Abstract
Summary: In our experience with the canine model, sidewall aneurysms made with an oblique-cut arteriotomy were less likely to thrombose than were those made with a standard technique.
Experimental sidewall aneurysms created in different species of animals have a tendency to thrombose spontaneously (1–3). We have developed a surgical modification to the construction of experimental sidewall aneurysms, called the oblique cut. We compared spontaneous thrombosis rates of standard experimental sidewall aneurysms to oblique-cut sidewall aneurysms in a canine model.
Methods
Forty-four male dogs were included in this retrospective study. These animals were used for other studies in which experimental aneurysms were required. Three neurosurgeons constructed the experimental aneurysms after a similar training period. The first 11 aneurysms made were standard sidewall aneurysms (group A; n=11). After noting spontaneous thrombosis in several of these aneurysms, one operator (Y.Y.) developed the oblique-cut aneurysm construction. Encouraged by the less-frequent occurrence of observed cases of spontaneous thrombosis, we continued to construct oblique-cut sidewall aneurysms, which comprise the latter-constructed experimental aneurysms (group B; n=33). For both groups of aneurysms, training and skill of operators, surgical operating conditions and materials, goals of construction and technique, handling and maintenance of animals, and angiographic evaluation were similar. The proportions of spontaneous thrombosis for each aneurysm group were compared.
Aneurysm Construction
All animal experiments were conducted in accordance with policies set by the Institutional Animal Care and Use Committee of St. Luke’s Roosevelt Hospital Center Animal Care Facility. All surgical and endovascular procedures were performed under general anesthesia. The animals were 3–4 years old, weighted 18–21 kg each, and were maintained on a standard laboratory diet. Anesthesia was induced with thiopental 15–20 mg/kg followed by endotracheal intubation and maintained with isoflurane 1–3% via the endotracheal tube. The neck and groin were prepped and draped in the usual sterile fashion. During surgery, the heart rate, blood pressure, O2 saturation, electrocardiogram, and depth of anesthesia were monitored. All sidewall aneurysms were constructed at the right carotid artery distally to the carotid bifurcation aneurysms, which were used for other studies. A 10-cm skin midline incision was made in the neck, and the right external jugular vein (EJV) was isolated. This segment was then excised by using double-ligation technique and severed to produce sections of the venous pouch. The distal portion of the right common carotid artery (CCA) was temporarily closed and an elliptical arteriotomy, approximately 6 mm in length, was created on the lateral end of the right CCA. The neck segment of the prepared EJV pouch was then sutured along the edges of the arteriotomy by using 6–0 Proline sutures, resulting in a berry-shaped sidewall aneurysm. For group A (standard) aneurysms, the aneurysm neck segment of the grafted venous pouch was cut vertically to the aneurysm long axis. For group B (oblique-cut) aneurysms, the neck segment was cut at an estimated 45° angle to long axis of the aneurysm (Fig 1). The axial length of the aneurysm was 12–25 mm. The graft suture segment was larger than the aperture of the artery, especially in group B aneurysms, because of oblique cut. The venous grafts were stitched gathering the suture plane to fit to the arterial aperture segment.
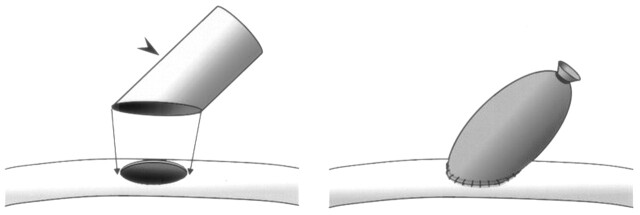
Fig 1.
Schematic drawing of the oblique-cut method of constructing a sidewall aneurysm.
The aneurysm neck segment of venous pouch harvested from the EJV was cut approximately 45° angle to the aneurysm long axis (arrowhead). An elliptical arteriotomy, approximately 6 mm long, is created on the lateral end of the right CCA. The neck segment of the pouch is then sutured along the edges of the arteriotomy by using 6–0 Proline sutures, resulting in a berry-shaped sidewall aneurysm.
Angiographic Analysis
All aneurysms were assessed by digital subtraction angiography (DSA) to document spontaneous aneurysm thrombosis. Aneurysms were classified into three groups, as patent, partially thrombosed, and complete thrombosed, at the last follow-up angiogram. Follow-up angiograms were obtained at 27–138 days after aneurysm construction (mean, 47 days). A patent aneurysm was defined as an aneurysm that was open with >10 mm contrast filling in its axial dimension. An aneurysm that was open with <10 mm axially filling or showed irregular dome filling was regarded as a partially thrombosed aneurysm. An aneurysm that showed signs such as irregularity of parent artery at the neck portion or no aneurysm filling was regarded as a completely thrombosed aneurysm.
