Abstract
Summary: We developed a method to produce tubular in vitro models of the cerebral vessels from real patient data. Three-dimensional data sets obtained from patients undergoing rotational angiography were used for stereolithographic biomodeling by using rapid prototyping technology. In a second step, tubular reproductions of the cerebral vessels were obtained by using the lost-wax technique. These reproductions can be useful for hemodynamic research and for the development and preclinical evaluation of new endovascular treatment methods.
Tubular vessel models are used for hemodynamic research and for the development and preclinical evaluation of new endovascular treatment methods (1–3). Ideally, a model should fulfill several criteria. First, it should reproduce the human vasculature reliably and realistic; second, it should rise no ethical concern; third, it should be easy to create and affordable. Although in vivo models are of high value for research and training purposes, the use of living animals presents disadvantages, such as high cost and difficult ethical considerations. The utility of simple in vitro models with different shapes (straight or curved segments, bifurcations) and materials (glass, plexiglass, silicone tubing) is confined mainly to fundamental hemodynamic investigations. In vitro models that better reproduce the complex cerebral vasculature have been created by using molding techniques that were applied on anatomic casts (2, 3).
We present a method to create tubular in vitro models of the cerebral vessels from actual patients. For that purpose, data from 3D rotational angiography were used for stereolithographic biomodeling (4) and the final replicas were produced by the lost-wax technique (5).
Technique
The consecutive steps of model creation are illustrated in Figures 1 and 2. In total, five models were obtained from five different patients.
Fig 1.
Flow chart of the consecutive steps of model preparation. The 3D data can be used for computer simulations likewise.
Fig 2.
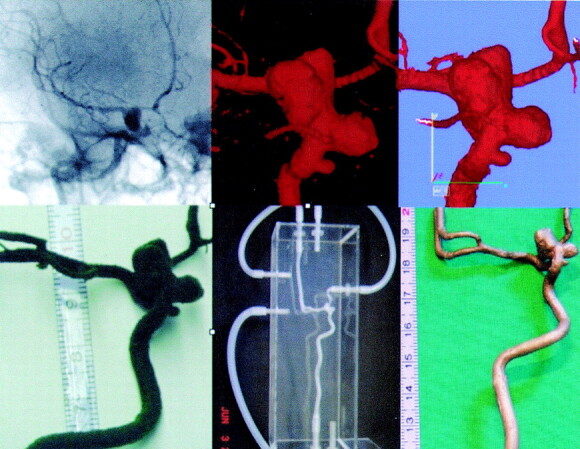
Illustration of the consecutive steps in model preparation. Left top, source image of rotational angiography; top center, 3D reconstruction (VRML); top right, postprocessing (STL); bottom left, wax model; bottom center, silicone model; bottom right, bronze model. Note that, on the wax model, the fine ophthalmic artery is no longer present.
Data Acquisition.
In all cases, models of the cerebral vasculature were created from patients undergoing clinically indicated conventional angiography with rotational data acquisition. Three-dimensional angiography was performed on a biplane C-arc unit (BV 3000; Philips Medical Systems, Best, the Netherlands). During rotational movement (180°) 100 images were acquired (12.5 frames/s), with a FOV of 17–30 cm, and a matrix of 1024 × 1024 (Fig 1, top left). The intraarterial bolus injection of contrast material was performed selectively for the artery of interest by using an injection pump (e.g., for the internal carotid artery, 2.5 mL/s; total volume 20 mL). The rotational run was then transferred to the angiography workstation (INTEGRIS 3D-RA, Philips Medical Systems) and a 3D reconstruction was performed. The workstation supports several different types of PC-based image formats, among them VRML (Virtual Reality Modeling Language), the international standard (ISO/IEC 14772) file format for describing 3D multimedia on the Internet. The entire data acquisition process was done without any modification from our routine clinical practice.
Postprocessing.
The 3D VRML file was send via the Intranet from the workstation to a PC—the feature to export data from the workstation was a custom, requested adaptation (provided by Philips Medical Systems)—and converted to the STL (Stereo Lithography format) by using Magics software (Magics RP 7.1; Materialise, Leuven, Belgium). At this stage the model was “cleaned” (i.e., nonconnected shells were removed, and parts of the cerebral vasculature that were not of interest for the final 3D model were removed). The STL file was then transferred, via compact disk, to a 3D printer.
Model Preparation.
