Abstract
BACKGROUND AND PURPOSE: This study was designed to determine whether calcification of the cavernous carotid artery (CCA) is associated with cerebrovascular infarcts in the same way that coronary artery calcification scores indicate myocardial infarctions. We sought to correlate the grade of CCA calcification with infarctions in the middle cerebral artery (MCA) distribution.
METHODS: Nonenhanced brain CT scans of 40 patients with MCA-distribution strokes, 34 with non-MCA–distribution strokes, and 94 age-matched control subjects were reviewed. Circumferential calcification and thickness of calcification were graded for the CCAs on head CT scans. Scores were determined for the left and right CCAs. Mann-Whitney tests and Spearman correlation coefficients were used to detect differences between patients and control subjects and between patients with MCA and those with non-MCA strokes.
RESULTS: CCA calcification scores did not significantly differ in the groups compared. The manner in which calcification was scored (by using circumference, thickness, or both) did not affect the results. No difference was noted between scores ipsilateral and those contralateral to the stroke.
CONCLUSION: Circumferential degree or thickness of cavernous artery calcification was not correlated with MCA or non-MCA infarctions. CCA calcification scores did not differ between patients with stroke and those without stroke.
The use of coronary artery calcification scoring to predict future myocardial infarction risk has gained wide acceptance in the medical community. However, the recent growth of the coronary artery calcification–scoring centers across the country has been mainly driven by commercialism. Coronary artery calcification reflects the total atherosclerotic burden on the body, but it also has predictive value in terms of future ischemic injury to the heart and brain (1–6). Nonetheless, the importance of calcification associated with atherosclerotic plaque has been debated, with some authors believing that calcification stabilizes plaque, while others conclude that it indicates an increased risk of thromboembolic disease (7–10).
The plaque burden in the intracranial circulation has not been comprehensively evaluated by measuring calcification of intracranial blood vessels with a method similar to that used in coronary arteries. Atherosclerotic calcification of the intracranial vertebrobasilar and internal carotid arteries (ICAs) is a frequent finding on nonenhanced brain CT. However, the importance of these calcifications is unknown and has only recently garnered attention because of the associated grading of the coronary arteries (11).
The purpose of this study was to examine patients with documented acute middle cerebral artery (MCA) infarctions to determine whether the degree of calcified atherosclerotic disease in the cavernous carotid arteries (CCAs) is greater in these patients than in an age-matched control population. We sought to determine whether CCA calcification was correlated with MCA thromoembolic disease. We hypothesized that patients with MCA strokes have more CCA calcification than those with non-MCA strokes or control subjects without strokes.
Methods
Medical records from the stroke neurology service of the Johns Hopkins Hospital, Baltimore, MD, were used to identify individuals with acute infarctions in the MCA distribution between January 2000 and March 2002. These individuals were designated as the MCA-stroke group. Patients with a clinical diagnosis of acute infarctions in other vascular distributions who were admitted in the same period were evaluated and combined into a group with non-MCA strokes. Only those with both clinically diagnosed stroke and positive diffusion-weighted MR images were included for analysis and assigned to these groups. Age-matched healthy control subjects without stroke were randomly selected in the same period. A stroke neurologist referred these control subjects, who had no clinically suspected infarction and negative diffusion-weighted MR images. Control subjects were not specifically screened for transient ischemic attacks (TIAs). All patients were required to have undergone MR imaging and head CT within 3 days of each other. Overall, 168 complete and retrievable cases of patients 40 years or older were reviewed: 40 with MCA strokes, 34 with non-MCA strokes, and 94 without stroke (control group).
Each CT study was performed with 3-mm-thick sections through the cavernous region. Bone windows were reviewed to determine the degree of CCA calcification. Table 1 shows the 5-point scoring system that was used here and in a previous study (11). This system enabled the assessment of both CCAs for absent calcification (grade 0), dots (grade 1), arcs (grade 2), and incomplete (grade 3) and complete (grade 4) circumferential calcification of the carotid artery. In addition, the thickness of the calcification was estimated by using the centimeter scale accompanying the radiograph (Fig 1). We looked at CT scores and CT thicknesses separately and together to maximize our ability to grade the atherosclerosis. Scores were determined for the left and right CCAs in patients with infarctions. In control subjects, only the higher of the two CCA scores was recorded, with no designation of the side.
