Abstract
BACKGROUND AND PURPOSE: The main clinical indication for functional MR imaging (fMRI) has been to preoperatively map the cortex. Motor paradigms to activate the cortex are simple and robust; however, language tasks show greater variability and difficulty. The aim of this study was to develop a language task with an adequate control task to engage the areas of the posterior temporal lobe responsible for sentence comprehension.
METHODS: We performed a cloze paradigm requiring silent reading of a visually presented sentence-completion task based on semantic meaning versus a letter-scanning epoch requiring the completion of nonlinguistic strings or a rest period. Before this task was clinically used in two patients epilepsy and cavernous angioma, its feasibility and accuracy were tested in 14 healthy right-handed participants.
RESULTS: Results showed significant activation of the posterior temporal cortex, including a broad area across the posterior left temporal cortex extending into the inferior parietal lobule. When the sentence completion–minus-letter string task was compared with the sentence completion–minus-rest task, increased activation was present in the posterior temporal lobe.
CONCLUSION: Decreased significant activation during the sentence completion–minus-rest contrast may be attributed to increased noise from intersubject variability in the rest period. Our results suggest that this task elucidates areas important to reading comprehension in the posterior and inferior temporal regions that verbal fluency and auditory discrimination tasks do not. Data from two cases are summarized to exemplify the input of this task for neurosurgery.
The enhancement of neurosurgical procedures is highly dependent on technological developments and support from the scientific community. State-of-the-art equipment has enabled surgeons to diagnose and treat problems with an increased accuracy and to improve surgical outcomes. At the forefront of these new techniques is human brain mapping with functional MR imaging (fMRI). The main clinical use of fMRI has been to preoperatively map the cortex near a brain tumor, arterial venous malformation, cavernous angioma, or seizure focus (1). Results of fMRI studies support the evaluation of cerebral organization as an essential step in predicting the risk of motor or language deficits in patients who have indications for surgical treatment (2). Further, the information that fMRI provides regarding brain plasticity in cerebral reorganization has major implications for surgical decisions.
The standard for functional localization of the eloquent cortex has been direct electrical cortical stimulation, which is an invasive (3, 4) brain-mapping technique that requires anesthesia, but more important, brain area for intraoperative cortical mapping is limited during this procedure. Brain localization with fMRI is highly correlated to that of electrical cortical stimulation (5–9). In addition, fMRI is a noninvasive procedure that engages the whole brain and that maps functions preoperatively and postoperatively. Furthermore, fMRI provides a 3D surgical roadmap that helps neurosurgeons plan procedures before surgery. Intraoperatively, fMRI results help orient the neurosurgeon to both the structural and functional anatomies within the limited view of the craniotomy.
The use of fMRI to localize language areas is complex, as evidenced by no existing one-to-one correspondence between activation and crucial language areas. Also, language paradigms vary in their ability to activate temporal-lobe areas and lateralize function (10, 11). Nevertheless, the fMRI paradigms highlight potentially important language areas for the neurosurgeon.
The purpose of this study was to expand and increase the efficacy of the existing battery of language tasks (12) for presurgical evaluation of patients with temporal lobe epilepsy, especially those with inferior/posterior temporal-lobe abnormalities. This reading comprehension task was designed to specifically focus on functions in the posterior temporal lobe; it involves the angular and supramarginal gyri, the posterior and inferior portions of the temporal gyrus, and other supplementary language areas for reading (13–16).
Because fMRI procedures are based on a subtraction method in which activation blocks are contrasted with control blocks, part of the difficulty in defining a precise language task is the construction of an adequate baseline or control condition. Many language paradigms are designed with rest periods as control epochs. In general, no mechanism is in place to monitor subjects’ thought processes during rest periods. Therefore, subjects may continue thinking, or they partially repeat the active blocks during rest periods, similar to findings reported by Stark and Squire (17).
We hypothesize that a condition requiring visual sentence-completion (18) contrasted to a control condition that requires the processing of letter-strings, will more accurately and effectively identify crucial reading comprehension areas and remove noise elicited by a resting control condition.
Methods
Subjects
Fourteen healthy right-handed participants (six men, eight women; mean age, 31.6 years; age range, 23–49 years) were recruited from the staff of the Long Island Jewish Medical Center, New Hyde Park, NY, and the surrounding community. Participation was restricted to dextral individuals; handedness was determined by means of a modified Edinburgh Handedness Inventory test (19). All subjects were screened to rule out psychological or neurologic disorders. Several patients with epilepsy were also included. (Two are reported in Results.) After completely explaining the study to the subjects, we obtained written informed consent from all. This study was approved by the institutional review board of the Long Island Jewish Medical Center.
