enhancement of the hypothalamus, brain stem, and central gray matter of the spinal cord (Fig 4C–E) and cervical nerve roots (Fig 5C) was demonstrated. No meningeal enhancement was noted.
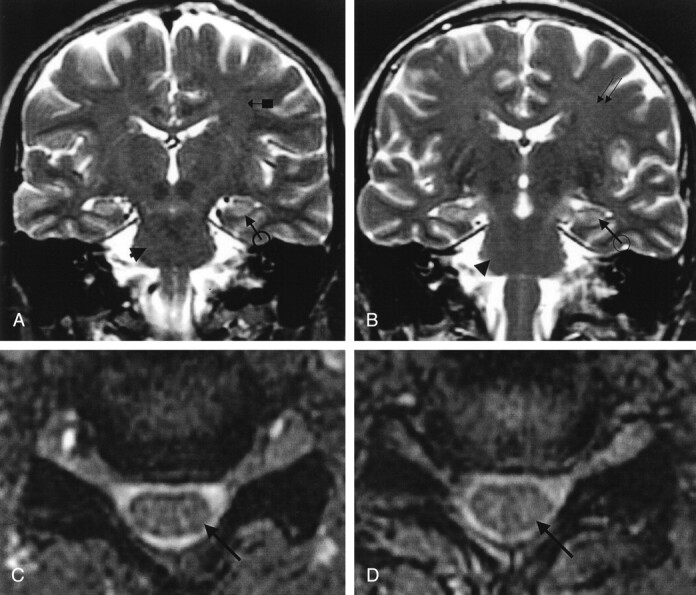
Fig 4.
Patient 4. Paralytic rabies.
A, Coronal and B, axial fast spin-echo T2-weighted images of the brain reveal ill-defined mild hyperintensity change at the posterior right paramedian lower medulla and hippocampi (arrowhead in A and B) and deep white matter of the brain (double arrows in A).
C, Axial and D and E, sagittal gadolinium-enhanced T1-weighted images of the hypothalami, brain stem, and upper cervical cord demonstrate mild to moderate enhancement at the hypothalami (arrow with circle in C and double arrow in D), tectal plate (thick arrow in C and D), periaqueductal gray matter (arrowhead in D), pons and medulla (arrows with block in D), and cervical cord (arrows in E). There is no meningeal enhancement. (Reprinted with permission from reference 3.)
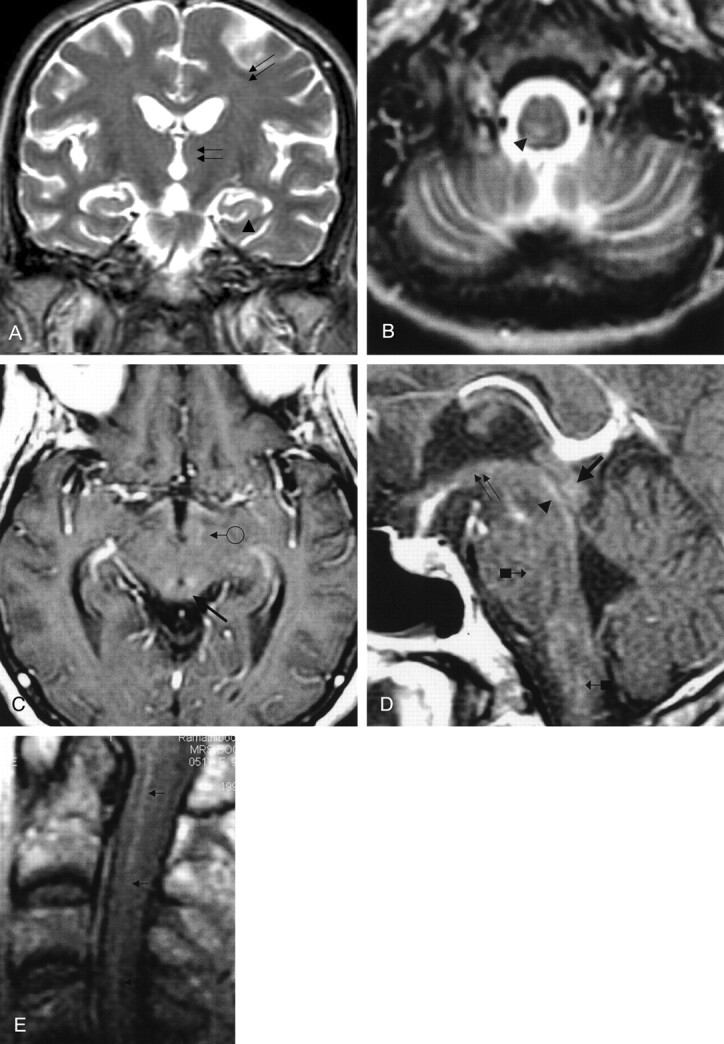
Fig 5.