TABLE 1:
CCA calcification scale
| Grade | Calcification |
|
|---|---|---|
| Extent | Thickness (mm) | |
| 0 | None | 0 |
| 1 | Dot | 1 |
| 2 | <90° of carotid wall circumference | 2 |
| 3 | 90–270° of circumference | 3 |
| 4 | 270–360° of circumference | >3 |
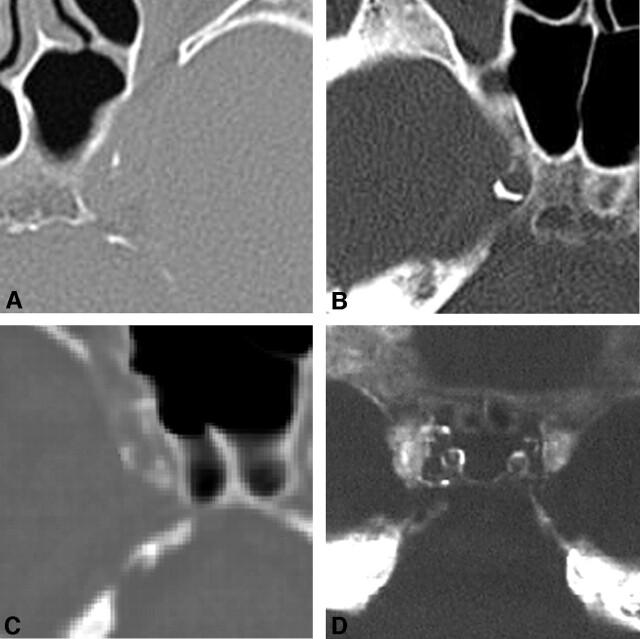
Fig 1.
Scoring system.
A, Grade 1. Small dot of calcification 1 mm thick is present in the left CCA wall. Grades for this plaque are 1 for extent and 1 for thickness.
B, Grade 2. CT scan of the right CCA shows an atherosclerotic, calcified plaque that is crescentic but <90° of the vascular circumference. Grades are 2 for extent and 1 (1 mm) for thickness.
C, Grade 3. Circumferential involvement of the right CCA calcification is 90° but <270°; therefore, the grade is 3 for extent.
D, Grade 4. Both carotid arteries show complete circumferential involvement with atherosclerotic plaque, hence grade 4.
A trained neuroradiologist with a Certificate of Added Qualification and 15 years of experience (D.M.Y.) reviewed the medical records, MR imaging findings, and MR reports in blinded and retrospective fashion to confirm or rule out an infarction of the MCA or other vascular distribution. The same neuroradiologist graded the CCA. Intraobserver variability was assessed by means of blinded re-review of 20 cases. In this blinded re-review, 18 patients (36 carotid arteries) had exactly the same grades as before, and in two patients, the values of one carotid artery deviated by one grade level; therefore, agreement was 95%.
The data were initially analyzed and found not to assume a normal distribution. Therefore, Mann-Whitney tests were used to determine differences among infarcts in the MCA and non–MCA distributions and nonischemic controls. All patients with stroke, both MCA and non-MCA groups, were compared with age-matched control subjects by using the two-sample Wilcoxon rank-sum (Mann-Whitney) test. Spearman correlation coefficients were used to access the relationship between the degree of calcification and the presence of infarction. Adjustments were made for age by using median regression, alternative to linear regression based on medians rather than means. Significance was defined at the level of 5% (P < .05). In addition, we used Fisher’s exact test to see if there was a difference in the CCA scores between the stroke side and the contralateral non-stroke side.
The Mann-Whitney test was used to test for differences between calcification scores in the patient groups. Comparisons were made between patients with MCA and non-MCA strokes and control subjects, between those with MCA stroke and those with non-MCA stroke, between those with MCA stroke and their age-matched control group, and between those with non-MCA stroke and their age-matched control group. In each comparison, six analyses were performed by using different calcification-score criteria. We looked at the mean and maximum grades of circumferential CCA involvement, the mean and maximum thickness of the degree of calcification, and mean and maximum of the combined grade of circumferential CCA involvement and thickness of the degree of calcification.
Results
Table 2 shows the mean ages of the patients in all three groups (Table 2). Tables 3 and 4 summarize the CT grades and thicknesses. Table 5 summarizes the results for circumferential calcium scores, calcium thicknesses, and the two combined.