Task Design
All subjects underwent a comprehensive battery of language tests, including tasks of verbal fluency, auditory word discrimination, and the investigational sentence-comprehension. Stimuli for the sentence comprehension task were programmed in E-Prime (Psychology Tools, Pittsburgh, PA) and visually presented to the subjects using MR imaging–compatible video goggles (Vision-2000; Resonance Technology, Northridge, CA). The reading activation blocks consisted of a cloze task with a visual display of simple sentences (all uppercase) with a blank space at the end, that required completion based on the semantic meaning of the sentences (e.g., SHE SAW A SHOOTING__). Subjects were asked to silently read each sentence and to retrieve a word to complete the stem based on the meaning of the sentence.
To subtract the nonsemantic components of the sentence-completion task, the control blocks consisted of strings of randomly generated letters (all uppercase) embedded with three consecutive letters of the alphabet following or preceding the blank, where one letter was missing (e.g., SPMQRABC_LFTN). The subjects were asked to silently scan the letter string, identify the three consecutive letters, and silently fill in the missing letter preceding or succeeding the letter string.
Rest blocks of 20 seconds were interleaved with the active sentence-completion and letter-string epochs. During the rest blocks, no other stimuli were presented, other than a blue background on the goggles. Subjects were instructed to keep their eyes open and to look at the blue screen but to try to inhibit any type of response or active thinking.
Before the reading task, a 16-second echo-planar image was acquired for T1 saturation and discarded. Sentences or letters strings were presented at a rate of one every 3 seconds with a 1-second delay between stimuli, and each block consisted of 10 trials. Therefore, a complete run consisted of the 16-second saturation phase, four 40-second sentence-completion blocks, four 40-second letter blocks, and nine 20-second rest blocks, resulting in a total task duration of 8 minutes 36 seconds.
For the subjects to have a complete understanding of the paradigm, practice conditions outside the scanner preceded the actual experiment. None of the practice trials was repeated in the recorded tasks. Gradient-damping headphones reduced the noise from the imaging unit. All subjects underwent routine clinical protocols in addition to the fMRI studies, and neuroradiology staff reviewed images to rule out structural brain abnormalities. On completion of the imaging session, subjects were interviewed to verify that they completed the task.
MR Acquisition Technique
All MR images were obtained by using a unit with a slew rate of 120T/m/s operating at 1.5 T (Horizon EchoSpeed; GE Medical Systems, Milwaukee, WI). fMRIs were acquired by using the blood oxygen level–dependent (BOLD) technique (epibold sequence) in a single-shot gradient-echo planar sequence (TR/TE = 4000/50, 64 × 64 matrix, 20-cm field of view, and band width = 64). This sequence provided 23 contiguous, 5-mm-thick sections in the axial plane parallel to the anterior commissure–posterior commissure line covering the whole brain. The 23 sections were acquired in 128 repeated phases in time. To permit T1 saturation, four complete brain volumes were acquired before the stimulus display was started. A T2-weighted sequence was acquired to cover the exact same section locations as those of the fMRI time series. To increase the accuracy of anatomic localization, T2 images were coregistered with functional images from the echo-planar series. The activation clusters were subsequently superimposed on T2 images to improve anatomic visualization.
Data Analysis
Raw image were automatically transferred from the MR host computer directly to a workstation (Ultra Sparc10; Sun Microsystems, Mountain View CA). The images were then reconstructed by using an off-line Fourier transform program (epirecon; GE Medical Systems) before statistical analysis. All reconstructed images were visually inspected and determined to be free of substantial radio-frequency inhomogeneity and ghosting artifacts and to confirm that images in the healthy participants were devoid of structural brain abnormalities.
SPM software (Welcome Department of Cognitive Neurology, London, England) was used to realign, smooth, coregister, and normalize the functional images. By using a rigid-body transformation, all subsequent images were realigned to the first image of the time series. Each realigned volume was then reconstructed by using sinc interpolation. No subject had translation motion greater than 1.0 mm or rotation greater than 1° in any dimension. Realigned images were then coregistered to high-resolution fast spin-echo images to correct any motion between the functional and structural images. Realigned images were spatially normalized into the common space of the Montreal Neurological Institute template, which has coordinates similar to those of the Talairach and Tournoux atlas (20). Spatial normalization was performed using a 12-parameter series of linear warps and 4 × 5 × 4 nonlinear warps to derive the optimal transformation of the fast spin-echo image to the T2-weighted Montreal Neurological Institute template. These warping parameters were then applied to the realigned and coregistered echo-planar images. Normalized images with 3-mm isotropic voxels were then reconstructed by using sinc interpolation. The normalized images were subsequently smoothed by using an 8-mm full width at half maximum gaussian filter (21). Image postprocessing and analysis was typically completed in 1 hour per subject.
