Abstract
BACKGROUND AND PURPOSE: Preoperative embolization of meningiomas is frequently used to facilitate surgery and to reduce intraoperative blood loss. The purpose of this study was to evaluate the frequency of procedure-related neurologic complications during and after particle embolization of intracranial meningiomas.
METHODS: Between 1996 and 2004, 185 consecutive patients underwent particle embolization of an intracranial meningioma. Devascularization was performed by means of superselective probing of the tumor-feeding vessels and ensuing free-flow embolization with spherical particles. All procedures were performed with systemic heparinization.
RESULTS: Six patients (3.2%) had ischemic events with neurologic deficit. Two had amaurosis, and four patients presented with hemiparesis. Hemorrhage occurred in six patients (3.2%). In five of these patients, rapid microsurgical tumor removal resulted in a favorable outcome without persistent neurologic deficit. In one patient, massive intratumoral, subarachnoid, and subdural hemorrhage was lethal.
CONCLUSION: Particle embolization of meningiomas is associated with a substantial risk of ischemic and hemorrhagic events. The individual risk-to-benefit ratio of embolization should be thoroughly considered.
Since Manelfe et al (1) first described preoperative embolization of intracranial meningiomas in 1973, this technique has increasingly been applied to reduce intraoperative blood loss and to facilitate microsurgical removal. However, the potential surgical benefit must be carefully weighted against risks of this preoperative procedure. Data on the frequency of procedure-related complications are limited. In small series of patients, neurologic deficit was absent (2), and as many as 9% patients in a selected series had skull-base meningiomas (3). Causes of neurologic complications include ischemia (3), hemorrhage (4), or tumoral swelling subsequent to embolization (3, 5).
The purpose of this study was to evaluate the frequency of procedure-related neurologic complications during and after particle embolization of intracranial meningiomas.
Methods
We systematically reviewed our institutional database and identified 185 subsequent embolization procedures performed to treat meningiomas between January 1996 and May 2004. Table 1 shows the patient data.
TABLE 1:
Data of 185 patients undergoing embolization for an intracranial meningioma
| Data | Value |
|---|---|
| Mean age (years)a | 63 ± 14 |
| F : M ratio | 112:73 |
| Fluoroscopy time (minutes)a | 47 ± 35 |
| Convexity | 63 |
| Falx | 53 |
| Sphenoid wing | 22 |
| Frontobasal | 19 |
| Petroclival | 12 |
| Posterior fossa | 16 |
Data are the mean ± standard deviation.
Embolization was performed with systemic heparinization (a bolus of 50 IU/kg body weight over 15 minutes followed by an infusion of 25 IU/kg/h). Experienced consultants (including M.B., L.S.) performed the procedures exclusively in the territory of the external carotid artery. After a guidecatheter (Envoy 5F or 6F; Cordis Endovascular, Miami, FL) was introduced, the tumor feeders were superselectively probed by using a standard microcatheter (Tracker 18; Target Therapeutics, San Jose, CA) and a standard microwire (GT; Terumo, Tokyo, Japan). We ensured that outflow at the catheter tip was unobstructed and that no collaterals to parenchymal or orbital branches were present. Spherical particles (Embospheres, 40–120 [n = 12] or 100–300 μm [n = 165], BiosphereMed, Marlborough, MA; or BeadBlock, 100–300 μm, [n = 8], Terumo) were mixed with nonionic contrast medium (Imeron 250; Bracco-Byk Gulden, Konstanz, Germany). Then, the tumor was devascularized by slowly injecting the particles under fluoroscopic control.
The procedure was terminated when stagnation of the contrast agent in the feeding artery was accomplished. An independent neurosurgeon neurologically assessed the patients after the intervention. In patients with neurologic complication due to embolization, MR images and angiograms were re-evaluated. Moreover, in patients presenting with hemorrhage, corresponding histologic specimens were stained with hematoxylin-eosin, Van Gieson stain, and Prussian blue. Actin and CD-34 (for vessel walls) immunohistologic staining was performed. For every meningioma, at least two meningiomas of the same histologic subtype without hemorrhage were also reevaluated.