Statistical Analysis
Proportions were compared using Fisher’s test and d tables for Fisher’s test (a=11, b = 0, c=1, d=11, at P = .01).
Results
At the last follow-up angiogram for each animal, 0/11 (0%) group A (standard) aneurysms and 32/33 (97.0%) group B (oblique-cut) aneurysms were patent (P < .01) (Table 1; Fig 2). One of 11 (9.1%) group A aneurysms and 1/33 (3.0%) group B aneurysms were partially thrombosed.
Group A=Standard. Group B=Oblique cut
| Mean follow up period | No. of patent aneurysms (%) | No. of partially thrombosed aneurysms (%) | No. of completely thrombosed aneurysms (%) | |
|---|---|---|---|---|
| Group A (n = 11) | 30 days | 0 (0%) | 1 (9.1) | 10 (90.9) |
| Group B (n=33) | 52 days | 32 (97%) | 1 (3) | 0 (0) |
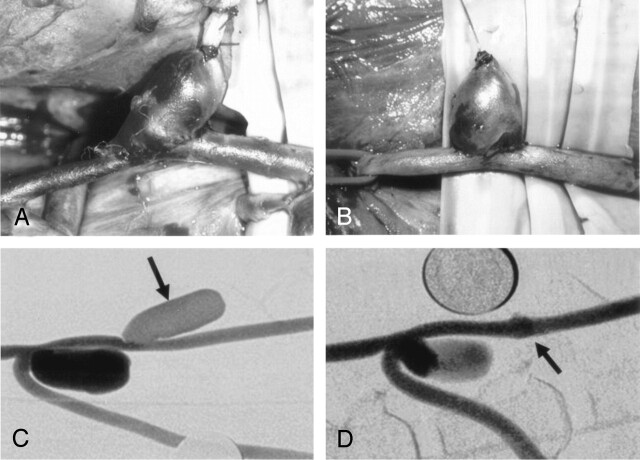
Fig 2.
Intraoperative view and DSA image of a sidewall aneurysm constructed obliquely to parent artery. The aneurysm remained patent on 30-day follow-up angiogram (A, C, arrow). Intraoperative view and DSA image of a sidewall aneurysm constructed conventionally perpendicular to the long axis of the parent vessel. The aneurysm was thrombosed 27 days after construction. (B, D, arrow).
Discussion
In a retrospective study in dogs, we found that oblique-cut sidewall aneurysms appeared less likely to thrombose spontaneously than did conventionally constructed experimental sidewall aneurysms. Many experimental aneurysm models have been described, mainly in rabbits, pigs, and dogs (4–8). The first reliable surgical construction technique for experimental aneurysms in laboratory animals was in a canine model, by German and Black, in 1954 (9). Black and German made observations on the relationship between the volume and the size of the orifice of experimental sidewall aneurysms in dogs (2). In evaluating 21 experimental sidewall aneurysms in canines, they found that 11/21 experimental sidewall aneurysms (52%) had spontaneously thrombosed within 2 weeks. Of the sidewall aneurysms that remained patent during this time, they noted that the volume-to-orifice area ratio was less then 23.6:1. Minimizing the aneurysm volume-to-orifice area ratio during construction has been helpful in reducing the incidence of spontaneous thrombosis; however, despite measures to minimize this ratio while creating a realistic aneurysm, conventional experimental sidewall aneurysm models continue to suffer from this important limitation. Nonetheless, the oblique-cut technique helps to minimize the aneurysm volume-to-orifice area ratio while allowing the creation of various sized aneurysms. The patency rate of 97% (32/33 oblique-cut aneurysms) for our group B sidewall aneurysms is higher than previously reported.
The occurrence, growth, thrombosis, and rupture of saccular aneurysms have been directly or indirectly related to hemodynamic forces (7). The exact nature of the change in hemodynamics that allows for less spontaneous thrombosis in our experimental oblique-cut sidewall aneurysm model is not known. It is hypothesized that the oblique-cut modification results in less turbulent and stagnant inflow and outflow within the aneurysm. Conventional experimental sidewall aneurysms have a different hemodynamic profile from those of bifurcation aneurysms (8). Characteristic stagnation of flow within a sidewall aneurysm can occur (7). The stagnation of blood flow in a sidewall aneurysm can result in the accumulation of both platelets and leukocytes along intimal surfaces, as well as in an impairment of the diffusion of oxygen and metabolites from blood to the vascular wall. Stagnation of blood flow may cause intimal damage and lead to thrombus formation followed by aneurysm occlusion (10).