For all analyses, low-frequency, periodic changes in signal intensity related to physiologic noise were eliminated by using a high-pass filter (22). Delayed-boxcar reference vectors were used to model the three types of task blocks (rest, sentence completion, letter string) for SPM analysis. Several contrasts were used to examine the language-related activations: sentence completion − rest, letter string − rest, and sentence completion − letter string. Each voxel was analyzed separately and considered activated if the strength of the regression coefficient on the specified contrast was statistically significant at P < .001, with a corrected extent threshold of P < .05. For the healthy control subjects, data analyses were conducted across the group. For the case studies, analyses were completed separately for each case.
Results
For all healthy control subjects, as well as for the two example patients, we observed no overlapping activation in the inferotemporal and posterotemporal areas among the tasks of verbal fluency, auditory word discrimination, and sentence comprehension. Sentence-comprehension results for each contrast in the healthy participants are discussed in detail, as they set the standard for the expected distribution of activated brain areas in control subjects. Images from two patients epilepsy cases are presented to exemplify the use of the sentence-comprehension task in patients with brain abnormalities. Sample data from healthy subjects are presented in neurologic convention (i.e., right is right) (Fig 1).Individual patient images are displayed in radiologic convention (right is left) (Fig 2).
Fig 1.
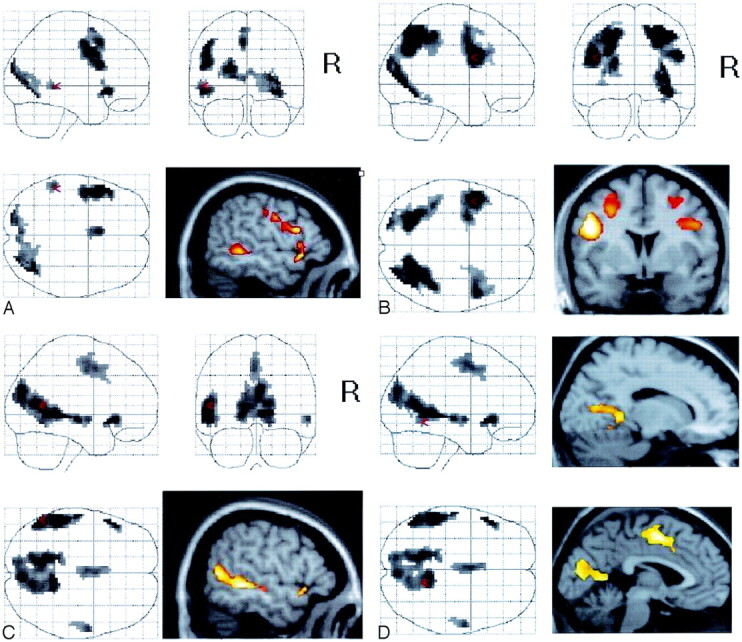
Sample contrasts from healthy subjects.
A, Sentence completion − rest.
B, Letter string − rest.
C, Sentence completion − letter string.
D, Mesial activation areas for sentence completion − letter string.
Fig 2.
Patient contrasts.
A, Case 1. Sentence completion − letter string.
B, Case 2. Sentence completion − letter string.
Healthy Participants
Sentence Completion − Rest
The group analysis of the contrast of sentence completion − rest showed five distinct activation patterns meeting our threshold criteria (Fig 1A, Table 1). We noted strong occipital activation with a large amount of activation in the right occipital lobe extending from the right cuneus (Brodmann area [BA] 18) to the tertiary visual cortex in the right middle occipital gyrus (BA 19). Some of the activation of this group extended inferiorly and medially near the lingual gyrus (BA 19) and posterior cingulate gyrus (BA 30). The activation also spread anterolaterally to the association cortex in the temporo-occipital region (BA 37). In the left hemisphere, the primary, secondary, and tertiary visual cortices were activated in the left cuneus and middle occipital gyri (BAs 17–19). The premotor cortex, representing the frontal eye fields (23), was strongly involved in the left hemisphere, with activation extending posteriorly to the left hemisphere primary motor cortex that controls eye movements (BA 17). Sentence completion − rest produced small activation in a region of the left inferior frontal gyrus (BAs 45 and 47) and a more anterior prefrontal region subsuming BA 9. We also observed a prominent area of activation in the left posterior middle temporal gyrus.