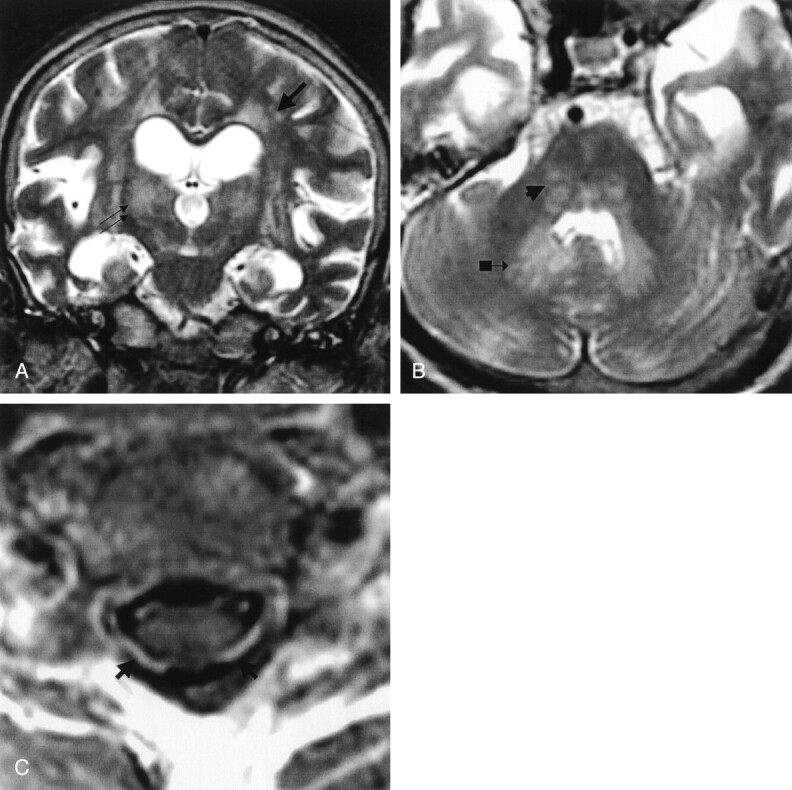
Patient 5. Paralytic rabies.
A, Coronal and B, axial fast spin-echo T2-weighted images of the brain, and C, gadolinium-enhanced axial T1-weighted image of the midcervical cord reveal ill-defined moderate hyperintensity changes of the deep gray matter (double arrows in A), white matter (single arrow in A), and brain stem and dentate nuclei (arrowhead and arrow with block in B). Vivid enhancement of the ventral and dorsal cervical nerve roots (arrows in C) is demonstrated. (Reprinted with permission from reference 3.)
Autopsy Findings
Postmortem studies were performed on the same day as the second MR imaging examination in patient 1 and one day after the MR examination in patient 5 (Table). There were scattered foci of mild to moderate degree of parenchymal infiltration distributed throughout the supra- and infratentorial structures. Dorsal root ganglionitis, particularly at the cervical region, was noted in both cases. Intense inflammation was found in the spinal cord, most marked at the cervical region, in patient 1. Only mild inflammation of the spinal nerve roots of all levels was seen. In patient 5, inflammatory reactions were found predominantly in the spinal nerve roots, especially of the ventral, at the cervical level, but were minimal in the spinal cord. Luxol fast blue stain showed demyelination of spinal nerve roots only in patient 5.