A limitation of our study is that the rate of patency of our conventional sidewall aneurysms was lower than that of a recent study (11). Kallmes et al (11) reported that the 81% of their sidewall aneurysms were patent at >2 weeks. The patency rate differences may be in part attributable to the relatively small number of animals used in each study (i.e., had we created more standard sidewall aneurysms, we may have found a lower rate of spontaneous thrombosis). These differences can also be secondary to different surgical methods of construction, aneurysm and/or neck size, method of assessing patency (angiography/sonography versus angiography), and durations of assessment. In particular, because the size and shape of aneurysm neck are known to affect the patency of sidewall aneurysms, this difference may correlate to the length of the elliptical arteriotomy for the arterial aperture. Their 10-mm-long spherical arteriotomy was greater than the 6 mm that was chosen for our study. The diameters of the canine CCA and the venous pouch from jugular vein are 4–5 mm and 8–9 mm, respectively. We chose a 6-mm length for the aperture (neck size) to minimize the risk of protrusion of an embolic device (e.g., detachable coil) into the parent artery for other experiments.
On the basis of our preliminary results, the next step would be a study where one standard sidewall aneurysm and one oblique-cut sidewall aneurysm is made in each of several dogs. This follow-up study can more rigorously test the hypothesis that the aneurysms made with the oblique cut have a lower incidence of spontaneous closure than do those made with a standard incision. Evaluation of this technique to other animal models, such as the porcine experimental sidewall aneurysm model (11, 12), which have a greater incidence of spontaneous thrombosis than in canine, is reasonable.
Conclusion
The oblique-cut modification of experimental sidewall aneurysm creation in canine appears to result in less spontaneous thrombosis than conventionally constructed sidewall aneurysms. Further studies to validate this new method of sidewall aneurysm construction are warranted.
References
- 1.Murayama Y, Vinuela F, Suzuki Y, et al. Ion implantation and protein coating of detachable coils for endovascular treatment of cerebral aneurysms: concepts and preliminary results in swine models. Neurosurgery 1997;40:1233–1243 [DOI] [PubMed] [Google Scholar]
- 2.Black SP, German WJ. Observations on the relationship between the volume and the size of the orifice of experimental aneurysms. J Neurosurg 1960;17:984–990 [DOI] [PubMed] [Google Scholar]
- 3.Desfaits AC, Raymond J, Muizelaar JP. Growth factors stimulate neointimal cells in vitro and increase the thickness of the neointima formed at the neck of porcine aneurysms treated by embolization. Stroke 2000;31:498–507 [DOI] [PubMed] [Google Scholar]
- 4.Forrest MD, O’Reilly GV. Production of experimental aneurysms at a surgically created arterial bifurcation. AJNR Am J Neuroradiol 1989;10:400–402 [PMC free article] [PubMed] [Google Scholar]
- 5.Massoud TF, Guglielmi G, Ji C, et al. Experimental saccular aneurysms. I. Review of surgically-constructed models and their laboratory applications. Neuroradiology 1994;36:537–546 [DOI] [PubMed] [Google Scholar]
- 6.Massoud TF, Ji C, Guglielmi G, et al. Experimental models of bifurcation and terminal aneurysms: construction techniques in swine. AJNR Am J Neuroradiol 1994;15:938–944 [PMC free article] [PubMed] [Google Scholar]
- 7.Strother CM, Graves VB, Rappe A. Aneurysm hemodynamics: an experimental study. AJNR Am J Neuroradiol 1992;13:1089–1095 [PMC free article] [PubMed] [Google Scholar]
- 8.Graves VB, Strother CM, Partington CR, Rappe A. Flow dynamics of lateral carotid artery aneurysms and their effects on coils and balloons: an experimental study in dogs. AJNR Am J Neuroradiol 1992;13:189–196 [PMC free article] [PubMed] [Google Scholar]
- 9.German WJ, Black SP. Experimental production of carotid aneurysms. N Engl J Med 1954;250:104–106 [DOI] [PubMed] [Google Scholar]
- 10.Nakatani H, Hashimoto N, Kang Y, et al. Cerebral blood flow patterns at major vessel bifurcations and aneurysms in rats. J Neurosurg 1991;74:258–262 [DOI] [PubMed] [Google Scholar]
- 11.Kallmes DF, Altes TA, Vincent DA, et al. Experimental side-wall aneurysms: a natural history study. Neuroradiology 1999;41:338–341 [DOI] [PubMed] [Google Scholar]
- 12.Murayama Y, Vinuela F, Tateshima S, et al. Bioabsorbable polymeric material coils for embolization of intracranial aneurysms: a preliminary experimental study. J Neurosurg 2001;94:454–463 [DOI] [PubMed] [Google Scholar]