TABLE 1:
MNI coordinates for activations during sentence completion − rest
| Region | X | Y | Z | Z Value | P Value |
|---|---|---|---|---|---|
| Frontal: L inferior frontal gyrus | −51 | 21 | −6 | 7.31 | <.000 |
| Frontal: L inferior frontal gyrus | −48 | 12 | 24 | 7.13 | <.001 |
| Occipital: L cuneus | −18 | −90 | 21 | 7.09 | <.000 |
| Occipital: L occipital | 39 | −66 | −6 | 6.66 | <.000 |
| Occipital: R middle occipital gyrus | 27 | −84 | 9 | 6.59 | <.000 |
| Frontal: L superior frontal gyrus | −6 | 12 | 48 | 6.32 | <.000 |
| Temporal: L middle temporal gyrus | −54 | −39 | −3 | 5.09 | <.005 |
| Frontal: L superior frontal gyrus | −6 | 3 | 63 | 4.91 | <.000 |
| Frontal:L inferior frontal gyrus | −54 | 15 | 3 | 4.18 | <.001 |
Letter String − Rest
The contrast of letter string − rest (Fig 1B, Table 2) also showed five areas of contiguous voxels meeting the threshold criteria. Occipital activation was significant, with more activations in the left occipital than right occipital lobe. Activated areas included the primary and secondary visual cortices in the left cuneus (BAs 17 and 18) and the tertiary visual cortex in the left middle occipital gyrus (BA 19). Activation also extended anteriorly into the parietal cortex, including the left superior parietal lobule, precuneus (BA 7), and supramarginal and angular gyri (BAs 39 and 40). In addition, activation spread bilaterally to more superior parietal areas (BAs 7 and 19) and the inferior temporo-occipital cortex (BAs 19 and 37).
TABLE 2:
MNI coordinates for activations during letter string − rest
| Region | X | Y | z | Z Value | P Value |
|---|---|---|---|---|---|
| Parietal: R superior parietal lobule | 24 | −66 | 51 | 8.30 | <.000 |
| Parietal: L precuneus | −21 | −66 | 51 | 7.97 | <.000 |
| Frontal: L inferior frontal gyrus | −48 | 6 | 30 | 7.76 | <.000 |
| Occipital: R middle occipital gyrus | 27 | −84 | 9 | 7.58 | <.000 |
| Occipital: R middle occipital gyrus | 27 | −84 | 9 | 7.31 | <.000 |
| Parietal: R inferior parietal lobule | 42 | −39 | 48 | 6.97 | <.000 |
| Frontal: R inferior frontal gyrus | 48 | 6 | 30 | 6.85 | <.000 |
| Occipital: L middle occipital gyrus | −24 | −90 | 18 | 6.52 | <.000 |
| Frontal: R middle frontal gyrus | 45 | 24 | 33 | 5.22 | <.000 |
| Frontal: R middle frontal gyrus | 45 | 24 | 24 | 4.47 | <.000 |
In the right hemisphere, activated visual areas included one region of the primary visual cortex in the right cuneus to the middle occipital gyrus (BA 17). The most salient activation was observed in the tertiary cortex of the middle occipital and lingual gyri (19). Activation was also present in the inferior temporo-occipital cortex, including the fusiform gyrus extending laterally and superiorly to the middle occipital cortex (BA 37). In addition, two areas of the frontal cortex were activated. In the left hemisphere, portions of the anterior frontal cortex (BA 9) extending to the posterior superior frontal premotor cortex (BA 6) and homologous regions in the right frontal lobe subsuming BAs 6 and 9. A relatively unique area representing neither the occipital nor frontal cortex was activated in the region of the right inferior parietal lobule (BA 40, angular gyrus).
Sentence Completion − Letter String
Activation included BAs 21 and 22 in the left middle and superior temporal gyri, extending into the angular gyrus in the left inferior parietal lobule (BA 39 [Fig 1C]). A smaller homologous region of activation was observed in the right middle temporal gyrus that did not extend into the parietal cortex.
The left inferior frontal gyrus, including BA 47, was also activated in this condition. We noted activation of left mesial frontal cortex, including the left anterior cingulate (BAs 24 and 32) extending to the medial frontal supplementary motor cortex (BA 6). The right hemisphere cortex was not activated in either of the lateral frontal or mesial frontal regions.
In addition to these mainly left temporal areas, the condition of sentence completion − letter string produced significant and unique activation in visual cortex. We observed activation in the mesial primary and secondary cortical regions in the left cuneus (BAs 18 and 19). The activation spread anteriorly to include the posterior cingulate cortex (BA 32). Activation was present in the right occipital cortex, including the right cuneus (BA 18). As in the left hemisphere, this activation involved more of the anterior mesial cortex, including the right posterior cingulate (BAs 31 and 32) and the lingual cortex (BA 19 [Fig 1D]).
Patient Studies
A neuropsychologist (K.P.) followed the two subjects with interviews, communication with neurosurgeons, and documentation from their medical records. The activation results of the contrast of sentence completion − letter string were superimposed on the patients’ high-resolution structural images acquired at the same location as their BOLD images.