Results
Twelve (6.5%) of 185 patients had a procedure-related complication during or within 24 hours after embolization of an intracranial meningioma. Of these, six patients presented with an assumable ischemic event (Table 2). In six patients, hemorrhage occurred (Table 3). In 10 patients, a 100–300-μm particle was used, and in one patient in each group, embolization was performed with ultrasmall 40–120-μm particles (Tables 2 and 3). The volume of particles applied was not associated with the risk of complications. In particular, patients presenting with ischemia or hemorrhage (mean, 1.1 ± 0.4 vials) did not receive more particles than patients without complications (mean, 1.3 ± 0.6 vials).
TABLE 2:
Data in six patients with an ischemic complication
| Patient/Age (years)/Sex | Localization | Fluoroscopy Time (minutes) | Embospheres (μm) | Deficit | Outcome |
|---|---|---|---|---|---|
| 1/61/F | Sphenoid wing | 55 | 100–300 | Hemiparesis, aphasia | Resolved after 5 days |
| 2/47/F | Frontal convexity | 31 | 40–120 | Hemiparesis | Persistent |
| 3/49/M | Frontal convexity | 48 | 100–300 | Hemiparesis, aphasia | Residual hemiparesis, aphasia improved |
| 4/71/F | Frontal convexity | 39 | 100–300 | Hemiparesis | Resolved after 48 hours |
| 5/74/F | Frontal convexity | 31 | 100–300 | Amaurosis | Persistent |
| 6/63/M | Parietal convexity | 69 | 100–300 | Amaurosis | Persistent |
TABLE 3:
Data in six patients with hemorrhage
| Patient/Age (years)/Sex | Localization | Fluoroscopy Time (minutes) | Particle (μm) | Hemorrhage | Deficity and Outcome | Histologic Subtype | Iron Deposition on Histology | Pathologic Vessels |
|---|---|---|---|---|---|---|---|---|
| 7/49/F | Fossa posterior | 25 | Embospheres, 40–120 | Intratumoral, subarachnoid | Headache, resolved | Rhabdoid, WHO III | Yes | Yes |
| 8/60/F | Frontal convexity | 22 | Embospheres, 100–300 | intratumoral | Headache, resolved | Atypical, WHO II | Yes | Yes |
| 9/66/F | Frontal convexity | 36 | Embospheres, 100–300 | Subdural, intratumoral | Hemiparesis, resolved | Fibromatous, WHO I | No | No |
| 10/72/F | Temporal convexity | 41 | Embospheres, 100–300 | Subdural, intratumoral | Hemiparesis, resolved | Fibromatous, WHO I | Yes | Yes |
| 11/75/F | Frontal convexity | 25 | Embospheres, 100–300 | Intratumoral, subarachnoid | Headache, resolved | Microcystic, WHO I | No | Yes |
| 12/81/F | Frontotemporal convexity | 32 | BeadBlock, 100–300 | Intratumoral, subarachnoid, subdural | Coma, death | Unknown† | Unknowna | Unknowna |
No autopsy.
Ischemic complications comprised two cases of amaurosis and four cases of hemiparesis. Two of the latter were accompanied by aphasia. In patients with amaurosis, retinal ischemia was verified on funduscopy. In two patients with persistent hemiparesis, follow-up CT or MR imaging revealed areas of supratentorial infarction (Fig 1). In two patients, hemiparesis resolved over several days, without evidence for infarction on follow-up CT. Angiograms of these patients were reviewed for collaterals to the ophthalmic artery or branches of the internal carotid artery. In one patient, an ophthalmic anastomosis of the middle meningeal artery was seen. The microcatheter was advanced further distally, and the ophthalmic branch never opacified during the embolization procedure. Nevertheless, this patient had ipsilateral amaurosis after the procedure. In the other patient, no collaterals of the external carotid artery to the ophthalmic or parenchymal vessels were seen. Patients with ischemic events did not differ in age (61 ± 11 vs 63 ± 14 years; P > .5, Mann-Whitney test) or fluoroscopy time (45.5 ± 14.9 vs 47 ± 35 minutes; P > .5, Mann-Whitney test) from the overall group of patients.
Fig 1.
