Abstract
Summary: Incontinentia pigmenti is a rare neurocutaneous disorder that may present with neurologic symptoms, in addition to a characteristic vesicular rash within the first days of life. We describe a neonate girl presenting with a rash and an encephalopathy who was first thought to suffer from a viral infection and was only later recognized as being affected by incontinentia pigmenti. Cerebral MR imaging showed extensive cortical necrosis in the acute period. Incontinentia pigmenti should be included in the differential diagnosis of encephalopathy and cutaneous involvement in neonates, after a viral infection has been ruled out.
Incontinentia pigmenti is a rare X-linked dominant phacomatosis that, in 30–50% of cases, affects the CNS (1,2). The diagnosis is usually made when the pathognomonic skin lesions, typically erupting in four stages—erythematous vesicular rash, verrucous patches, swirling hyperpigmentation, and atrophic scarring—are present. Mutations in the NEMO gene, located on Xq28, have been shown to cause this disease (3); a large intragenic deletion is found in 80–90% of cases (4). The role of NEMO is manifold, because it acts via the activation of NF-κB, a transcription factor protecting against apoptosis and controlling immune and inflammatory responses, cell adhesion, and the response to various cytokines (5).
The exact pathogenesis and timing of neurologic involvement are not well known. It often (40%) manifests in the neonatal period with seizures, thus paralleling the eruption of the vesicular rash (1). We present the case history and imaging findings of a female neonate with extensive hemorrhagic cortical necrosis and subcortical white matter involvement. First she was thought to suffer from viral encephalitis, but later, after the typical skin manifestations had developed, was diagnosed with incontinentia pigmenti.
Case Report
An apparently healthy girl was born at term as the first child of consanguineous parents. Pregnancy and birth were normal. On the 2nd day of life, the baby developed recurrent apnoeic spells, prompting transfer to the neonatology intensive care unit, where a polymorphous, partly vesicular, partly maculopapulous rash was noted; the general physical examination was otherwise normal. Neurologic examination revealed muscular hypotonia, absent fixation, and poor sucking. Electroencephalography first showed frequent multifocal seizures and then evolved into a burst-suppression pattern. Treatment with Phenobarbital was started, as was antibiotic and antiviral treatment. CSF studies performed before the start of therapy—including cell count, protein, glucose, culture, and polymerase chain reaction for herpes simplex virus (HSV) types I and II—were normal. Differential blood count including eosinophils was normal. Blood cultures and serum C-reactive protein were negative, and there were no signs of systemic involvement. Ophthalmologic examination was normal. Extensive metabolic investigations did not show abnormalities. MR imaging on the day after presentation showed already extensive cortical necrosis with hyperintense patches in adjacent white matter on T2-weighted images, which suggested viral encephalitis with hemorrhagic necrosis (Fig 1A–D). These lesions did not show contrast enhancement.
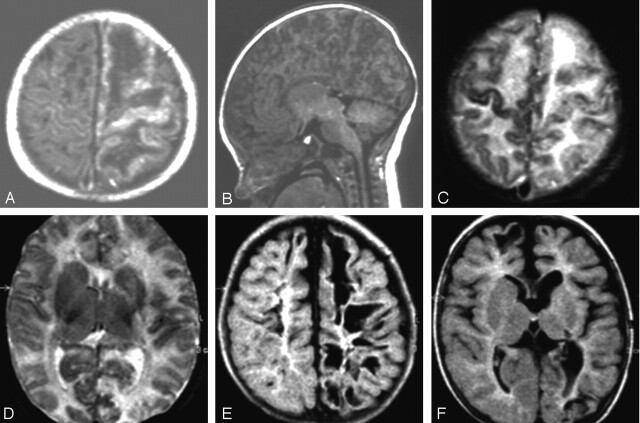
Fig 1.
MR imaging on the 3rd day of life (A–D) and at the age of 5 months (E and F).