TABLE 2:
Population demographic data by infarction type
| Data | MCA Group (n = 40) | Non-MCA Group (n = 34) | Control Group (n = 94) |
|---|---|---|---|
| Cerebrovascular infarction | Yes | Yes | No |
| Patient age (y) | |||
| Mean ± SD | 64.3 ± 13.2 | 64.2 ± 13.3 | 63.8 ± 12.0 |
| Range | 41–86 | 42–83 | 41–86 |
TABLE 3:
CT scores and thicknesses by infarction group
| CT Score and Thickness (mm) | MCA Group (n = 40) |
Non-MCA Group (n = 34) |
Control Group (n = 94) | ||
|---|---|---|---|---|---|
| Left | Right | Left | Right | ||
| CT score 0 | 12 | 14 | 10 | 10 | 27 |
| CT score 1 | |||||
| 1 | 7 | 4 | 8 | 6 | 18 |
| 2 | 0 | 2 | 3 | 3 | 6 |
| 3 | 0 | 0 | 0 | 0 | 0 |
| 4 | NA | NA | 1 | 0 | NA |
| CT score 2 | |||||
| 1 | 5 | 1 | 0 | 3 | 7 |
| 2 | 2 | 5 | 0 | 2 | 5 |
| 3 | 0 | 0 | 0 | 1 | 0 |
| 4 | NA | NA | NA | NA | 0 |
| 5 | NA | NA | NA | NA | 2 |
| CT score 3 | |||||
| 1 | 2 | 5 | 3 | 3 | 10 |
| 2 | 3 | 1 | 3 | 3 | 4 |
| 3 | 1 | 0 | 1 | 0 | 2 |
| 4 | 1 | 0 | NA | NA | NA |
| Ct score 4 | |||||
| 1 | 2 | 4 | 0 | 1 | 7 |
| 2 | 3 | 3 | 4 | 2 | 4 |
| 3 | 2 | 1 | 1 | 0 | 1 |
| 4 | NA | NA | NA | NA | 1 |
TABLE 4:
Sumary of calcification scores
| Grade and Group | Mean | Maximum |
|---|---|---|
| CT score | ||
| MCA | 1.73 ± 1.45 (0–4) | 1.98 ± 1.53 (0–4) |
| Non-MCA | 1.53 ± 1.37 (0–4) | 1.74 ± 1.46 (0–4) |
| Control | NA | 1.62 ± 1.42 (0–4) |
| CT thickness (mm) | ||
| MCA | 1.05 ± 0.82 (0–3) | 1.28 ± 1.04 (0–4) |
| Non-MCA | 1.13 ± 0.82 (0–3) | 1.35 ± 1.01 (0–4) |
| Control | NA | 1.10 ± 1.03 (0–5) |
| CT score and thickness | ||
| MCA | 2.78 ± 2.16 (0–7) | 3.26 ± 2.38 (0–7) |
| Non-MCA | 2.66 ± 2.04 (0–6) | 3.09 ± 2.25 (0–7) |
| Control | NA | 2.72 ± 2.17 (0–8) |
Note.—NA = not applicable; for control cases only the higher (maximum) calcification score of the left and right side was recorded.
TABLE 5:
Summary of calcification scores in the MCA plus non-MCA groups
| Grade | Infarction Side | Contralateral Side | P Value * |
|---|---|---|---|
| CT score | 1.5 ± 1.48 (0–4) | 1.47 ± 1.49 (0–4) | .76 |
| CT thickness (mm) | 1.15 ± 1.09 (0–4) | 0.97 ± 0.90 (0–4) | .67 |
| CT score and thickness | 2.68 ± 2.40 (0–7) | 2.44 ± 2.21 (0–7) | .50 |
Note.—Data are the value ± SD (range).
Fisher exact test.
Of the infarctions in the MCA distribution, 27 were left sided, 11 were right sided, and two were bilateral. Infarctions in non-MCA distributions had 14 strokes on the right side, eight on the left side, and two were bilateral. In addition, in the non-MCA group, 8 patients had documented cerebrovascular events in the cerebellum and two in the brainstem.
On our Mann-Whitney analysis, no significant differences were detected between the various groups compared (Table 6). Statistical analysis showed no difference in the grades of CCA calcifications for the three groups. The circumferential degree or thickness of CCA calcification was not correlated with the presence or absence of infarction. When age was as a covariate on median regression, we found no evidence of a relationship between the degree of CCA atherosclerosis and the presence of MCA stroke, non-MCA stroke, or no stroke (control) groups.