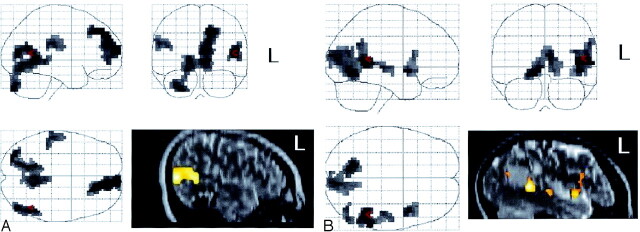
Case 1.— A right-handed 16-year-old male adolescent who presented with seizures underwent a CT and subsequent MR revealing a cavernous angioma in the left posterior temporal lobe. The patient had been receiving antiepileptic medications. fMRI during the sentence-comprehension task results showed no activation in and around the lesion. Activation with sentence-completion − letter string suggested that the angioma posteriorly displaced a region involved in reading (Fig 2A). The glass brain in Fig 2A shows that sentence completion − letter string produced bilateral activation of the temporal lobes, as well as extensive frontal activation.
Results of the battery of language tasks were reported to the neurosurgical team. Frameless stereotactic surgery was performed, and the cavernous angioma was excised. Nine months after the surgery, an interview of the patient elicited no evidence of seizure activity. The patient had no deficits in his visual fields or difficulty reading or performing his schoolwork.
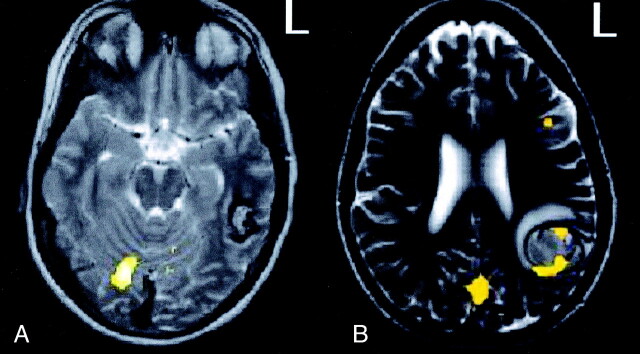
Case 2.—A 26-year-old right-handed woman had epilepsy caused by a cavernous angioma in the left inferior parietal region. The patient was taking various antiepileptic medications. Fig 2B shows the results with sentence completion − letter string. The glass brain in Fig 2B shows only left temporal activation and little frontal activation for sentence comprehension. Unlike the previous patient (Fig 3A), this patient had left-lateralized clusters of activation in and around the area of the cavernous angioma (Fig 3B).
Fig 3.
Activation with sentence completion − letter string. Image for case 1 (A) shows no area of activation around the cavernous angioma, whereas that for case 2 (B) shows clear involvement.
Several days after the fMRI procedure, the patient had hemorrhage and underwent emergency surgery for evacuation of the hematoma and resection of the cavernous angioma. After surgery, she had anomia, mild alexia, and agraphia. Two weeks after the operation, she was admitted to the emergency department with Wernicke aphasia. Imaging revealed no new hemorrhaging, but EEG showed partial status epilepticus. Three years after surgery, she continued to have mild deficits, especially in complex reading.
Discussion
This reading-comprehension task activated the left posterior temporal lobe. The sentence completion − letter string contrast produced more activation in this area of interest than the sentence completion − rest contrast. This finding supports the hypothesis that a paradigm involving a letter-string task isolates the effects of sentence reading moreso than a paradigm relying on rest periods as contrast. In case 2, the sentence-comprehension task activated areas around the cavernous angioma in the inferior and posterior temporal lobe, whereas verbal-fluency and auditory word-discrimination tasks did not.
In general, at least two processes are required to perform a reading task. First, the subject must visually read the sentence; second, he or she must comprehend its meaning. On the basis of known brain behavior and according to the literature on electrical cortical stimulation, the first process should involve inferior temporo-occipital regions involved in the visual processing of the words and in sentence comprehension mediated by the posterosuperior temporal regions (13, 16, 24, 25).
The regions activated in this study corresponded to these activities. An extensive area of activation was seen in the left posterior temporo-occipital and parieto-occipital regions. These areas, which include BAs 37 and 40, overlap with regions from other functional reading studies that show activation when verbal comprehension of visually presented material is required (10, 13, 14). The extension of the activated region is consistent with findings from functional imaging studies by Just et al (26), who showed activation in BAs 20 and 21 for reading and identifying words, lexical processes involved in reading, and answering questions requiring accurate verbal comprehension (26). The participation of the posterosuperior temporal and inferior parietal cortex during sentence reading and comprehension is also supported by results of fMRI studies; the findings show prominent activation in these regions for word recognition, regardless of input technique (26–28), and semantic comprehension independent of visual or phonologic characteristics of the stimuli presented (26, 29).