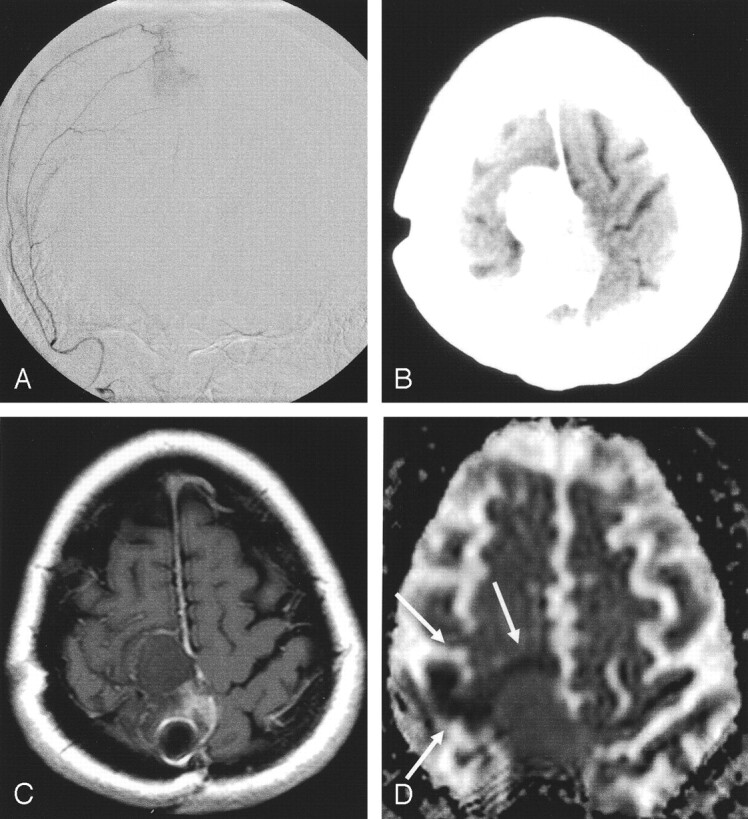
Patient 2. Peritumoral ischemia after embolization (Embospheres, 40–120 μm) of a recurrent frontal meningioma.
A, Predominant blood supply by the ipsilateral middle meningeal artery was embolized with spherical particles.
B, After the procedure, the patient had left-sided hemiparesis. CT shows attenuating pooling of contrast medium in the tumor.
C, Next day, T1-weighted spin-echo MR image shows no contrast enhancement, indicating complete devascularization of the tumor.
D, DC map shows a small, hypointense rim of brain parenchyma around the meningioma, indicating cytotoxic edema (arrows). This was interpreted as particles passing into the surrounding brain tissue via leptomeningeal collaterals.
Hemorrhage occurred and was primarily intratumoral in one patient, both intratumoral and subarachnoid in two (Fig 2), and additionally subdural in three (Fig 3). Clinically, three patients had sudden-onset headache, and two had progressive hemiparesis. One patient was asymptomatic during and 2 hours after embolization but was then found comatose. CT revealed massive intratumoral, subarachnoid, and subdural hemorrhage with signs of brainstem herniation (Fig 3). Because of the patient’s age and clinical symptoms, surgery was not performed. The patient died 1 day later. In the other patients, rapid surgical removal of the tumor resulted in good clinical outcomes without symptoms related to the hemorrhage. Angiograms of patients with hemorrhage were reviewed for the presence of atypical vascularization, and in all, typical blush of the meningioma was seen, without evidence of pathologic vessels or arteriovenous shunts (Figs 2 and 3). MR images of these patients revealed a typical pattern consistent with that of a meningioma.
Fig 2.