Discussion
After rabies virus is taken up by the unmyelinated nerve ending at the bitten site, the virus reaches the central nervous system (CNS) by retrograde axoplasmic flow (1, 2). The prodromal stage begins when the virus moves from the periphery to dorsal root ganglia (causing neuropathic pain) and to the CNS. Dissemination within the CNS is rapid. Mental or limbic dysfunctions and autonomic stimulation signs can be seen in patients with encephalitic rabies, as well as in some with the paralytic form, but to a much greater degree in the encephalitic group. Mental status alternates between normal periods and progressively more severe agitation and depression. Irritability is gradually followed by deterioration of consciousness and coma. A relative sparing of consciousness with weakness of all extremities dominates the clinical picture in the case of paralytic rabies. The latter phase of rabies cycle involves a centrifugal spread of the virus to organs with highly innervated nerve fibers, particularly the salivary glands. The pathologic manifestation of rabies in the brain and spinal cord and nerve roots is variable from a very mild to severe encephalitis, myelitis, and dorsal root ganglionitis. These findings are nonspecific except for dorsal root ganglionitis, which correlates to local neuropathic pain and a high incidence of segmental demyelination and remyelination in peripheral nerves relative to the incidence of axonal loss in patients with paralytic rabies (10–12).
No association has been found between the two distinct clinical patterns of human rabies and the site of bite, species of responsible vector, presence or absence of history of previous immunization, and the incubation period (2, 12, 13). Similar to prior reports, all rabies cases in our study were associated with dog exposures in Bangkok and nearby provinces; only one patient (patient 5) received postexposure rabies prophylaxis. Leg, upper limb, and face bites in our patients could be associated with either the encephalitic or the paralytic form. Further, the incubation period was variable. We have shown that the rabies virus distribution in the CNS cannot explain this clinical diversity (13). Rabies virus antigen is found mainly in the brain stem, spinal cord, and thalamus, in both forms. Moreover, rabies virus antigen is rarely detected in the hippocampus. All of these suggest a differential functional impairment rather than a direct process of virus infection at a certain region. Patients with intact T cell immunity to rabies virus, with high concentrations of serum interleukin-2 receptor and interleukin-6, die earlier and present with encephalitic rabies, whereas those lacking such responses survive longer and present with paralytic rabies (2). Findings in rodents are similar. Degree of muscarinic acetylcholine receptor functional changes in the brain does not correlate with the amount of virus (14).
Results of this study are in support of the idea of differential functional impairment, in that patients with the paralytic and those with the encephalitic form of rabies had similar MR imaging patterns. Such MR findings were noted in an earlier report of a bat-related rabies case (8) in which the patient had clinical features substantially different from those reported herein. Furthermore, in patient 1 in our study, MR imaging demonstrated abnormal findings in the brain even though the patient did not yet show any cardinal sign of rabies except for local neuropathic pain at the left arm (Figs 1 and 3). Also, by that time, moderate enhancement of the left brachial plexus could be clearly seen. Enhancement was not found in other structures of the brain at that time. This finding may suggest inflammatory reactions in response to the presence of rabies virus during its retrograde axoplasmic flow. Brachial plexus tissue was not obtained for postmortem study; however, there was evidence of dorsal root ganglionitis at the cervical level in this patient. Patient 2, with encephalitic rabies, received a large dose of intravenous HRIG and later developed paralytic complications. There was a prominent ill-defined hyperintensity change in the brain including the cortical gray matter on T2-weighted images in this patient. It is still intriguing how this treatment can exaggerate the clinical severity. Findings of mesial temporal and hippocampal atrophy and ventricular dilatation in patient 5 might be preexistent conditions and not related to the rabies process. Survival time in this case was 21 days, and seizure history was not obtained in any of our patients.
Hypothetical mechanisms in encephalitic rabies, thus, can be proposed (2). Production of proinflammatory molecules results from rabies-infected neuronal processes in the brain stem. These substances, in turn, lead to functional modification of the limbic system and stimulation of the hypothalamic-pituitary-adrenal (HPA) axis. In encephalitic rabies, p55 tumor necrosis factor-α receptors may also be activated. Rabies virus antigen in the CNS is thus recognized. Subsequently, recruitment of immune cells and intensification of limbic symptoms and HPA stimulation follow. Once Vβ-8 T cells are stimulated by rabies-virus nucleocapsid superantigens, these cytokine cascades are reamplified, exaggerating the disturbance of the limbic and sympathetic systems.