A and B, T1-weighted images. The sagittal view (B) illustrates the extensive cortical damage including cerebellar cortex.
C and D, Axial T2-weighted images, demonstrating high signal intensity in the subcortical white matter mainly of the left hemisphere and also signal intensity changes of almost the entire left cortex. On T1-weighted images, these white matter areas appear hypointense (A). E and F, At follow-up, fluid-attenuated inversion recovery images show residual cystic lesions, more on the left than on the right, involving mainly subcortical white matter and also cortical areas. There is no normal signal intensity of myelination. Basal ganglia appear atrophic on the left (F).
The seizures were difficult to treat and—because of recurrent apnoea, intubation, and ventilation—treatment was necessary for 9 days. A second MR imaging scan performed on the 9th day of life showed lesions demarcated more clearly but revealed essentially no new findings. The child became seizure-free under topiramate but showed muscular hypotonia and stimulus-sensitive myoclonus. The skin rash waxed and waned over the following weeks but never completely disappeared.
The child was reviewed at the age of 5 months, when infantile spasms with hypsarrhythmia had developed. Psychomotor development was severely delayed; muscle tone was increased more on the right than on the left side with opisthotonus and pyramidal signs. Head control and fixation were absent, and the child did not grasp objects or turn over. At this time, the rash had transformed into the swirled hyperpigmentations characteristic of incontinentia pigmenti. There were still no retinal abnormalities. The spasms stopped under vigabatrine. Follow-up MR imaging showed residua of the described lesions and, in addition, no progress of myelination (Fig 1E, -F). Careful examination of the mother did not reveal any abnormalities, although she probably suffered from a seizure disorder as a child.
Discussion
Although neurologic involvement, in particular epilepsy, is frequent in older patients with incontinentia pigmenti, severe neurologic symptoms are rare and have been described in only 7.5% of patients in a cohort of 40 children (1). Manifestation of neurologic symptoms, usually with seizures, is in almost half of the cases already within the first weeks of life (1). Our patient presented with an acute encephalopathy in the neonatal period and now shows a severe residual neurologic impairment. The MR imaging supports this clinical evolution: in the neonatal period, it demonstrated extensive acute cortical necrosis and subcortical hemorrhage, later on residual lesions.
We initially suspected a generalized HSV infection presenting as fulminant encephalitis; the MR imaging appearance also favored this diagnosis. The appropriate treatment was initiated. As all investigations remained negative, we had to dismiss this diagnosis. It is interesting to note that two cases of neonates with incontinentia pigmenti and concomitant HSV infection have been described elsewhere (6,7). One of them also presented with an encephalitis-like picture, albeit with a much milder course than our patient. Because in this patient the CSF was also negative for HSV (6), we speculate that, as in our patient, the neurologic symptoms might have been secondary to incontinentia pigmenti and not to HSV infection.
MR imaging findings in older children with incontinentia pigmenti include periventricular leukomalacia (8,9), hypoplasia of the corpus callosum, gray matter heterotopia (9), cerebellar abnormalities (10), encephalomalacia (11), multiple cerebral infarctions (12), and vessel abnormalities (13). In one child, acute occlusion of the right middle cerebral artery at the age of 4 years has been described (14). Patients without neurologic symptoms generally display normal imaging results. The findings compatible with cerebral infarction (12–14) and of a relation between retinal and cerebral vascular involvement (13) especially support the hypothesis that vascular disease plays a major role in the pathogenesis of cerebral lesions and thus neurologic involvement in incontinentia pigmenti.