The sentence-comprehension task requires the subject to use the process of word retrieval. This type of word retrieval is mediated by regions in the superior, middle, and inferior temporal lobes (13, 14, 16), as well as areas extending to BA 37 in the temporo-occipital region (13, 14, 27). The areas activated during this task closely correspond with the nodes of reading and word retrieval, as Schwartz et al showed in electrical cortical stimulation studies (4). Therefore, the previous literature has not differentiated the areas used for visual reading, reading comprehension, and word retrieval. Similarly, the current sentence-comprehension task did not isolate these processes. The identification of cortex needed for neurologically important activities is the aim of presurgical planning, whereas the differentiation of specific areas for language processes holds little utility. Our findings suggest that subtraction methods based on only a rest period may minimize areas important in clinical evaluation.
Several other areas of activation were localized to the basal temporal cortex. Luders et al (30) first described the basal temporal language area by presenting results of cortical stimulation in the basal aspect of the inferior temporal gyrus, fusiform gyrus, and parahippocampal gyrus. The reading disturbance included speech slowing, complete speech arrest while subjects read aloud, and an inability to identify or comprehend written words, though visual perception of the words was normal. In addition, studies of PET (31) and event-related potentials (32) show a contribution of the fusiform gyrus in reading; the basal temporal language areas may be similar to premotor or supplementary motor areas that can tolerate resection without permanent deficit. Activation in the basal temporal area was present in both the sentence completion − letter string and the sentence completion − rest contrasts. Activation in the former suggests that the letter-string condition does not overlap crucial reading comprehension functions that are present in the sentence-comprehension condition (Fig 1).
In addition, activation was present in the precentral gyrus region, mediating eye movements, and the frontal eye fields attributed to the directed saccades and the foveation of targets, is evident. These areas are well reported in the fMRI and lesion literature (33–38) to be crucial in providing the initial sensory and perceptual input to the lexical and semantic processing regions of the left hemisphere that mediate reading comprehension. The visually presented sentences are reported to involve several posterior sites in the left hemisphere, including the angular gyrus, supramarginal gyrus, and fusiform gyrus in the occipitotemporal region (39, 40).
An area of activation also suggested participation of the supplementary motor area or cingulate in reading. The left supplementary motor area is known to be activated when initiation of a linguistic task is required; therefore, the activation in this region is not unanticipated. Moreover, the cingulate activation is consistent with several reports of similar activation (41).
When the visual demands of the tasks were controlled by subtracting the letter-string condition (sentence completion − letter string), the only areas that were activated were those mediating visual processing (BAs 18 and 19), with possible anterior spread into the mesial aspect to the posterior cingulate (BA 30). Although the sentence completion − rest contrast resulted in significant frontal activation, the sentence completion − letter string contrasts largely diminished the effects, which emphasized the fact that the sentence completion − letter string paradigm taps into the posterior temporal regions.
Of note, the current study had multiple limitations. The two cases presented are insufficient for drawing valid results and conclusions. In case 2, numerous factors confound interpretation of the results. The patient underwent emergency surgery and may have had an ongoing seizure disorder; therefore, we cannot tell the extent to which the deficits were due to hemorrhage, seizure disorder, or resection. Studies in additional patients are needed to deduce whether the task has direct clinical implications for surgical decisions and outcomes. Also, included only right-handed individuals; therefore, our results cannot be generalize to left-handed people. Furthermore, additional tasks are needed to comprehensively capture language processing. On a methodologic basis, further research is necessary to determine whether processes of visual reading, reading comprehension, and word retrieval can be isolated using more discriminate tasks. Of importance, future research needs to correlate interoperative maps with results from fMRI tasks. fMRI paradigms with rest periods might include additional control epochs for subtraction procedures.
Conclusion
The sentence-comprehension task with both a rest period and a letter-string subtraction method activates posterior temporal areas for language processes more comprehensively than other fMRI paradigms, such as verbal fluency and auditory word-discrimination tasks. For the aims of clinical utility, our results suggest that all of the contrasts of the sentence-comprehension task can be informative for mapping significant neurologic activation.
TABLE 3:
MNI coordinates for activations during sentence completion − letter string
| Region | X | Y | Z | Z Value | P Value |
|---|---|---|---|---|---|
| Temporal: L middle temporal gyrus | −54 | −39 | −3 | 7.31 | <.000 |
| Frontal: L inferior front gyrus | −51 | 21 | −6 | 6.79 | <.001 |
| Occipital: L lingual gyrus | −15 | −51 | 0 | 6.57 | <.000 |
| Temporal: L middle temporal gyrus | −54 | −60 | 9 | 6.55 | <.000 |
| Anterior: R culmen | 6 | −45 | 0 | 6.35 | <.000 |
| Limbic lobe: L posterior cingulate | −12 | −57 | 6 | 6.30 | <.000 |
| Temporal: L middle temporal gyrus | −60 | −18 | −6 | 5.97 | <.000 |
| Frontal: L medial frontal gyrus | 0 | 0 | 51 | 5.10 | <.000 |
| Temporal: R middle temporal gyrus | 60 | −6 | −6 | 5.04 | <.002 |
| Frontal: L cingulate gyrus | −3 | 15 | 36 | 3.55 | <.000 |
| Temporal: R middle temporal gyrus | 54 | −18 | −9 | 3.50 | <.002 |
Footnotes
Supported in part by grants from the National Institute of Mental Health (MH-60374-01) and a Faculty Award (to M.A.) from the Long Island Jewish Medical Center.