TABLE 6:
Comparison of calcification groups
| Comparison and Calcification Criterion |
P Value |
|
|---|---|---|
| Mann-Whitney Test, Unadjusted | Median Regression, Adjusted for Age | |
| All stroke vs all controls | ||
| Maximum | ||
| Circumference | .230 | .550 |
| Thickness | .089 | <.001 |
| Circumference + thickness | .156 | .552 |
| Mean | ||
| Circumference | .881 | .999 |
| Thickness | .511 | .776 |
| Circumference + thickness | .873 | .594 |
| MCA stroke vs non-MCA stroke | ||
| Maximum | ||
| Circumference | .391 | .308 |
| Thickness | .194 | .857 |
| Circumference + thickness | .328 | .799 |
| Mean | ||
| Circumference | .365 | .141 |
| Thickness | .183 | .309 |
| Circumference + thickness | .254 | .238 |
| MCA stroke vs MCA controls | ||
| Maximum | ||
| Circumference | .075 | .446 |
| Thickness | .107 | .390 |
| Circumference + thickness | .060 | .326 |
| Mean | ||
| Circumference | .250 | .791 |
| Thickness | .369 | .999 |
| Circumference + thickness | .329 | .784 |
| Non-MCA stroke vs non-MCA controls | ||
| Maximum | ||
| Circumference | .636 | .353 |
| Thickness | .449 | .999 |
| Circumference + thickness | .963 | .388 |
| Mean | ||
| Circumference | .272 | .342 |
| Thickness | .992 | .999 |
| Circumference + thickness | .395 | .256 |
Discussion
In studies of stroke incidence rates, ischemic stroke is divided into subtypes to account for its causes, clinical presentations, risk factors, and outcomes. Large-artery atherosclerosis, disease of small vessels (lacunae), cardioembolism, other determined etiologies, and strokes of unknown cause are the categories most frequently encountered in the literature (12–14). The subtype of large-artery atherosclerosis includes ischemic strokes due to cervical or intracranial narrowing of the lumen of the carotid artery. We hypothesized that an evaluation of CCA calcification on head CT can be used to screen for individuals at risk for or with cerebral infarction as a result of intracranial stenosis. The literature attributes 16%–21% of all strokes to large-artery atherosclerosis (12–14), and we hypothesized that intracranial stenosis, manifested as calcification of carotid siphon, is correlated with MCA or non-MCA infarction.
We were not attempting to portray atheroma of the CCA as the source of the embolism or thrombotic event. We sought to determine whether cavernous carotid disease is a marker for widespread systemic disease, affecting the neck as it might affect the heart or other locations. Our objective was to determine if CCA calcification has a predictive value for stroke similar to that of coronary calcification for stroke or acute myocardial infarction (1–6).
When nonenhanced brain CT scans are reviewed, the reader often notices calcification of the vertebral artery, basilar artery, or ICA. Calcification of branch vessels and distal cerebral vessels is less common and often associated with chronic renal failure or other significant risk factors for atherosclerosis. The importance of reporting these findings has not been addressed despite the fact that this finding in frequently included in the report.
When discussing the degree of atherosclerosis in the carotid circulation, most authors use a measurement of stenosis that demonstrates vascular narrowing. The relationship of the degree of stenosis and the degree of calcified plaque has not been fully evaluated in terms of the intracranial atherosclerotic burden. Whether calcification of carotid vessels is an independent risk factor for future infarction beyond the degree of stenosis is unclear. A previous study demonstrated that the degree of CCA calcification is not correlated with the overall burden of white matter disease seen on MR imaging after the collected data are adjusted for age (11). In that study, the effect of age was dominant, as both the degree of CCA atherosclerosis and the degree of white-matter ischemic burden (graded by means of cardiovascular health–study scoring) increased with age. Atherosclerosis was not a specific independent predictor of the severity of white matter disease.