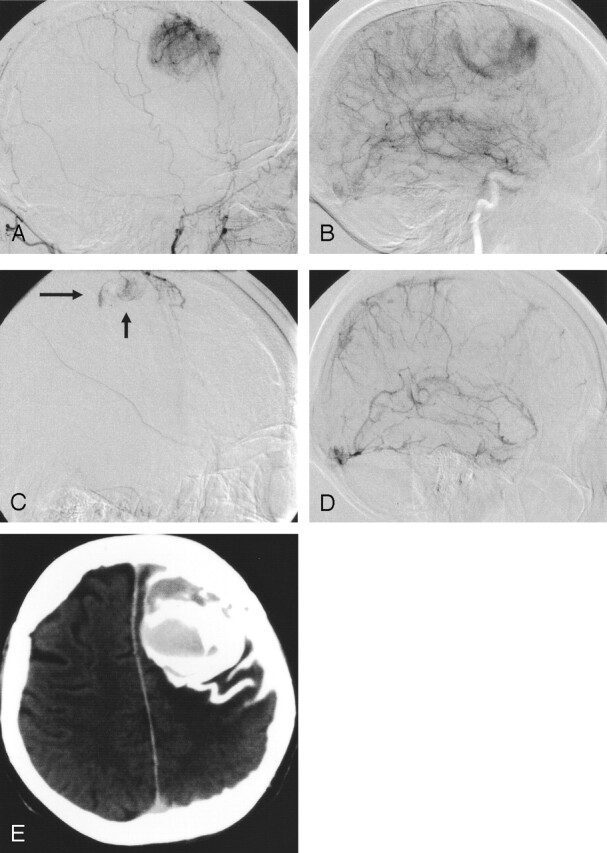
Patient 11. Subarachnoid and intratumoral hemorrhage during embolization (Embospheres, 100–300 μm) of a right frontal-convexity meningioma.
A and B, Images show blood supply by the ipsilateral middle meningeal artery (A), which was subsequently devascularized with particles, and leptomeningeal branches of the middle cerebral artery (B).
C and D, At the end of the procedure, patient had sudden-onset headache. Angiograms show subarachnoid extravasation of contrast medium (arrows in C). Control run in the internal carotid artery (D) shows disappearance of the leptomeningeal supply, indicating complete tumor devascularization.
E, Postprocedural CT shows intratumoral and subarachnoid hemorrhage. At surgery, bleeding from intratumoral vessels were slight; the fresh intratumoral clot and tumor were easily removed. The patient recovered completely.
Fig 3.
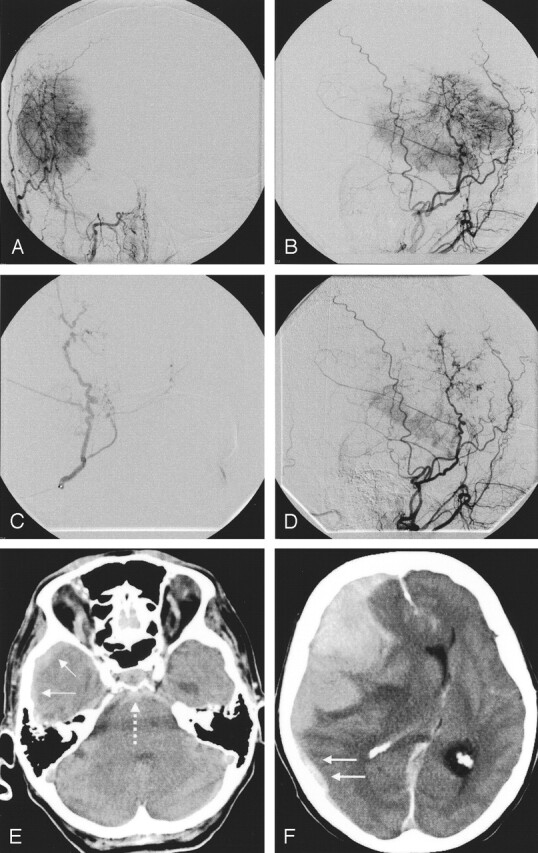
Patient 12. An 81-year-old woman with fatal subdural, subarachnoid, and intratumoral hemorrhages after embolization (Bead Block,100–300 μm).
A and B, Embolization of a large, right temporal meningioma with a predominant middle meningeal arterial supply.
C, Ipsilateral middle meningeal artery was superselectively probed and embolized with spherical particles.
D, Procedure was abandoned after the application of one vial because the patient had back pain. Control image reveals marked tumoral devascularization.
E and F, Afterward, the patient had no new neurologic symptoms, but 2 hours later, she was comatose with fixed, dilated pupils. CT shows extensive subdural (solid arrows), subarachnoid (dotted arrow) and intratumoral hemorrhage. Because of her age and clinical state, she did not undergo surgery and died the next day.