During the late or comatose phase, there was mild to moderate enhancement of areas in the brain, brain stem, spinal cord, and dorsal and ventral cervical spinal nerve roots as seen in two patients with paralytic rabies (Figs 4 and 5). Of interest, intense inflammation and demyelination were found in spinal nerve roots in patient 5 that corresponded to enhanced nerve root lesions on MR images (Fig 5C). Such demyelination of the spinal nerve roots was not evident in patient 1, who had encephalitic rabies. This agrees with a previous pathology report (11) and raises the possibility of peripheral nerve injury or demyelination in causing weakness in patients with paralytic rabies. It remains to be seen whether such enhanced nerve root lesions can be found exclusively in patients with paralytic rabies since the beginning of weakness of extremities. An autoimmune phenomenon rather than a bystander effect may be a responsible mechanism, since rabies neutralizing antibody could not be demonstrated in the CSF of the three patients with paralytic rabies reported herein (data not shown),
Because the brain stem appeared to be preferentially involved in the MR findings in both forms of rabies, the differential diagnoses include Japanese encephalitis, eastern equine encephalitis, rhombencephalitis due to various pathogens such as Listeria monocytogenes, herpes simplex virus, adenovirus, and acute hemorrhagic leukoencephalitis, a severe form of acute disseminated encephalomyelitis that may manifest with fever and multiple sclerosis (1, 2, 15–19). The MR imaging findings in Japanese and eastern equine encephalitis were similar to those of rabies in terms of location, but without enhancement. However, a much more prominent nonenhancing hyperintensity change on T2-weighted images was found in the former, with only a mild degree of cortical gray and white matter involvement (15, 16). Foci of hemorrhages were frequently demonstrated on MR images and even on CT scans in the case of Japanese encephalitis (15). This is unusual in rabies (3–9). No evidence of hemorrhage in the brain was found at autopsy in the two patients reported on herein or in another seven patients in our previous report (13). The abnormality seen in rhombencephalitis is composed of more prominent moderate hyperintensity change involving the brain stem and cerebellum with patchy enhancement along the periphery of the involved areas on T2-weighted images (17). The abnormality seen in acute hemorrhagic leukoencephalitis and acute disseminated encephalomyelitis and multiple sclerosis consisted of bilateral symmetrical abnormal hyperintensity changes on T2-weighted images that selectively involved the white matter of the supratentorial structures, cerebellar peduncle, brain stem, and cervical cord (18, 19). Clinical findings in Behcet disease, Wernicke encephalopathy, and Leigh disease may also be confused with rabies, although fever is only evident in the latter. Involvement of basal ganglia and thalamus, absence of predominance of white matter lesions, and involvement of the central part of the pons favor a diagnosis of Behcet disease (19). Hyperintense area around the third ventricle and cerebral aqueduct on T2-weighted and fluid-attenuated inversion-recovery images are characteristics of acute Wernicke encephalopathy (20). A patient with Leigh syndrome had brain stem, cerebellum, and subthalamic nuclei, as well as putaminal lesions (21). However, thalamus and subcortical white matter were spared. Intracranial vasospasm, as previously reported (5), was not evaluated in our patients. In our experience, CT of the brain was insufficient to enable detection of abnormal parenchymal changes in rabies, as reported elsewhere (4, data not shown).
Conclusion
Our results show that the pattern of ill-defined mild hyperintensity changes on T2-weighted images that affect the brain stem, hippocampi, thalami, and white matter in a clinical setting of acute encephalitis can be used to suggest rabies and to differentiate rabies from other mimicking conditions. Enhancement appears only at the coma stage with the exception of brachial plexus, which can be enhanced during the prodromal phase of the disease.
Acknowledgments
We are indebted to the medical and neurology residents, nurses, and technicians at Chulalongkorn University Hospital and at the neuroimaging center at Ramathibodi Hospital for taking care of these patients.
Footnotes
Supported in part by grants from Ramathibodi Foundation, Thai Red Cross Society, and The Thailand Research Fund.