Thromboembolic events resulting from atherosclerotic disease in the proximal ICA and carotid bulb are common causes of stroke. Large multicenter, randomized trials, such as North American Symptomatic Carotid Endarterectomy Trial, the European Carotid Surgery Trial, and the Asymptomatic Carotid Atherosclerosis Study, showed that the risk of stroke can be assessed on the basis of carotid atherosclerosis by measuring the degree of stenosis (15–17). The extent to which calcium in plaques at the carotid bifurcation parallels the degree of luminal narrowing is unclear. Available reports of radiographic and CT analysis of the carotid arteries suggest that distribution of calcium varies; however, at the same time, asymptomatic patients have increased amounts of calcification, which might have a stabilizing effect (18–19). At our institution, neck CT angiography (CTA) is not routinely performed as a part of the stroke workup in the emergency department due to concerns about radiation exposure, the proximity to the radiosensitive thyroid gland, and the presence of noncalcified plaque that is invisible without contrast enhancement. Therefore, we were unable to obtain any data that would have helped us in evaluating calcification levels in the neck as a marker of atherosclerosis and stroke risk. Instead, we looked at the calcification of the CCA region and considered it as a surrogate marker for systemic atherosclerosis.
In a comparison of carotid-siphon calcification detected with CT and carotid-siphon stenosis assessed with CTA, Woodcock et al showed that sensitivity and specificity, respectively, were 86% and 98% for bone windows, and 100% and 0% for brain windows (20). The positive predictive value for angiographic stenosis greater than 50% due to CCA calcium burden was 86% with CT bone windows and 11% with brain windows and levels. These data justified our reliance on bone windows for nonenhanced brain CT scans in our analysis of calcium deposits in the CCA. Woodcock et al did not correlate the data on CT calcium and CTA stenosis with the development of strokes, which was the emphasis of our study.
Stenosis in the intracranial ICA has been suggested as a marker of an increased risk of stroke and of extensive cerebrovascular and systemic atherosclerotic disease (21). Marzewski et al followed up 66 patients with intracranial ICA stenosis of at least 50% (maximal luminal compromise in any single angiographic plane) for 5 years and observed a significant increase in the rate of cerebrovascular events in these patients compared with the general population (21). Of the 66 patients, 14 had intrapetrous stenosis, 65 had intracavernous stenosis, and six had supraclinoid stenosis. In a similar study, Craig et al retrospectively reviewed 58 patients with angiographically proved intracranial ICA stenosis (22). During 30- month follow-up, 33% were free of subsequent cerebrovascular events, and 43% died: 36% due to stroke and 44% due to cardiac disease. Two-thirds of the strokes were in the territory of the stenotic vessel. The cavernous segment was the most common site of intracranial ICA stenosis and contained plaque in 42 patients. Nine patients had stenosis in the petrous segment, and seven had stenosis in the supraclinoid portion. The severity of the intracranial lesions was at least 50% in 35 patients and between 30% and 50% in 23. These studies indicate that patients with intracranial ICA stenosis are at high risk for stroke and that, in most, the intracranial atherosclerotic burden is located in the CCA.
In an radiographic analysis of calcification of carotid siphon, calcification of the plaque did not always correspond to the atherosclerotic narrowing of the lumen (23). In that study, calcification involved a highly variable percentage of the cross-sectional area of the plaque, from almost 0% to as much as 50%. Shallow plaques appeared to be more heavily calcified than thick ones. At times, the thickest segment of the plaque was not calcified, whereas an adjacent thin sector was uniformly calcified. However, on histologic examination, the degree of calcification of the carotid siphon paralleled the degree of atherosclerosis of the aorta and the carotid and cerebral arteries. In addition, siphon calcification seemed to be related to cerebral thrombosis. Of the 26 cases with calcification of grade 3 (the second-highest severity), 23 involved cerebrovascular lesions.
The major limitation of our study is that the grading scale may have represented different forms of calcification rather than increasing degrees of calcification, making the mean calcification grades of questionable value. Virmani et al (24) describes two classifications of calcified plaque in coronary arteries: the calcified nodule and the fibrocalcific plaque. The calcified nodule is a attenuating nodule of calcium protruding into the lumen through a disrupted thin, fibrous cap, whereas a fibrocalcific plaque has a thick, fibrous cap overlying extensive calcification in the intima. The difference in appearance between these forms of calcification may result in a difference in their grades of severity, which is a misrepresentation of their clinical behavior. Fibrocalcific plaques are considered healed and stable, whereas calcified nodules are associated with acute rupture and luminal thrombi.