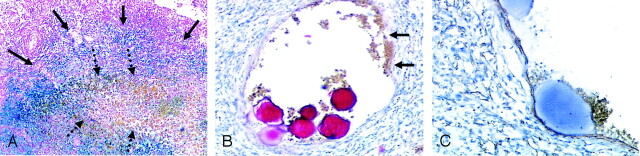
Histopathologic examination of the five meningiomas with hemorrhage revealed atypical meningiomas (World Health Organization [WHO] grades II or III) in two patients (Table 3). Iron deposition consistent with previous hemorrhage was present in three (Fig 4A). Pathologic intratumoral vessels with a thin wall relative to a large lumen were found in four patients. These vessels had walls of variable thickness sporadically mixed with a thin tunica muscularis (Fig 4B). The vessels were positive for actin on immunohistologic stain (Fig 4C). In all of these vessels, particles were found. Similar findings were not observed in control meningiomas without hemorrhage.
Fig 4.
Representative histologic findings after periprocedural hemorrhage.
A, Patient 8. Section shows massive iron deposition (blue) indicating previous intratumoral hemorrhage (solid arrows) around acute hemorrhage (brown, dotted arrows) (hematoxylin-eosin and Prussian blue, original magnification ×8).
B and C, Patient 11. Pathologic vessels with variable wall thickness were seen in four of five patients (B, hematoxylin-eosin, original magnification ×20). In some areas, the wall is atypically thin relative to the lumen (arrows in B). These vessels were positive for actin, indicating arteries (C, original magnification ×50). Similar vessels, also filled with particles, were seen in other patients with hemorrhage.
Discussion
The treatment of choice for intracranial meningiomas is microsurgical tumor resection. However, this procedure is associated with a substantial mortality rate of 7%–14%, as shown in an unselected series of meningiomas (6). This rate is even increases in large meningiomas or in meningiomas with a difficult surgical approach, such as those in the skull base (3). Preoperative embolization of hypervascularized intracranial lesions such as meningiomas has become a well-established preoperative procedure to facilitate surgery and to reduce intraoperative blood loss. However, a thorough risk-benefit analysis is mandatory (7). In the literature, data on the benefit of embolization followed by surgery compared with surgery without embolization are limited. Two retrospective studies showed a beneficial effect in terms of estimated blood loss at surgery (8, 9). In a prospective study, we compared embolized and nonembolized meningiomas treated with surgery and found difference in intraoperative blood loss only in completely devascularized meningiomas, whereas the other parameters did not significantly differ (10).
Data on the neurologic risk of embolization are also sparse and controversial. In some small, unselected studies, neurologic complications were absent (2) or occurred at rates of 12% (8) or 16% (11). In a large, selected series of skull-base meningiomas, neurologic deficit occurred in as many as 21.6% of patients, with permanent deficits in 9% (3). In the present series of 185 unselected meningiomas, the overall rate of neurologic adverse events was 6.5%, with persistent neurologic deficit occurring in 2.2% and death, in 0.5%. Ischemic or hemorrhagic events cause neurologic complications during or after embolization. Ischemia may be due to thromboembolism or unintentional particle obstruction of parenchymal branches. Although we performed all procedures by using anticoagulation with heparin and though we cautiously injected the particles under permanent fluoroscopic control, six ischemic events occurred. Two were territorial infarctions, presumably caused by thromboembolic events. In one patient (patient 2, Fig 1) ischemia was most likely caused by particles passing from the external carotid artery to the internal carotid artery through leptomeningeal anastomoses, as follow-up diffusion MR imaging showed a rim of ischemic brain tissue surrounding the embolized tumor. In this patient ultrasmall particles of 40–120 μm were used. We speculate that these particles passed through arterial-arterial anastomosis, resulting in this pattern of ischemia. Therefore, ultrasmall particles may increase the risk for ischemic events during embolization.
In both patients with amaurosis, no orbital branches opacified during embolization; therefore, the reason for the complication (thromboembolism or particle embolism) remains uncertain. In retrospect, all ischemic events were unpredictable and not related to factors increasing the risk, such as age or long fluoroscopy times.