So far MR imaging findings have been described in six neonates with incontinentia pigmenti: in two children with neonatal presentation, CT and MR imaging results were comparable with those of our patient (15,16), one child showed diffuse cerebral hemorrhagic ischemia mainly involving the central, minus the subcortical white matter (11), and one child had small hemorrhagic infarcts within the periventricular white matter (17). In the latter, MR angiography displayed decreased branching and impaired filling of the intracerebral vessels, thus indicating that vascular abnormalities might be the cause of the cerebral disease. The involvement mainly of the white matter in this case has been explained with the particular vulnerability of this compartment to ischemic insults during the neonate period, but our patient and two other cases described earlier illustrate that injuries to the cortex can be more prominent than those to the white matter. Recently, two sisters with neonatal seizures and involvement of mainly the subcortical white matter at the age of 2 and 3 weeks have been described; in one of them these lesions showed contrast enhancement. Further neurologic development in both appears normal (18). The MR imaging appearance of all these cases including ours is similar to infants with acute infections of the CNS, which suggests that an inflammatory process might also contribute to the cerebral disease of incontinentia pigmenti.
There are only few reports on histopathologic changes in this disorder. In one 3-month-old infant who had presented with neonatal seizures and an accompanying vesicular rash, ulegyria and necrotic regions of the white matter and the adjacent cortex were described. In addition, a diffuse inflammatory process was found with perivascular accumulation of lymphocytes, histiocytes, and eosinophils. Overt vascular abnormalities were absent. The authors argue that the brain might be involved in the specific inflammatory process, which also gives rise to the vesicular skin eruptions (19). In another report of a child who had died of an acute episode of encephalitis at the age of 2.4 years, fresh hemorrhagic necrosis was described (20). A third case displayed unilateral micropolygyria and multiple focal neuronal losses in the cortex (21). The malformations described in children with incontinentia pigmenti—ulegyria and polymicrogyria—could have resulted from ischemic injuries during fetal life.
Conclusion
Altogether, our findings and the published histopathologic changes suggest that, in the pathogenesis of the cerebral lesions of incontinentia pigmenti, an inflammatory process leading to a compromise of microcirculation seem to play an important role. Similar to the pathogenesis of retinal involvement, microvascular abnormalities might also be important, although there is as yet no histopathologic evidence.
As NEMO, whose function is abolished in typical incontinentia pigmenti mutations, is important not only for apoptosis, but also via interleukin-1, for immunologic processes and, via vascular endothelial growth factor receptor 3, for angiogenesis, there is a theoretical basis for this hypothesis (5,22). Strategies to reduce long-term neurologic sequelae must take these factors into account and start early in the neonatal period.
References
- 1.Hadj-Rabia S, Froidevaux D, Bodak N, et al. Clinical study of 40 cases of incontinentia pigmenti. Arch Dermatol 2003;139:1163–1170 [DOI] [PubMed] [Google Scholar]
- 2.Carney RG. Incontinentia pigmenti: a world statistical analysis. Arch Dermatol 1976;112:535–542 [PubMed] [Google Scholar]
- 3.Smahi A, Courtois G, Vabres P, et al. Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti: The International Incontinentia Pigmenti (IP) Consortium. Nature 2000;405:466–472 [DOI] [PubMed] [Google Scholar]