Our understanding of coronary plaque vulnerability is applicable to carotid atherosclerosis because coronary and carotid bifurcation plaques have a common mechanism of plaque rupture, which includes thinning and foam-cell infiltration of the fibrous cap (25). However, stroke is predominantly embolic, whereas coronary infarction is generally occlusive because of the small caliber of the coronary arteries. Kane et al (26) reported that the diameter of the CCA on MR imaging is 4.62 ± 0.68 mm, a value that probably lies somewhere between the diameter of the carotid bulb and that of the coronary artery, although closer to the latter. Therefore, one might expect the risk of CCA occlusion to be similar to that of coronary occlusion, and coronary atherosclerosis may be a good model for understanding the behavior of plaque in the CCA. However, other differences between the two types of vessels, such as higher blood velocities in the CCA than in the coronary arteries, that might prevent occlusion by thrombus, could lead to a poor correlation between calcification and cerebral ischemic events.
Our control subjects were not specifically screened for TIAs, but they were examined for a positive diagnosis of ischemic infarction based on diffusion-weighted MR studies. Gladstone et al reported the treatment and outcomes of Canadian patients with TIA and concluded that such patients have a high risk of immediate- and short-term stroke and therefore might share some of the characteristics of patients with stroke (27). However, more than 75% of the nonenhanced head CT studies performed at our emergency department are overwhelmingly attributed to head trauma, alcoholism, and changes in mental status related to intravenous drug use and not TIA. Also, the 5% increased risk of stroke in patients with TIA is associated with the first 30 days after discharge, and one-half of these infarctions occur within the first 2 days. Because our study involved a retrospective evaluation of CT scans performed at least 60 days after imaging and because members of our neurology department updated and verified our patients’ infarction status during the evaluation, we believe that we limited any effect that including patients with TIA in our control group had on our analyses.
The relationship between CCA atherosclerosis and carotid bifurcation disease and their aggregate effect on a patient’s prognosis has been evaluated. In one study, patients were separated into a group with only carotid bifurcation stenosis and a group with stenoses of both the carotid bifurcation and the cavernous carotid siphon (28). About one-half of the patients with cerebrovascular symptoms had intracranial atherosclerotic lesions, and approximately 20% of them had a greater degree of stenosis in the carotid siphon than in the ICA. In another study, 393 patients were followed up for 10 years after carotid endarterectomy, and the influence of concurrent carotid-siphon stenosis on short- and long-term outcomes was analyzed (29). Carotid-siphon stenosis significantly increased the risk of late death and lowered the stroke-free survival rate.
Although the ability assess the degree of CCA stenosis in all patients with suspected stroke would be useful, nonenhanced CT is the most common imaging studies ordered in the initial evaluation of these patients. Therefore, the only vascular information available is the calcification of the intracranial vessels. One cannot measure the degree of stenosis on a nonenhanced CT scan; therefore, we proposed that the degree of calcification may serve as a surrogate marker for stenosis. Results of our study, which should be viewed as a preliminary assessment in which other relevant clinical variables (hypertension, diabetes mellitus, smoking) were not addressed, suggest that CCA calcification itself is not worthwhile indicator of the risk of stroke.
CTA has emerged as a technique for rapidly assessing luminal narrowing and one that can be applied to CCA disease. Performed with contrast material, CTA is accurate for localizing narrowing and occlusions of intracranial vessels. Some departments successfully use a protocol that combines nonenhanced CT, CTA, and CT perfusion for acute stroke imaging to gather information about the vascularization, pathophysiology, and extent of ischemia (30). Our retrospective study was performed by using CT studies before CTA was widely applied and before multidetector CT scanners were available throughout our department. In the setting of acute stroke, CTA adds some risk associated with contrast agent and renal impairment, and therefore, we require written informed consent from our patients. Our algorithm involves diffusion-weighted imaging and head and neck MR angiography in the acute setting, which can be performed at low risk to the patient. Hence, we do not have data from the CTA/CT perfusion protocol and advocate the use of MR imaging over CT.
Ptak et al attempted to correlate the presence of calcium in the CCA to the known predictors of vascular disease (31). They looked at sex, age, hypertension, diabetes, smoking, hypercholesterolemia, cardiac disease, and alcohol and intravenous drug abuse. In contrast to our study, theirs did not address the distribution or degree of calcification by using a grading scale. Instead, they analyzed calcification in binary fashion by assigning 0 and 1 to patients with and those without detected calcium, respectively. Ptak et al found a significant correlation between CCA calcification and diabetes, hypercholesterolemia, or hypertension and therefore suggested that that carotid siphon calcification is a predictor of existing systemic disease.