In the literature, we identified nine cases of hemorrhage due to embolization (3, 4, 12–18). In this study, we had an unexpectedly high rate of hemorrhagic events during or after embolization of 3.2%. Spontaneous hemorrhage occurs in about 1.3% of meningiomas (19) and is more frequent in atypical meningiomas than in typical ones (20). About 10% of all meningiomas are atypical (21). In our, series, two (40%) of five patients had an atypical meningioma. Moreover, pathologic vessels with a thin wall relative to the lumen were found in four of five patients. Last, iron deposition, which indicates previous intratumoral hemorrhage, was seen in three patients. Similar findings were not observed in any meningioma of the same histologic type without hemorrhage. These results clearly indicated that hemorrhage in embolized meningiomas was associated with a distinct histologic profile (ie, atypical histology), pathologic vessels with a thin wall, and previous hemorrhage. Before embolization, T2-weighted gradient-echo MR imaging, may be an option to detect these previous hemorrhages and thereby identify patients at risk.
Hemorrhage in embolized meningiomas may be caused by particles passing from the arterial side to the venous side via arteriovenous shunts. In this case, venous outflow is obstructed, while some arterial input remains, resulting in increased transmural pressure and subsequent hemorrhage. However, we observed particles only in arterial vessels, as indicated on actin stains.
Tumor infarction has been suggested as another possible mechanism of spontaneous hemorrhage (22). Likewise, intratumoral ischemia and subsequent necrosis due to vascular obstruction by particles might cause intratumoral hemorrhage during or after embolization. Both histology (23) and MR imaging (24) have demonstrated extensive areas of necrosis after embolization of meningiomas; these areas were more extensive with spherical particles than with polyvinyl alcohol particles of different sizes (23). It cannot be excluded that the superiority of spherical particles in terms of distal vessel occlusion causing subsequent tissue necrosis may result in an increased rate of hemorrhagic events. However, all cases reported so far involved embolization with polyvinyl alcohol particles, Gelfoam powder, or gelatin sponges (3, 4, 12–18).
Conclusion
The overall neurologic complication rate of 6.5% underscores the need for thorough risk-benefit analysis before meningioma embolization. Periprocedural hemorrhage was more frequent than previously thought and can be managed with rapid surgical evacuation of the hematoma and removal of the tumor. However, to recognize this potentially life-threatening complication early enough to treat it, thorough postprocedural clinical surveillance is advised.
Acknowledgments
We would like to thank Professor W. Roggendorf, MD, for evaluating the histologic specimen and Sonja Bendszus for collecting the patient data.
References
- 1.Manelfe C, Guiraud B, David J, et al. Embolisation par catherterisme des meningiomes intracranienes. Rev Neurol 1973;128:339–351 [PubMed] [Google Scholar]
- 2.Hieshima GB, Everhart FR, Mehringer CM, et al. Preoperative embolization of meningiomas. Surg Neurol 1980;14:119–127 [PubMed] [Google Scholar]
- 3.Rosen CL, Ammerman JM, Sekhar LN, Bank WO. Outcome analysis of preoperative embolization in cranial base surgery. Acta Neurochir (Wien) 2002;144:1157–1164 [DOI] [PubMed] [Google Scholar]
- 4.Yu SC, Boet R, Wong GK, et al. Postembolization hemorrhage of a large and necrotic meningioma. AJNR Am J Neuroradiol 2004;25:506–508 [PMC free article] [PubMed] [Google Scholar]
- 5.Wakhloo AK, Juengling FD, Van Velthoven V, et al. Extended preoperative polyvinyl alcohol microembolization of intracranial meningiomas: assessment of two embolization techniques. AJNR Am J Neuroradiol 1993;14:571–581 [PMC free article] [PubMed] [Google Scholar]