Presented in part at the conferences of the Organization for Human Brain Mapping, Sendai, Japan, June 2–7, 2002, and the 10th International Society of Magnetic Resonance Imaging, Honolulu, Hawaii, May 17–23, 2002.
References
- 1.Atlas S, Howard R, Maldjian J, et al. Functional MRI of regional brain activity in patients with intracerebral gliomas: findings and implications for clinical management. Neurosurgery 1996;38:329–338 [DOI] [PubMed] [Google Scholar]
- 2.Baciu M, Le Bas JF, Segebarth C, Benabid AL. Presurgical fMRI evaluation of cerebral reorganization and motor deficit in patients with tumors and vascular malformations. Eur J Radiol 2003;46:139–46 [DOI] [PubMed] [Google Scholar]
- 3.Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere: an electrical stimulation mapping investigation in 117 patients. J Neurosurg 1989;71:316–326 [DOI] [PubMed] [Google Scholar]
- 4.Schwartz TH, Devinsky O, Doyle W, Perrine K. Function-specific high-probability nodes identified in posterior language cortex. Epilepsia 1999;40:575–583 [DOI] [PubMed] [Google Scholar]
- 5.FitzGerald DB, Cosgrove GR, Ronner S, et al. Location of language in the cortex: A comparison between functional MR imaging and electrocortical stimulation. AJNR Am J Neuroradiol 1997;18:1529–1539 [PMC free article] [PubMed] [Google Scholar]
- 6.Yetkin FZ, Mueller WM, Morris GL, et al. Functional MR activation correlated with intraoperative cortical mapping. AJNR Am J Neuroradiol 1997;18:1311–1315 [PMC free article] [PubMed] [Google Scholar]
- 7.Rutten GJ, van Rijen PC, van Veelen CW, Ramsey NF. Language area localization with three-dimensional functional magnetic resonance imaging matches intrasulcal electro-stimulation in Broca’s area. Ann Neurol 1999;46:405–408 [DOI] [PubMed] [Google Scholar]
- 8.Schlosser MJ, Luby M, Spencer DD, et al. Comparative localization of auditory comprehension by using functional magnetic resonance imaging and cortical stimulation. J Neurosurg 1999;1:626–635 [DOI] [PubMed] [Google Scholar]
- 9.Lurito JT, Lowe MJ, Sartorius C, Mathews VP. Comparison of fMRI and intraoperative direct cortical stimulation in localization of receptive language areas. J Comput Assist Tomogr 2000;24:1j99–105 [DOI] [PubMed] [Google Scholar]
- 10.Deblaere K, Backes WH, Hofman P, et al. Developing a comprehensive presurgical functional MRI protocol for patients with intractable temporal lobe epilepsy: a pilot study. Neuroradiology 2002;44:667–673 [DOI] [PubMed] [Google Scholar]
- 11.Ojemann JG, Buckner RL, Akbudak E, et al. Functional MRI studies of word-stem completion: reliability across laboratories and comparison to blood flow imaging with PET. Hum Brain Mapp 1998;6:203–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashtari M, Lencz T, Zuffante P, et al. Left middle temporal gyrus activation during a phonemic discrimination task. Neuroreport 2004;15:389–393 [DOI] [PubMed] [Google Scholar]
- 13.Price CJ. The anatomy of language: contributions from functional neuroimaging. J Anat 2000;197:335–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamadori A. Neuropsychological model of reading based on Japanese experiences. Psychologia 2000;43:1–14 [Google Scholar]
- 15.Stowe LA, Broere CA, Paans AM, et al. Localizing components of a complex task: sentence processing and working memory. Neuroreport 1998;9:2995–2999 [DOI] [PubMed] [Google Scholar]
- 16.Damasio A. Aphasia. N Engl J Med 1992;326:531–539 [DOI] [PubMed] [Google Scholar]
- 17.Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A 2001;98:12760–12766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kircher TT, Bulimore ET, Brammer MJ. Differential activation of temporal cortex during sentence completion in schizophrenic patients with and without formal thought disorder. Schizophr Res 2001;50:27–40 [DOI] [PubMed] [Google Scholar]
- 19.Oldfield R. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9:97–113 [DOI] [PubMed] [Google Scholar]