The 3D printer (ModelMaker II; Solidscape, Merrimack, NH) formed wax copies of the vascular tree. The resolution of the build layer was 0.076 mm. This single wax model was then used to form the final model, by using the lost-wax technique as described in detail by Gailloud et al (3). In short, the wax model was embedded in a clear liquid resin (EP 4101; Eager Plastics, Chicago, IL) that solidified into a solid transparent block. Holes were drilled in the block to reach the wax at different points, allowing for subsequent evacuation of the wax by heating. After evacuation of the wax, a model consisting of a hollow reproduction of the cast within the resin block was obtained (silicone model). Alternatively, the wax model was embedded in plaster and used to form a bronze model that could be then used to form replicas of the original form.
Accuracy Determination.
To determine the geometric accuracy of the models, two randomly chosen silicone model were connected to a circulatory pump (as shown in Figure 2) and a rotational angiographic acquisition of the 3D model was performed by using the same imaging parameters as for patients. The 3D VRML data sets obtained directly from the patient and from the patient’s model were compared qualitatively, and all models were used to simulate endovascular procedures (e.g., microcatheterization, stent placement, and coiling of aneurysms) by two senior and junior neuroradiologists in our group, to evaluate whether the model provided a practical environment for these procedures.
Results
With experience, the entire postprocessing step was performed in <10 minutes. The printing time for the wax models depended on the size of the 3D data set and was on the order of hours, and the preparation of the final in vitro model that could be connected to a circulatory pump was on the order of days.
Arteries with a diameter of >1 mm were reproduced anatomically accurately on the wax model. Smaller branches, however, broke off on the rather fragile wax model (Figs 2 and 3).
Fig 3.
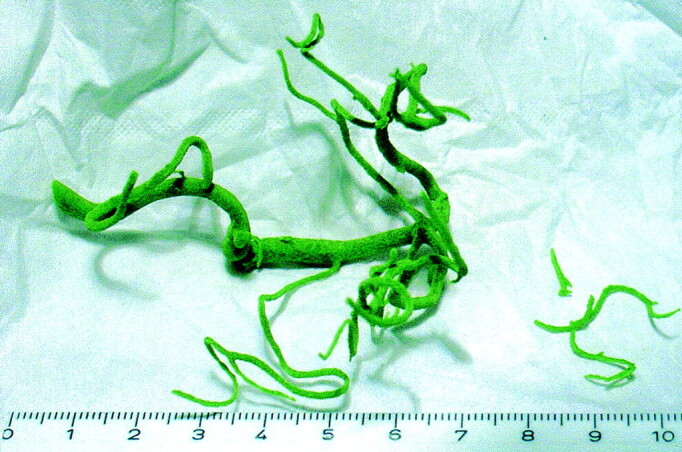
Wax model formed from a different data set that was obtained after contrast injection into the left vertebral artery. Note the details of the model, including the left posterior cerebellar artery and the right anterior cerebellar artery. Some smaller branches, however, have broken off.
All observers agreed that the 3D VRML data sets obtained from the patient’s model closely resembled the anatomy as depicted on the original VRML data sets obtained directly from the patient and that the models were helpful and practical to train in endovascular procedures and to evaluate new devices. Compared with in vivo catheterization, the in vitro model was found to be more rigid and to have a higher surface friction.
Discussion
Within the past decade, image-guided minimally invasive endovascular treatment of cerebral vascular diseases—foremost aneurysms—has become a clinical mainstay (6). The constantly ongoing technological developments in the field of endovascular implants require evaluation of the properties of newly designed implants (7, 8). In clinical routine, training of endovascular operators with new methods is of high importance. Both of these issues can be addressed by use of vascular models.
The presented method to obtain tubular vascular models of the cerebral vasculature from real-patient data were feasible by a combination of stereolithographic modeling and the lost-wax technique. Stereolithographic modeling, a rapid prototyping technology pioneered by Hull (9), allows one to obtain replicas of highly complex objects and was first reported by Mankovich et al (10) for replication of human anatomic structures. More recently, Wurm et al (11) described the use of this technique, which provides the neurosurgeon with new visualization options. We used the stereolithographic solid wax model as the mold to obtain the final tubular vessel model by applying the lost-wax technique. This molding technique is adapted from dentistry prosthetic devices (Elastrat, Geneva, Switzerland), and has been previously applied for biomodeling by Gailloud et al (3), who used corrosion casts from human specimens as the initial mold.