Couple with our findings, results published thus far suggest that the CCA calcified plaque burden has a weak or no correlation with subsequent or current infarction, although it might seem like an important finding on nonenhanced CT (7–11). Therefore, assigning a calcification score to the common carotid artery bifurcation rather than the CCAs, where such calcification is seen, may be relevant. However, the neck is not routinely scanned as part of the emergency department protocol to evaluate the patient for acute stroke. As an alternative, narrowing rather than calcifying plaque may the only significant risk factor on carotid (as opposed to coronary) CT or CTA. Oliver et al (32) demonstrated that plaque attenuation on CTA can be used to identify features of plaque vulnerability that predispose the patient to cerebral ischemia; an example is a large necrotic or lipid core compared with a predominantly fibrous plaque. In addition, because some believe that the type of calcification (eg, unstable fibrocalcific plaques and stable calcified nodules) is critical, future work must provide a method that allows us to distinguish between the two and to score them independently. In this way, calcium grading of the CCA might become a more reliable indicator of the risk of infarction.
Conclusion
In patients with acute cerebral artery strokes, the degree of CCA calcification did not significantly differ among control subjects, patients with infarctions in the MCA distribution, and patients with infarctions of other vascular territories. In addition, the degree of CCA calcification did not differ between patients with and those without documented cerebral infarctions. We believe that the extent of CCA calcification reflects the total atherosclerotic burden.
Footnotes
Supported by the Johns Hopkins University Howard Hughes Summer Research Program.
References
- 1.Rafkin RD, Parisi AF, Folland E. Coronary calcification in the diagnosis of coronary artery disease. Am J Cardiol 1979;44:141–147 [DOI] [PubMed] [Google Scholar]
- 2.Agatston AS, Janowitz WH, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–832 [DOI] [PubMed] [Google Scholar]
- 3.Shemesh J, Apter S, Rozenman J, et al. Calcification of coronary arteries: detection and quantification with double-helix CT. Radiology 1995;197:779–783 [DOI] [PubMed] [Google Scholar]
- 4.Stanford W, Thomson BH. Coronary atherosclerosis and its effect on cardiac structure and function: evaluation by electron beam computed tomography. Clin Chem 1998;44 (8 pt 2):1871–1881 [PubMed] [Google Scholar]
- 5.Stanford W. Coronary artery calcification as an indicator of preclinical coronary artery disease. Radiographics 1999;19 (6):1409–1419 [DOI] [PubMed] [Google Scholar]
- 6.Vliegenthart R, Hollander M, Breteler MM, et al. Stroke is associated with coronary calcification as detected by electron-beam CT: the Rotterdam Coronary Calcification Study. Stroke 2002;33 (2):462–465 [DOI] [PubMed] [Google Scholar]
- 7.Burke AP, Weber DK, Kolodgie FD, Farb A, Taylor AJ, Virmani R. Pathophysiology of calcium deposition in coronary arteries. Herz 2001;26:239–244 [DOI] [PubMed] [Google Scholar]
- 8.Hunt KJ, Pankow JS, Offenbacher S, et al. B-mode ultrasound-detected carotid artery lesions with and without acoustic shadowing and their association with markers of inflammation and endothelial activation: the atherosclerosis risk in communities study. Atherosclerosis 2002;162:145–155 [DOI] [PubMed] [Google Scholar]
- 9.Duncan BB, Metcalf P, Crouse JR III, et al. Risk factors differ for carotid artery plaque with and without acoustic shadowing: Atherosclerosis Risk in Communities Study Investigators. J Neuroimaging 1997;7:28–34 [DOI] [PubMed] [Google Scholar]
- 10.Huang H, Virman R, Younis H, Burke AP, Kamm RD, Lee RT. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation 2001;103:1051–1056 [DOI] [PubMed] [Google Scholar]
- 11.Babiarz LS, Yousem DM, Wasserman BA, Wu C, Bilker W, Beauchamp NJ Jr. Cavernous carotid artery calcification and white matter ischemia. AJNR Am J Neuroradiol 2003;24 (5):872–877 [PMC free article] [PubMed] [Google Scholar]