References
- 1.Rupprecht CE, Hanlon CA, Hemachudha T. Rabies re-examined. Lancet Infect Dis 2002;2:327–343 [DOI] [PubMed] [Google Scholar]
- 2.Hemachudha T, Laothamatas J, Rupprecht CE. Human rabies: a disease of complex neuropathogenetic mechanisms and diagnostic challenges. Lancet Neurol 2002;1:101–109 [DOI] [PubMed] [Google Scholar]
- 3.Laothamatas J, Hemachuddha T, Tulayadaechanont S, Mitrabhakdi E. Neuroimaging in paralytic rabies. Rama Med J 1997;20:141–148 [Google Scholar]
- 4.Awasthi M, Parmar H, Patankar T, Castillo M. Imaging findings in rabies encephalitis. AJNR Am J Neuroradiol 2001;22:667–680 [PMC free article] [PubMed] [Google Scholar]
- 5.Sing TM, Soo MY. Imaging findings in rabies. Australas Radiol 1996;40:338–341 [DOI] [PubMed] [Google Scholar]
- 6.Hantson P, Guerit JM, de Tourtchaninoff M, et al. Rabies encephalitis mimicking the electrophysiological pattern of brain death: a case report. Eur Neurol 1993;33:212–217 [DOI] [PubMed] [Google Scholar]
- 7.Roine RO, Hillbom M, Valle M, et al. Fatal encephalitis caused by a bat-borne rabies-related virus: clinical findings. Brain 1988;111:1505–1516 [DOI] [PubMed] [Google Scholar]
- 8.Pleasure SJ, Fischbein NJ. Correlation of clinical and neuroimaging findings in a case of rabies encephalitis. Arch Neurol 2000;57:1765–1769 [DOI] [PubMed] [Google Scholar]
- 9.Desai RV, Jain V, Singh P, Singhi S, Radotra BD. Radiculomyelitic rabies: can MR imaging help? AJNR Am J Neuroradiol 2002;23:632–634 [PMC free article] [PubMed] [Google Scholar]
- 10.Tangchai P, Vejjajiva A. Pathology of the peripheral nervous system in human rabies: a study of nine autopsy cases. Brain 1971;94:299–306 [DOI] [PubMed] [Google Scholar]
- 11.Chopra JS, Banerjee AK, Murthy JMK, Pal SR. Paralytic rabies: a clinicopathological study. Brain 1980;103:789–802 [DOI] [PubMed] [Google Scholar]
- 12.Hemachudha T. Rabies. In: Vinken PJ, Bruyn GW, Klawans HL, eds. Handbook of clinical neurology. Viral Disease. Amsterdam: Elsevier Science Publishers;1989;383–404
- 13.Tirawatnpong S, Hemachudha T, Manutsathit S, Shuangshoti S, Phanthumchinda K, Phanuphak P. Regional distribution of rabies viral antigen in central nervous system of human encephalitis and paralytic rabies. J Neurol Sci 1989;92:91–99 [DOI] [PubMed] [Google Scholar]
- 14.Dumrongphol H, Srikiatkhachorn A, Hemachudha T, Kotchabhakdi N, Govitrapong P. Alteration of muscarinic acetylcholine receptors in rabies viral-infected dog brains. J Neurol Sci 1996;137:1–6 [DOI] [PubMed] [Google Scholar]
- 15.Kalita J, Misra UK. Comparison of CT scan and MRI findings in the diagnosis of Japanese encephalitis. J Neurol Sci 2000;174:3–8 [DOI] [PubMed] [Google Scholar]
- 16.Deresiewicz RL, Thaler SJ, Hsu L, Zamani AA. Clinical and neuroradiographic manifestations of eastern equine encephalitis. N Engl J Med 1997;26:1867–1874 [DOI] [PubMed] [Google Scholar]
- 17.Zagardo MT, Shanholtz CB, Zoarski GH, Rothman MI. Rhombencephalitis caused by adenovirus: MR imaging appearance. AJNR Am J Neuroradiol 1998;19:1901–1903 [PMC free article] [PubMed] [Google Scholar]
- 18.Vartanian TK. Case records of the Massachusetts General Hospital: weekly clinicopathological exercises–case 1–1999. A 53-year-old man with fever and rapid neurologic deterioration. N Engl J Med 1999;340:127–135 [DOI] [PubMed] [Google Scholar]
- 19.Falini A, Kesavadas C, Pontesilli S, Rovaris M, Scotti G. Differential diagnosis of posterior fossa multiple sclerosis: neuroradiological aspects. Neurol Sci 2001;22:S79–S83 [DOI] [PubMed] [Google Scholar]
- 20.Kashihara K, Irisawa M. Diffusion-weighted magnetic resonance imaging in a case of acute Wernicke’s encephalopathy. J Neurol Neurosurg Psychiatry 2002;73:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farina L, Chiapparini L, Uziel G, Bugiani M, Zeviani M, Savoiardo M. MR findings in Leigh syndrome with COX deficiency and SURF-1 mutations. AJNR Am J Neuroradiol 2002;23:1095–1100 [PMC free article] [PubMed] [Google Scholar]