- 6.Black PM. Meningiomas. Neurosurgery 1993;32:643–657 [DOI] [PubMed] [Google Scholar]
- 7.Latchaw RE. Preoperative intracranial meningioma embolization: technical considerations affecting the risk-to-benefit ratio. AJNR Am J Neuroradiol 1993;14:583–586 [PMC free article] [PubMed] [Google Scholar]
- 8.Dean B, Flom RA, Wallace RC, et al. Efficacy of endovascular treatment of meningiomas: evaluation with matched samples. AJNR Am J Neuroradiol 1993;15:1675–1680 [PMC free article] [PubMed] [Google Scholar]
- 9.Macpherson P. The value of pre-operative embolisation of meningioma estimated subjectively and objectively. Neuroradiology 1991;33:334–337 [DOI] [PubMed] [Google Scholar]
- 10.Bendszus M, Rao G, Burger R, et al. Is there a benefit of preoperative meningioma embolization? Neurosurgery 2000;47:1306–1312 [PubMed] [Google Scholar]
- 11.Richter HP, Schachenmayr W. Preoperative embolization of intracranial meningiomas. Neurosurgery 1983;13:261–268 [DOI] [PubMed] [Google Scholar]
- 12.Hayashi T, Shojima K, Utsunomiya H, et al. Subarachnoid hemorrhage after preoperative embolization of a cystic meningioma. Surg Neurol 1987;27:295–300 [DOI] [PubMed] [Google Scholar]
- 13.Suyama T, Tamaki N, Fujiwara K, et al. Peritumoral and intratumoral hemorrhage after gelatin sponge embolization of malignant meningioma: case report. Neurosurgery 1987;21:944–946 [DOI] [PubMed] [Google Scholar]
- 14.Watanabe K, Matsumura K, Matsuda M, Handa J. Meningioma with intratumoral and subdural hemorrhage as an immediate complication of therapeutic embolization: case report. Neurol Med Chir (Tokyo) 1986;26:904–907 [DOI] [PubMed] [Google Scholar]
- 15.Kallmes DF, Evans AJ, Kaptain GJ, et al. Hemorrhagic complications in embolization of a meningioma: case report and review of the literature. Neuroradiology 1997;39:877–880 [DOI] [PubMed] [Google Scholar]
- 16.Beretta L, Dell’Acqua A, Giorgi E, et al. Complications during preoperative embolization in intracranial meningioma. Minerva Anestesiol 1992;58:111–114 [PubMed] [Google Scholar]
- 17.Motozaki T, Otuka S, Sato S, et al. Preoperative embolization with gelfoam powder for intracranial meningioma causing unusual peritumoral hemorrhage: with reference to the mechanism of hemorrhage. No Shinkei Geka 1987;15:95–101 [PubMed] [Google Scholar]
- 18.Shojima K, Hayashi T, Higashihara H, et al. Cystic meningioma associated with intratumor and subarachnoid hemorrhage during embolization: a case report. No Shinkei Geka 1986;14:919–924 [PubMed] [Google Scholar]
- 19.Modesti LM, Binet EF, Collins GH. Meningiomas causing spontaneous intracranial hematomas. Neurosurgery 1976;45:437–441 [DOI] [PubMed] [Google Scholar]
- 20.Helle TL, Conley FK. Hemorrhage associated with meningioma: a case report and review of the literature. J Neurol Neurosurg Psychiatry 1980;43:725–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buccoliero AM, Caldarella A, Taddei A, et al. Atypical, aplastic, and unusual meningiomas: morphology and incidence in 300 consecutive cases. Pathologica 2003;95:83–87 [PubMed] [Google Scholar]
- 22.Kim DG, Park CK, Paek SH, et al. Meningioma manifesting intracerebral haemorrhage: a possible mechanism of haemorrhage. Acta Neurochir (Wien) 2000;142:165–168 [DOI] [PubMed] [Google Scholar]
- 23.Bendszus M, Klein R, Burger R, et al. Efficacy of trisacryl gelatin microspheres versus polyvinyl alcohol particles in the preoperative embolization of meningiomas. AJNR Am J Neuroradiol 2000;21:255–261 [PMC free article] [PubMed] [Google Scholar]
- 24.Bendszus M, Warmuth-Metz M, Klein R, et al. Sequential MRI and MR-spectroscopy (MRS) in embolized meningiomas: correlation with surgical and histopathological findings. Neuroradiology 2002;44:77–82 [DOI] [PubMed] [Google Scholar]