- 12.Yip PK, Jeng JS, Lee TK, et al. Subtypes of ischemic stroke: a hospital-based stroke registry in Taiwan (SCAN-IV). Stroke 1997;28 (12):2507–2512 [DOI] [PubMed] [Google Scholar]
- 13.Petty GW, Brown RD Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke 1999;30 (12):2513–2516 [DOI] [PubMed] [Google Scholar]
- 14.Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke 2001;32 (11):2559–2566 [DOI] [PubMed] [Google Scholar]
- 15.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis; North American symptomatic carotid endarterectomy trial collaborators. N Engl J Med 1991;325:445–453 [DOI] [PubMed] [Google Scholar]
- 16.MRC European carotid surgery trial: interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. European carotid surgery trialists’ collaborative group. Lancet 1991;337:1235–1243 [PubMed] [Google Scholar]
- 17.Endarterectomy for asymptomatic carotid artery stenosis: Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995;273:1421–1428 [PubMed] [Google Scholar]
- 18.Deneke T, Grewe PH, Ruppert S, Balzer K, Muller KM. Atherosclerotic carotid arteries–calcification and radio-morphological findings. Z Kardiol 2000;89 (suppl 2):36–48 [DOI] [PubMed] [Google Scholar]
- 19.Shaalan WE, Cheng H, Gewertz B, et al. Degree of carotid plaque calcification in relation to symptomatic outcome and plaque inflammation. J Vasc Surg. 2004;40 (2):262–269 [DOI] [PubMed] [Google Scholar]
- 20.Woodcock RJ Jr, Goldstein JH, Kallmes DF, Cloft HJ, Phillips CD. Angiographic correlation of CT calcification in the carotid siphon. AJNR Am J Neuroradiol 1999;20:3:495–499 [PMC free article] [PubMed] [Google Scholar]
- 21.Marzewski DJ, Furlan AJ, St Louis P. Intracranial internal carotid artery stenosis: long-term prognosis. Stroke 1982;13:821–824 [DOI] [PubMed] [Google Scholar]
- 22.Craig DR, Meguro K, Watridge C, Robertson JT, Barnett HJM, Fox AJ. Intracranial internal carotid artery stenosis. Stroke 1982;13:825–828 [DOI] [PubMed] [Google Scholar]
- 23.Fisher CM, Gore I, Okabe N, White PD. Calcification of the carotid siphon. Circulation 1965;32:538–547 [DOI] [PubMed] [Google Scholar]
- 24.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000;20 (5):1262–1275 [DOI] [PubMed] [Google Scholar]
- 25.Carr S, Farb A, Pearce WH, Virmani R, Yao JS. Atherosclerotic plaque rupture in symptomatic carotid artery stenosis. J Vasc Surg 1996;23 (5):755–765 [DOI] [PubMed] [Google Scholar]
- 26.Kane AG, Dillon WP, Barkovich AJ, et al. Reduced caliber of the internal carotid artery: a normal finding with ipsilateral absence or hypoplasia of the A1 segment. AJNR Am J Neuroradiol 1996;17 (7):1295–1301 [PMC free article] [PubMed] [Google Scholar]
- 27.Gladstone DJ, Kapral MK, Fang J, Laupacis A, Tu JV. Management and outcomes of transient ischemic attacks in Ontario. CMAJ 2004;170 (7):1113–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuler JJ, Flanigan DP, Leonardo LT, et al. The effect of carotid siphon stenosis on stroke rate, death and relief of symptoms following elective carotid endarterectomy. Surgery 1982;92:1058–1067 [PubMed] [Google Scholar]
- 29.Mattos MA, van Bemmelen PS, Hodgson KJ. The influence of carotid siphon stenosis on short- and long-term outcome after carotid endarterectomy. J Vasc Surg 1993;186:902–911 [PubMed] [Google Scholar]
- 30.Ezzeddine MA, Lev MH, McDonald CT, et al. CT angiography with whole brain perfused blood volume imaging: added clinical value in the assessment of acute stroke. Stroke 2002;33 (4):959–966 [DOI] [PubMed] [Google Scholar]
- 31.Ptak T, Hunter GH, Avakian R, Novelline RA. Clinical significance of cavernous carotid calcifications encountered on head computed tomography scans performed on patients seen in the emergency department. J Comput Assist Tomogr 2003;27 (4):505–509 [DOI] [PubMed] [Google Scholar]
- 32.Oliver TB, Lammie GA, Wright AR, et al. Atherosclerotic plaque at the carotid bifurcation: CT angiographic appearance with histopathologic correlation. AJNR Am J Neuroradiol 1999;20 (5):897–901 [PMC free article] [PubMed] [Google Scholar]