- 20.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-D proportional system: an approach to cerebral imaging. New York: Thieme Medical Publishers;1988
- 21.Friston JK, Frith CD, Turner R, Frackowiak RS. Characterizing evoked hemodynamics with fMRI. Neuroimage 1995;2:157–165 [DOI] [PubMed] [Google Scholar]
- 22.Friston K, Holmes AP, Price CJ, et al. Multisubject fMRI studies and conjunction analyses. Neuroimage 1999;10:385–396 [DOI] [PubMed] [Google Scholar]
- 23.Maldjian J, Atlas SW, Howard RS II, et al. Functional magnetic resonance imaging of regional brain activity in patients with intracerebral arteriovenous malformations before surgical or endovascular therapy. J Neurosurg 1996;84:477–483 [DOI] [PubMed] [Google Scholar]
- 24.Ischebeck A, Indefrey P, Usui N, et al. Reading in a regular orthography: an FMRI study investigating the role of visual familiarity. J Cogn Neurosci 2004;16:727–741 [DOI] [PubMed] [Google Scholar]
- 25.Joubert S, Beauregard M, Walter N, et al. Neural correlates of lexical and sublexical processes in reading. Brain Lang 2004;89:9–20 [DOI] [PubMed] [Google Scholar]
- 26.Just MA, Carpenter PA, Keller TA, et al. Brain activation modulated by sentence comprehension. Science 1996;274(5284):114–116 [DOI] [PubMed] [Google Scholar]
- 27.Dhond RP, Buckner RL, Dale AM, et al. Spatiotemporal maps of brain activity underlying word generation and their modification during repetition priming. J Neurosci 2001;21:3564–3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Binder JR, Rao SM, Hammeke TA, et al. Functional magnetic resonance imaging of human auditory cortex. Ann Neurol 1994;35:662–672 [DOI] [PubMed] [Google Scholar]
- 29.Bookheimer SY, Zeffiro T, Blaxton TA, et al. Activation of language cortex with automatic speech tasks. Neurology 2000;55:1151–1157 [DOI] [PubMed] [Google Scholar]
- 30.Luders H, Lesser RP, Hahn J, et al. Basal temporal area demonstrated by electrical stimulation. Neurology 1986;36:505–510 [DOI] [PubMed] [Google Scholar]
- 31.Price C, Wise RJ, Watson JD, et al. Brain activity during readings: the effects of exposure duration and tasks. Brain 1994;117:1255–1269 [DOI] [PubMed] [Google Scholar]
- 32.Nobre AC, Allison T, McCarthy G. Word recognition in the human inferior temporal lobe. Nature 1994;372:260–263 [DOI] [PubMed] [Google Scholar]
- 33.Pierrot-Deseilligny CH, Ploner CJ, Muri RM, et al. Effects of cortical lesions on saccadic eye movements of humans. Ann N Y Acad Sci 2002;956:216–229 [DOI] [PubMed] [Google Scholar]
- 34.Paus T. Location and function of the human frontal eye-fields: a review. Neuropsychologia 1996;34:475–483 [DOI] [PubMed] [Google Scholar]
- 35.Cornelissen FW, Kimmig H, Schira M, et al. Event-related fMRI responses in the human frontal eye fields in a randomized pro- and antisaccade task. Exp Brain Res 2002;145:270–274 [DOI] [PubMed] [Google Scholar]
- 36.Petit L, Dubois S, Tzourio N, et al. PET study of the human foveal fixation system. Hum Brain Mapp 1999;8:28–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pierrot-Deseilligny C, Milea D, Muri RM. Eye movement control by the cerebral cortex. Curr Opin Neurol 2004;17:17–25 [DOI] [PubMed] [Google Scholar]
- 38.Koyama M, Hasegawa I, Osada T, et al. Functional magnetic resonance imaging of macaque monkeys performing visually guided saccade tasks: comparison of cortical eye fields with humans. Neuron 2004;41:795–807 [DOI] [PubMed] [Google Scholar]
- 39.Kronbichler M, Hutzler F, Wimmer H, et al. The visual word form area and the frequency with which words are encountered: evidence from a parametric fMRI study. Neuroimage 2004;21:946–953 [DOI] [PubMed] [Google Scholar]
- 40.Constable RT, Pugh KR, Berroya E, et al. Sentence complexity and input modality effects in sentence comprehension: an fMRI study. Neuroimage 2004;22:11–21 [DOI] [PubMed] [Google Scholar]
- 41.Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex: the evolution of an interface between cognition and emotion. Ann N Y Acad Sci 2001;935:107–117 [PubMed] [Google Scholar]