We chose to use data from 3D rotational angiography as the imaging source. The high-quality, densely sampled data obtained from this technique could be easily postprocessed. It provides a higher spatial resolution than obtained with CT angiography or MR angiography, techniques that in principle could be likewise the imaging source to form the tubular models. In light of the high resolution of the 3D angiographic data, and the high resolution available on the rapid prototyping machine, the degree of anatomic accuracy was, as expected, high. For two observers, differences between caliper measurements on the rotational data sets (VRML files) obtained directly from the patient and from the patient’s model did not exceed 10% and measurement errors did not point in a systematic direction. Repeated measurements by the same observer (intraobserver variability) on both the original VRML file as well as on the VRML file obtained reached up to 10% for both observers as well. Thus, the image threshold that influences the visualization of vascular diameters (11) and not inaccuracies in model preparation remained to be the major potential error. Furthermore, we opted for rotational angiography because the high-quality VRML data sets were used likewise as an ideal basis to perform computer flow simulations. Findings from computer simulation depend to a large degree on the input parameters chosen, and slight alterations might cause large computational differences in the output. Thus, a validation of the simulations is warranted, whereby the silicon models form rotational angiography can be used for the in vitro flow experiments.
The fragility is a disadvantage of the initial wax mold, so small branches smaller than 1 mm could not be displayed in the final model. To minimize the printer time, we focused the 3D printing on regions of interest (e.g., an aneurysm and the adjacent arteries) and formed proximal and more distal vessel parts manually, showing the vessel course in an only approximate fashion in these areas. The costs for such custom made models depend on size and are in the range of $1800–$2200 (U.S.). This includes 3D printing from based on the STL file, one tubular silicone model copy of the vascular area of interest, connection tubes, and a transparent Plexiglas box to connect the model lumen to a circulation circuit (delivery time, 10–15 working days).
The final tubular model when connected to a circulatory pump was practical to use for the simulation of endovascular procedures. The major drawback was the relatively higher friction of the silicone models compared with in vivo catheterization, limiting the impression of a realistic procedure. Models prepared with polyvinylalcohol hydrogel have recently been reported to have lower surface friction and less stiffness compared with silicone models (12). Use of this material in conjunction with our proposed modeling process might improve the naturalistic impression of catheterization.
In conclusion, we described the feasibility to create tubular in vitro models of the cerebral vessels with high accuracy by using only commercially available tools.
Footnotes
Supported, in part, by a grant from the Swiss National Science Foundation (grant 3200-066634).
References
- 1.Gobin YP, Counord JL, Flaud P, Duffaux J. In vitro study of haemodynamics in a giant saccular aneurysm model: influence of flow dynamics in the parent vessel and effects of coil embolisation. Neuroradiology 1994;36:530–536 [DOI] [PubMed] [Google Scholar]
- 2.Gailloud P, Pray JR, Muster M, et al. An in vitro anatomic model of the human cerebral arteries with saccular arterial aneurysms. Surg Radiol Anat 1997;19:119–121 [PubMed] [Google Scholar]
- 3.Gailloud P, Muster M, Piotin M, et al. In vitro models of intracranial arteriovenous fistulas for the evaluation of new endovascular treatment materials. AJNR Am J Neuroradiol 1999;20:291–295 [PMC free article] [PubMed] [Google Scholar]
- 4.D’Urso PS, Thompson RG, Atkinson RL, et al. Cerebrovascular biomodelling: a technical note. Surg Neurol 1999;52:490–500 [DOI] [PubMed] [Google Scholar]
- 5.Sugiu K, Martin JB, Jean B, et al. Artificial cerebral aneurysm model for medical testing, training, and research. Neurol Med Chir (Tokyo) 2003;43:69–72; discussion 73 [DOI] [PubMed] [Google Scholar]
- 6.Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;360:1267–1274 [DOI] [PubMed] [Google Scholar]
- 7.Wakhloo AK, Lanzino G, Lieber BB, Hopkins LN. Stents for intracranial aneurysms: the beginning of a new endovascular era? Neurosurgery 1998;43:377–379 [DOI] [PubMed] [Google Scholar]
- 8.Lylyk P, Ceratto R, Hurvitz D, Basso A. Treatment of a vertebral dissecting aneurysm with stents and coils: technical case report. Neurosurgery 1998;43:385–388 [DOI] [PubMed] [Google Scholar]
- 9.Hull CW. Apparatus for production of three-dimensional objects by stereolithography. US patent no 4,575,330. Washington, DC: US Patent Office;1986
- 10.Mankovich NJ, Cheeseman AM, Stoker NG. The display of three-dimensional anatomy with stereolithographic models. J Digit Imaging 1990;3:200–203 [DOI] [PubMed] [Google Scholar]
- 11.Wurm G, Tomancok B, Pogady P, et al. Cerebrovascular stereolithographic biomodeling for aneurysm surgery: technical note. J Neurosurg 2004;100:139–145 [DOI] [PubMed] [Google Scholar]
- 12.Ohta M, Handa A, Iwata H, et al. Poly-vinyl alcohol hydrogel vascular models for in vitro aneurysm simulations: the key to low friction surfaces. Technol Health Care 2004;12:225–233 [PubMed] [Google Scholar]