- 4.Aradhya S, Woffendin H, Jakins T, et al. A recurrent deletion in the ubiquitously expressed NEMO (IKK-gamma) gene accounts for the vast majority of incontinentia pigmenti mutations. Hum Mol Genet 2001;10:2171–2179 [DOI] [PubMed] [Google Scholar]
- 5.Smahi A, Courtois G, Rabia SH, et al. The NF-kappaB signalling pathway in human diseases: from incontinentia pigmenti to ectodermal dysplasias and immune-deficiency syndromes. Hum Mol Genet 2002;11:2371–2375 [DOI] [PubMed] [Google Scholar]
- 6.Fromer ES, Lynch PJ. Neonatal herpes simplex and incontinentia pigmenti. Pediatr Dermatol 2001;18:86. [DOI] [PubMed] [Google Scholar]
- 7.Stitt WZ, Scott GA, Caserta M, Goldsmith LA. Coexistence of incontinentia pigmenti and neonatal herpes simplex virus infection. Pediatr Dermatol 1998;15:112–115 [DOI] [PubMed] [Google Scholar]
- 8.Shah SN, Gibbs S, Upton CJ, et al. Incontinentia pigmenti associated with cerebral palsy and cerebral leukomalacia: a case report and literature review. Pediatr Dermatol 2003;20:491–494 [DOI] [PubMed] [Google Scholar]
- 9.Mangano S, Barbagallo A. Incontinentia pigmenti: clinical and neuroradiologic features. Brain Dev 1993;15:362–366 [DOI] [PubMed] [Google Scholar]
- 10.Pascual-Castroviejo I, Roche MC, Martinez F, V, et al. Incontinentia pigmenti: MR demonstration of brain changes. AJNR Am J Neuroradiol 1994;15:1521–1527 [PMC free article] [PubMed] [Google Scholar]
- 11.Fiorillo L, Sinclair DB, O’Byrne ML, Krol AL. Bilateral cerebrovascular accidents in incontinentia pigmenti. Pediatr Neurol 2003;29:66–68 [DOI] [PubMed] [Google Scholar]
- 12.Kasai T, Kato Z, Matsui E, et al. Cerebral infarction in incontinentia pigmenti: the first report of a case evaluated by single photon emission computed tomography. Acta Paediatr 1997;86:665–667 [DOI] [PubMed] [Google Scholar]
- 13.Lee AG, Goldberg MF, Gillard JH, et al. Intracranial assessment of incontinentia pigmenti using magnetic resonance imaging, angiography, and spectroscopic imaging. Arch Pediatr Adolesc Med 1995;149:573–580 [DOI] [PubMed] [Google Scholar]
- 14.Pellegrino RJ, Shah AJ. Vascular occlusion associated with incontinentia pigmenti. Pediatr Neurol 1994;10:73–74 [DOI] [PubMed] [Google Scholar]
- 15.Chatkupt S, Gozo AO, Wolansky LJ, Sun S. Characteristic MR findings in a neonate with incontinentia pigmenti. AJR Am J Roentgenol 1993;160:372–374 [DOI] [PubMed] [Google Scholar]
- 16.Shuper A, Bryan RN, Singer HS. Destructive encephalopathy in incontinentia pigmenti: a primary disorder? Pediatr Neurol 1990;6:137–140 [DOI] [PubMed] [Google Scholar]
- 17.Hennel SJ, Ekert PG, Volpe JJ, Inder TE. Insights into the pathogenesis of cerebral lesions in incontinentia pigmenti. Pediatr Neurol 2003;29:148–150 [DOI] [PubMed] [Google Scholar]
- 18.Porksen G, Pfeiffer C, Hahn G, et al. Neonatal seizures in two sisters with incontinentia pigmenti. Neuropediatrics 2004;35:139–142 [DOI] [PubMed] [Google Scholar]
- 19.Hauw JJ, Perie G, Bonnette J, Escourolle R. [Neuropathological study of incontinentia pigmenti: anatomical case report] (author’s translation). Acta Neuropathol (Berl) 1977;38:159–162 [DOI] [PubMed] [Google Scholar]
- 20.Siemes H, Schneider H, Dening D, Hanefeld F. Encephalitis in two members of a family with incontinentia pigmenti (Bloch-Sulzberger syndrome). The possible role of inflammation in the pathogenesis of CNS involvement. Eur J Pediatr 1978;129:103–115 [DOI] [PubMed] [Google Scholar]
- 21.O’Doherty NJ, Norman RM. Incontinentia pigmenti (Bloch-Sulzberger syndrome) with cerebral malformation. Dev Med Child Neurol 1968;10:168–174 [DOI] [PubMed] [Google Scholar]
- 22.Aradhya S, Nelson DL. NF-kappaB signaling and human disease. Curr Opin Genet Dev 2001;11:300–306 [DOI] [PubMed] [Google Scholar]